Abstract
Objective
To determine the associations of FGF23 with death, HF, and CVD and investigate the influence of CKD in a general community-living population.
Background
FGF23 increases renal phosphorus excretion and inhibits vitamin D activation. In ESRD, high FGF23 levels are associated with mortality. The associations of FGF23 with death, heart failure (HF), and cardiovascular disease (CVD) in teh general population are unknown.
Methods
Plasma FGF23 was measured in 3,107 community-living persons ≥ 65 years in 1996–97, and participants were followed through 2008. HF and CVD events were adjudicated by a panel of experts. Associations of FGF23 with each outcome were evaluated using Cox proportional hazards models, and we tested whether associations differed by CKD status.
Results
Both lower eGFR and higher urine ACR were associated with high FGF23 at baseline. During 10.5 years (median) follow-up, there were 1,730 deaths, 697 incident HF events, and 797 incident CVD events. Although high FGF23 concentrations were associated with each outcome in combined analyses, the associations were consistently stronger for those with CKD (P interactions all < 0.006). In the CKD group (n=1,128), the highest FGF23 quartile had adjusted hazards ratios (HR) of 1.87 (1.47, 2.38) for all-cause death, 1.94 (1.32, 2.83) for incident HF, and 1.49 (1.02, 2.18) for incident CVD events compared to the lowest quartile. Corresponding HRs in those without CKD (n=1,979) were 1.29 (1.05, 1.59), 1.37 (0.99, 1.89), and 1.07 (0.79, 1.45).
Conclusions
FGF23, a hormone involved in phosphorous and vitamin D homeostasis, is independently associated with all-cause death and incident HF in community-living older persons. These associations appear stronger in persons with CKD.
Keywords: Fibroblast growth factor-23, kidney disease, mineral metabolism, cardiovascular disease, heart failure, elderly
INTRODUCTION
Fibroblast growth factor-23 (FGF23) is a 32KDa hormone secreted into blood from bone osteocytes. Its two functions are to induce urinary phosphorus excretion and to inhibit activation of vitamin D; both actions occur in the renal proximal tubule. Rare human disorders of FGF23 excess are characterized by high renal phosphorus excretion, low serum phosphorus levels, calcitriol deficiency, osteomalacia and spontaneous fractures.(1) Recent in vitro and rodent studies also suggest that FGF23 may directly influence cardiac myocytes to hypertrophy.(2)
Circulating FGF23 concentrations are often several orders of magnitude higher in patients with end-stage renal disease (ESRD), and are strongly associated with mortality risk in that setting.(3) The association of FGF23 with mortality was recently extended to individuals with moderate to severe chronic kidney disease (CKD), and to populations with known prevalent cardiovascular disease (CVD).(4–7) Little is known about FGF23 levels in community-living individuals without CKD or CVD.(8)
The purpose of this study was to examine the relationship of FGF23 with all-cause death, incident HF, and incident CVD events in a free living population of older persons with long-term follow-up, and considerable numbers of events. We aimed to determine the strength of the association of FGF23 with kidney function at the baseline visit, and to examine the extent to which associations of FGF23 with longitudinal outcomes were dependent on presence of concomitant CKD. Because FGF23 exerts its main biological effects at the renal proximal tubule,(9–11) and is tightly linked with kidney dysfunction,(12) we hypothesized that FGF23 might provide novel insights into mechanisms linking CKD with adverse outcomes in community living older persons.
METHODS
Participants
The Cardiovascular Health Study (CHS) is a community-based study of older adults, designed to evaluate risk factors for CVD. The study design and protocols have been described previously.(13,14) In brief, eligibility required age ≥ 65 years, expectation to remain in the area for 3 years after recruitment, no active cancer treatment, and the ability to provide consent. Between 1989–90, 5201 participants were recruited from four US communities using Medicare eligibility lists. An additional 687 African-Americans were recruited in 1992–93. In person examinations were performed annually through 1998–99 and again in 2005–06. Telephone interviews were conducted semiannually from 1989 to 1999 and biannually thereafter. We conducted FGF23 measurements at the 1996–97 study visit, selected because it was the first visit at which urine albumin to creatinine ratios (ACR) was measured. Among 3,406 individuals who participated, we excluded individuals with insufficient blood specimens for FGF23 measurement (n=69), missing creatinine (n=1), cystatin C (n=0), urine ACR (n=92), or covariate data (n=137) resulting in a final analytic sample of 3,107 participants for this analysis.
Measurements
Fibroblast growth factor-23
Fasting (8-hour) EDTA specimens collected at the 1996–97 study visit were stored at −70° Celsius until 2010 when they were thawed and measured for FGF23 using a C-terminal ELISA kit (Immutopics, www.immutopicsintl.com).(15) Our estimates of the intra-assay and inter-assay coefficients of variation (CVs) ranged from 7.4 and 10.6%.
Outcomes
Methods of ascertainment for adjudication of death, HF, and CVD events have been described previously.(16–19) In brief, deaths were identified by review of obituaries, medical records, death certificates, the Centers for Medicare and Medicaid Services health care-utilization database for hospitalizations and from household contacts; 100% complete follow-up for ascertainment of mortality status was achieved.
All HF and CVD events were adjudicated by the CHS Events Committee. Participants with history of HF were excluded in analyses evaluating incident HF, and likewise, those with prevalent CVD (history of myocardial infarction [MI], percutaneous coronary intervention, coronary artery bypass graft surgery, transient ischemic attack, stroke, or claudication) were excluded in incident CVD events analyses. Incident HF required a physician’s diagnosis of HF, and adjudication by the Events Committee required symptoms, signs, chest radiographic findings, and treatment of heart failure.(16,17) We evaluated a composite CVD outcome defined as incident fatal or non-fatal MI, incident fatal or non-fatal stroke, and CVD death; whichever came first. MI was ascertained from hospital records and was indicated by a clinical history of cardiac symptoms, elevated cardiac enzyme concentrations, and serial electrocardiographic changes.(17) Cases of possible stroke were adjudicated by a committee of neurologists, neuroradiologists, and internists on the basis of interviews with patients, medical records, and brain imaging studies.(18) CVD death was defined as death caused by coronary heart disease, HF, peripheral arterial disease, or cerebrovascular disease.(19)
Other Measurements
Information on baseline confounders were obtained at the 1996–97 study visit concurrent with FGF23 and included age, sex, race, self-reported health status; and CVD risk factors including hypertension (systolic blood pressure ≥ 140mmHg, diastolic blood pressure ≥ 90mmHg, or use of anti-hypertensive medications), impaired fasting glucose (fasting glucose 100–125 mg/dL), diabetes (fasting glucose ≥ 126 mg/dL or use of anti-glycemic medications or insulin), smoking (current, former, or never), body mass index, total cholesterol, use of lipid lowering medications, and C-reactive protein concentrations.(20)
Cystatin C concentrations were measured using a BN II nephelometer (Siemens; www.siemens.com) (10) Estimated glomerular filtration rate (eGFR) was calculated using the equation eGFR=76.7 * cystatin C (mg/L) −1.19.(21) In companion analyses, we calculated eGFR using serum creatinine and the CKD-Epidemiology equation.(22) A random morning urine sample was obtained and measured for urine albumin by rate nephelometry and creatinine using a Kodak Ektachem 700 Analyzer, and urine ACR was calculated in mg/g.
Statistical Analysis
We categorized participants into quartiles based on the distribution of FGF23 in the study sample, and evaluated the distribution of confounders across FGF23 categories. Next, we evaluated the unadjusted correlation of eGFR and ACR with FGF23 using Spearman correlation coefficients. Next, we constructed natural piecewise cubic spline functions with FGF23 as the dependent variable, and with pre-specified knots placed at the quartiles of the distributions of eGFR and ACR. The spline function evaluating eGFR as the predictor of FGF23 adjusted for age, sex, race, and ACR; conversely, the spline of ACR predicting FGF23 was adjusted for age, sex, race, and eGFR.
Cox regression was used to evaluate the association of FGF23 with each outcome. In models evaluating incident HF and CVD, persons with prevalent disease at baseline were excluded. The primary analyses evaluated FGF23 quartiles with the lowest as the reference category. We also evaluated FGF23 as a continuous variable after log base 2 transformation, interpreted as “per doubling”. For each outcome, an initial model was adjusted for age, sex, and race. A second model added self-reported health status, estrogen use among women,(23) prevalent CVD and HF (where applicable), traditional CVD risk factors, and C-reactive protein levels. A final model added eGFR and natural log-ACR. Next, we stratified patients by eGFR < 60ml/min/1.73m2 vs. greater and tested interactions of FGF23 by eGFR for each outcome. Similarly, we tested interactions by urine ACR dichotomized at 30mg/g. In both cases, associations were stronger in persons with CKD, so we defined a single variable of CKD (eGFR < 60ml/min/1.73m2 or ACR ≥ 30mg/g) and evaluated an FGF23*CKD interaction term in the final adjusted model for each outcome. All analyses were also repeated in a sensitivity analysis using eGFR by the CKD-Epidemiology collaboration equation(22) in place of eGFR by cystatin C.
RESULTS
Among the 3,107 participants, mean age was 78±5 years and 60% were female. Nine percent had a history of HF and 29% had a history of CVD at baseline. The mean eGFR was 71±19 ml/min/1.73m2, and median urine ACR was 8.8 (interquartile range [IQR] 4.7–20.4) mg/g. The distribution of FGF23 was right skewed, with a median 70 (IQR 53–99) RU/ml.
Compared to participants in the lowest FGF23 quartile, those with higher levels were older, more frequently female and Caucasian, reported poorer health status, and had more than 2-fold greater prevalence of HF, MI, and claudication at baseline, whereas the prevalence of prior stroke was similar across quartiles (Table 1). Individuals with higher FGF23 concentrations also had a higher prevalence of most traditional CVD risk factors, lower eGFR, and higher ACR.
Table 1.
Baseline Characteristics by Quartiles of Fibroblast Growth Factor-23. The Cardiovascular Health Study
| Range (RU/ml) | Fibroblast Growth Factor-23 Quartiles
|
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| < 51 | 51–70 | 71–100 | > 100 | |
| Number of Participants | 792 | 785 | 774 | 756 |
| Demographics | ||||
| Age (years) ± SD | 77 ± 5 | 77 ± 5 | 78 ± 5 | 79 ± 5 |
| Female n(%) | 421 (53%) | 444 (57%) | 491 (63%) | 503 (67%) |
| Black race n(%) | 180 (23%) | 115 (15%) | 96 (12%) | 106 (14%) |
| Self reported health fair or poor n(%) | 137 (17%) | 152 (19%) | 139 (18%) | 266 (35%) |
| Prevalent HF and CVD | ||||
| History of HF n(%) | 25 (3%) | 41 (5%) | 55 (7%) | 150 (20%) |
| History of MI n(%) | 60 (8%) | 59 (8%) | 93 (12%) | 127 (17%) |
| History of Stroke n(%) | 42 (5%) | 52 (7%) | 38 (5%) | 58 (8%) |
| History of Claudication n(%) | 16 (2%) | 16 (2%) | 27 (4%) | 39 (5%) |
| CVD Risk Factors | ||||
| Hypertension n(%) | 457 (58%) | 482 (61%) | 492 (64%) | 523 (69%) |
| Glycemia Status n(%) | ||||
| Impaired Fasting Glucose | 38 (5%) | 51 (7%) | 66 (9%) | 60 (8%) |
| Diabetes | 81 (10%) | 103 (13%) | 114 (15%) | 155 (21%) |
| Current smoker n(%) | 42 (5%) | 46 (6%) | 77 (10%) | 75 (10%) |
| Body mass index (kg/m2) ± SD | 26.3 ± 4.3 | 26.7 ± 4.2 | 27.3 ± 4.6 | 27.5 ± 5.3 |
| Total cholesterol (mg/dl) ± SD | 200 ± 37 | 202 ± 38 | 204 ± 41 | 203 ± 42 |
| Lipid lowering medication n(%) | 72 (9%) | 80 (10%) | 106 (14%) | 106 (14%) |
| C-reactive protein (mg/L), median [IQR] | 1.83 [0.91, 3.89] | 2.19 [0.99, 4.64] | 2.85 [1.28, 5.64] | 3.12 [1.50, 6.79] |
| Kidney Function | ||||
| eGFR (ml/min/1.73m2) ± SD | 81 ± 18 | 74 ± 17 | 69 ± 16 | 58 ± 19 |
| Urine ACR (mg/g), median (IQR) | 6.86 [4.28, 14.33] | 8.37 [4.50, 17.51] | 8.34 [4.71, 18.97] | 13.36 [6.01, 54.41] |
Abbreviations: HF=heart failure; CVD=cardiovascular disease; MI=myocardial infarction; eGFR=estimated glomerular filtration rate; ACR=albumin to creatinine ratio.
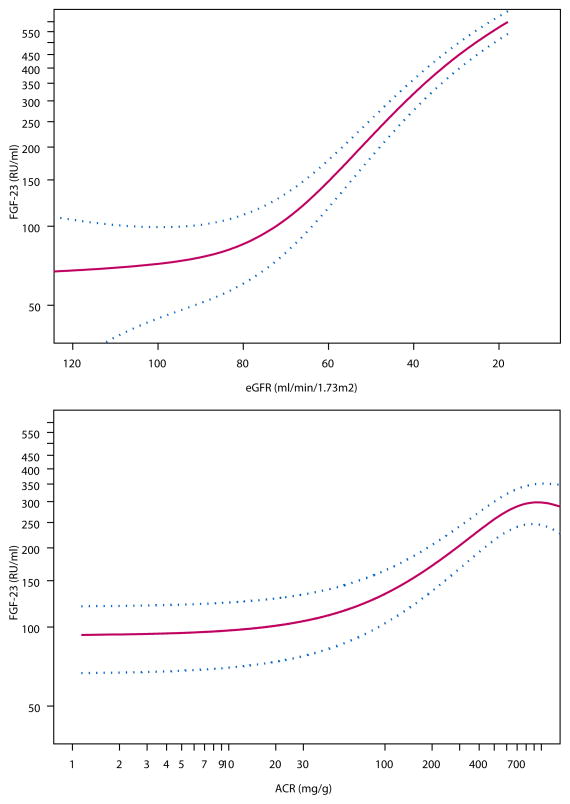
The unadjusted Spearman correlation of FGF23 with eGFR and= ACR were −0.42 and 0.20, respectively (P< 0.001 for each). Spline functions demonstrated that the relationships of eGFR and ACR with FGF23 were independent of one another. The relationship of eGFR with FGF23 appeared to increase consistently at eGFR levels less than 80ml/min/1.73m2, independent of age, sex, race, or urine ACR (Figure 1A). Similarly, urine ACR levels greater than 30mg/g were associated with progressively higher FGF23 levels, independent of age, sex, race, and eGFR (Figure 1B).
Figure 1. Relationship of Estimated Glomerular Filtration Rate (1A) and Urine Albumin to Creatinine Ratio (1B) with Fibroblast Growth Factor-23 in Community-Living Individuals.
Cubic spline function demonstrating the adjusted cross-sectional association of (A) eGFR estimated by cystatin C and (B) ACR with plasma FGF-23 levels. Red lines represent the adjusted point estimates, and blue lines represent the 95% confidence intervals. The y-axis demonstrates the change in log FGF23. The spline functions for eGFR (Figure 1A) was adjusted for age, sex, race, and urine ACR. Spline function for ACR (Figure 1B) was adjusted for age, sex, race, and eGFR., The extreme 2.5% of the distribution of eGFR and ACR were excluded to avoid improbable extrapolations based on extremes of the data.
During a median 10.5 (IQR 5.9–11.5) years of follow-up, 1,730 participants died, 697 had incident HF, and 797 had incident CVD events. There was a graded relationship of higher FGF23 levels with higher event rates for each outcome (Table 2). Associations were particularly strong for all-cause mortality and HF. These associations were modestly attenuated with adjustment for traditional CVD risk factors. Further adjustment for eGFR and urine ACR attenuated each association by about half. Nonetheless, compared to the lowest FGF23 quartile, the highest quartile remained associated with 40% greater death risk and 50% greater HF risk compared to the lowest quartile. The association with incident CVD was weaker by the point estimate and not statistically significant after adjustment for eGFR and ACR. Results were similar when eGFR was estimated using creatinine instead of cystatin C (data not shown).
Table 2.
Association of FGF23 with Incident Heart Failure, Cardiovascular Disease, and All-Cause Death. The Cardiovascular Health Study
| FGF23 Range (RU/mL) | FGF23 Quartiles
|
Linear model
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Per Doubling of FGF23 | P-value§ | |
| < 51 | 51–70 | 71–100 | > 100 | |||
| All-Cause Mortality | ||||||
| Annual event rate (# events/ # at risk) | 4.8% (350/792) | 5.6% (397/785) | 6.2% (419/774) | 10.3% (554/756) | ||
| Age, sex, race adjusted; HR (95% CI) | 1.00 (ref) | 1.18 (1.02, 1.36) | 1.29 (1.12, 1.48) | 2.29 (2.00, 2.62) | 1.65 (1.53, 1.77) | <0.001 |
| + CVD risk factors*; HR (95% CI) | 1.00 (ref) | 0.98 (0.77, 1.25)0 | 1.16 (0.92, 1.47)0 | 1.87 (1.49, 2.36) | 1.49 (1.31, 1.69) | <0.001 |
| + kidney function**; HR (95% CI) | 1.00 (ref) | 1.03 (0.89, 1.19) | 1.05 (0.90, 1.21) | 1.42 (1.22, 1.65) | 1.25 (1.14, 1.36) | <0.001 |
| Incident Heart Failure † | ||||||
| Annual event rate (# events/ # at risk) | 2.2% (147/767) | 2.7% (169/744) | 3.0% (172/719) | 5.3% (209/606) | ||
| Age, sex, race adjusted; HR (95% CI) | 1.00 (ref) | 1.28 (1.02, 1.60) | 1.37 (1.10, 1.71) | 2.47 (1.99, 3.07) | 1.76 (1.57, 1.97) | <0.001 |
| + CVD risk factors*; HR (95% CI) | 1.00 (ref) | 1.21 (0.97, 1.51) | 1.27 (1.01, 1.59) | 2.07 (1.66, 2.58) | 1.62 (1.44, 1.83) | <0.001 |
| + kidney function**; HR (95% CI) | 1.00 (ref) | 1.10 (0.88, 1.38) | 1.10 (0.88, 1.39) | 1.55 (1.22, 1.97) | 1.41 (1.23, 1.61) | <0.001 |
| Incident Cardiovascular Disease ‡ | ||||||
| Annual event rate (# events/ # at risk) | 3.7% (188/600) | 4.5% (213/587) | 4.8% (205/558) | 6.5% (191/461) | ||
| Age, sex, race adjusted; HR (95% CI) | 1.00 (ref) | 1.23 (1.01, 1.50) | 1.31 (1.07, 1.60) | 1.74 (1.42, 2.14) | 1.36 (1.20, 1.53) | <0.001 |
| + CVD risk factors*; HR (95% CI) | 1.00 (ref) | 1.17 (0.96, 1.43) | 1.21 (0.99, 1.48) | 1.50 (1.22, 1.86) | 1.24 (1.10, 1.41) | 0.001 |
| + kidney function**; HR (95% CI) | 1.00 (ref) | 1.14 (0.93, 1.39) | 1.13 (0.92, 1.40) | 1.30 (1.04, 1.63) | 1.12 (0.98, 1.29) | 0.10 |
Abbreviations: FGF23=Fibroblast growth factor-23; SD=standard deviation; HR=hazard ratio; CI=confidence interval.
P-value for the linear Cox model.
Excludes 271 participants with prevalent heart failure at baseline.
Excludes 901 participants with prevalent cardiovascular disease at baseline
Adjusted for age, sex, race, health status (fair or poor vs. better), current smoking, prior stroke, prior MI, prior HF, prior claudication, hypertension, diabetes (nl, ifg, dm), bmi, estrogen use (women), total chol, lipid med use, natural log (CRP).
Adjusted for CVD risk factor model (“*”) and eGFR and natural log (ACR).
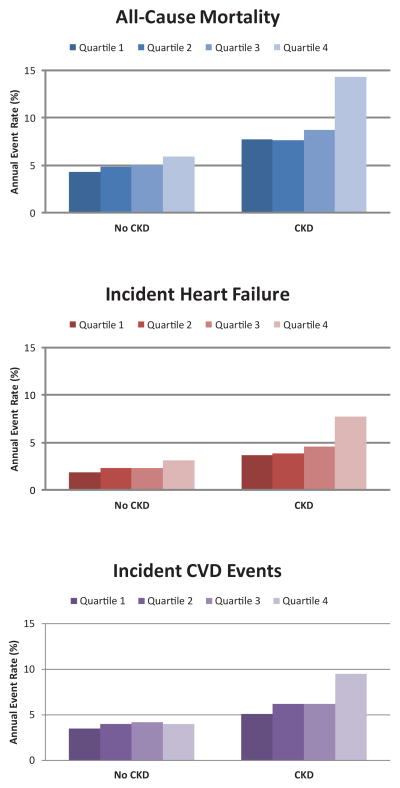
We observed that the associations of FGF23 with each outcome were significantly stronger among individuals with CKD (all p interactions < 0.006; Figure 2). Among persons without CKD, statistically significant associations were observed for FGF23 with all-cause mortality and incident HF, but no association was observed for incident CVD events (Table 3). In contrast, among those with CKD, associations were particularly strong for death and incident HF, and were weaker for incident CVD events. When evaluating the components of the composite incident CVD outcome, high FGF23 had the strongest association with CVD death, followed by incident stroke. No association was observed between FGF23 and incident MI irrespective of CKD status (Supplemental Table 1).
Figure 2. Associations of Fibroblast Growth Factor-23 Quartiles and Risk of All-Cause Mortality, Incident Heart Failure, and Incident CVD Events: The Cardiovascular Health Study.
Annual event rates of (A) all-cause mortality, (B) incident heart failure, and (C) incident cardiovascular disease events by quartiles of FGF23, stratified by chronic kidney disease status. Patients with prevalent heart failure and cardiovascular disease at the baseline examination were excluded from figures 2B and 2C respectively.
Table 3.
Association of FGF23 with Incident Heart Failure, Cardiovascular Disease, and All-Cause Death Stratified by CKD. The Cardiovascular Health Study€
| FGF23 Range (RU/mL) | FGF23 Quartiles
|
Linear model
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Per Doubling of FGF23 | P-value§ | |
| < 51 | 51–70 | 71–100 | > 100 | |||
| Chronic Kidney Disease (N=1,128) | ||||||
| All-Cause Mortality | ||||||
| Annual event rate (total # events/total # at risk) | 7.7 (90/141) | 7.6 (150/235) | 8.7 (191/285) | 14.3 (404/467) | ||
| Adjusted* HR (95% CI) | 1.00 (ref) | 0.97 (0.75, 1.26) | 1.09 (0.84, 1.41) | 1.87 (1.47, 2.38) | 1.41 (1.30, 1.52) | <0.001 |
| Incident Heart Failure † | ||||||
| Annual event rate (total # events/total # at risk) | 3.7 (38/136) | 3.8 (63/213) | 4.6 (81/256) | 7.7 (145/350) | ||
| Adjusted* HR (95% CI) | 1.00 (ref) | 0.97 (0.64, 1.46) | 1.16 (0.78, 1.73) | 1.94 (1.32, 2.83) | 1.52 (1.33, 1.72) | <0.001 |
| Incident Cardiovascular Disease ‡ | ||||||
| Annual event rate (total # events/total # at risk) | 5.1 (39/104) | 6.2 (71/156) | 6.2 (76/184) | 9.5 (126/257) | ||
| Adjusted* HR (95% CI) | 1.00 (ref) | 1.09 (0.74, 1.63) | 1.09 (0.73, 1.62) | 1.49 (1.02, 2.18) | 1.24 (1.09, 1.43) | 0.002 |
| No Chronic Kidney Disease (N=1,979) | ||||||
| All-Cause Mortality | ||||||
| Annual event rate (total # events/total # at risk) | 4.3 (270/651) | 4.8 (247/550) | 5.0 (228/489) | 5.9 (150/289) | ||
| Adjusted* HR (95% CI) | 1.00 (ref) | 1.10 (0.93, 1.32) | 1.15 (0.96, 1.38) | 1.29 (1.05, 1.59) | 1.07 (0.98, 1.17) | 0.15 |
| Incident Heart Failure † | ||||||
| Annual event rate (total # events/total # at risk) | 1.9 (109/631) | 2.3 (106/531) | 2.3 (91/463) | 3.1 (64/256) | ||
| Adjusted* HR (95% CI) | 1.00 (ref) | 1.19 (0.90, 1.56) | 1.11 (0.84, 1.48) | 1.37 (0.99, 1.89) | 1.17 (1.02, 1.33) | 0.023 |
| Incident Cardiovascular Disease ‡ | ||||||
| Annual event rate (total # events/total # at risk) | 3.5 (149/496) | 4.0 (142/431) | 4.2 (129/374) | 4.0 (65/204) | ||
| Adjusted* HR (95% CI) | 1.00 (ref) | 1.12 (0.89, 1.42) | 1.16 (0.91, 1.42) | 1.07 (0.79, 1.45) | 0.99 (0.87, 1.13) | 0.899 |
Abbreviations: FGF23=Fibroblast growth factor-23; SD=standard deviation; HR=hazard ratio; CI=confidence interval; CKD=Chronic Kidney Disease
CKD defined as either eGFR < 60 ml/min/1.73m2 or urine albumin to creatinine ratio > 30mg/g.
P-values for interaction in the adjusted model were < 0.001 for all cause mortality, 0.008 for incident HF, and 0.006 for incident CVD
P-value for the linear Cox model.
Excludes 271 participants with prevalent heart failure at baseline.
Excludes 901 participants with prevalent cardiovascular disease at baseline
Adjusted for age, sex, race, health status (fair or poor vs. better), current smoking, prior stroke, prior MI, prior HF, prior claudication, hypertension, diabetes (nl, ifg, dm), bmi, estrogen use (women), total chol, lipid med use, natural log (CRP).
We conducted sensitivity analyses where we used the CKD-Epi equation rather than cystatin C to estimate GFR. In all cases, results were similar when results were adjusted for and stratified by CKD-Epi defined eGFR (data not shown). Last, because high FGF23 was strongly correlated with CKD severity, we tested whether the stronger associations within the CKD stratum might reflect differing severity of CKD. To investigate this possibility, we evaluated the final model with additional adjustment for eGFR and urine ACR within the CKD strata. Results were similar to the stratified analyses presented previously (HR per doubling of FGF23 1.29 [1.19, 1.41] for all-cause death, 1.36 [1.18, 1.57] for incident HF, and 1.15 [0.99, 1.35] for incident CVD events).
DISCUSSION
We demonstrate that high FGF23 levels are associated with all-cause mortality and incident HF; associations that were particularly strong among persons with CKD. Comparatively, the association of FGF23 with incident CVD events was more modest, and driven principally through its association with CVD death. No independent association was observed between FGF23 and incident MI irrespective of CKD status.
We believe the findings of stronger associations in CKD are robust, in part because of the highly significant p-values for interaction. In parallel, a recent study found that high FGF23 was associated with greater left ventricular mass in a European cohort, and this association was also stronger in persons with CKD.(24) Yet the mechanisms responsible for stronger associations in CKD are uncertain. It is possible that the processes manifested or caused by higher FGF23 levels may be particularly harmful in persons with CKD. For example, recent studies suggest that FGF23 may cause increases in left ventricular mass in vitro and in rodent models.(2) Higher FGF23 may also indicate an elevated dietary phosphorus load, and thus a greater need to excrete phosphorus into the urine.(25) Alternatively, high FGF23 may limit conversion of 25hydroxyvitamin D to the active hormone calcitriol.(26) Higher phosphorus load and calcitriol deficiency may be particularly harmful in patients with CKD. Recent studies have identified both higher serum phosphorus and calcitriol deficiency as independent risk factors for CVD events,(27–29) and in some of our prior studies, we have observed that hyperphosphatemia and vitamin D deficiency are more strongly associated with subclinical CVD in individuals with CKD than in those without.(30,31)
Another possibility is that high FGF23 may reflect a component of kidney dysfunction that is not captured by the standard clinical markers of eGFR and urine ACR; renal endocrine resistance to FGF23 action. While eGFR and ACR reflect different aspects of kidney function, neither is a marker of renal endocrine responsiveness. ACR and eGFR are both strongly and independently associated with CVD events,(11,32) and measuring both in combination has improved the ability to identify risk of death and CKD progression.(33,34) Here, we demonstrated that despite FGF23’s correlation with both markers, it provided unique risk information about death, HF, and CVD events. Moreover, the relative strengths of association of FGF23 with each outcome have striking similarities to our prior work evaluating eGFR by cystatin C and creatinine in community-living cohorts. Elevated concentrations of these markers are consistently more strongly associated with death and incident HF than with MI. For example, in CHS, we previously showed that the highest quintile of cystatin C was associated with a more than doubling in risk for all-cause mortality and incident HF, compared to only a 30% higher relative risk for MI.(9,10,35) These findings have proven consistent in many other studies and settings.(36–40) Much less is known about the comparative strength of associations of FGF23 with these outcomes. However, in a prior study evaluating FGF23 in a cohort with prevalent CVD and with a spectrum of kidney function similar to individuals evaluated here, we also observed that high FGF23 was more strongly associated with all-cause mortality and HF than with recurrent MI (adjusted hazard ratios were 1.54, 1.31, and 1.05, respectively).(6) The consistency of these findings and the similar pattern to that observed for eGFR lead us to propose the novel hypothesis that high FGF23 may be marking a novel axis of kidney dysfunction, and that its measurement may provide information about other dimensions of kidney function above and beyond that obtained by measurement of eGFR and ACR.
The two pathways hypothesized above linking FGF23 with adverse outcomes are not mutually exclusive. At any level of eGFR and ACR, a higher FGF23 may indicate impaired renal endocrine resistance. At the same time, higher FGF23 may directly lead to adverse cardiovascular consequences by mechanisms of calcitriol deficiency, altered phosphorus homeostasis, or direct effects on cardiac myocytes.(2,41,42) The mechanisms linking CKD to HF, CVD, and death have remained elusive despite intensive research(43) and widespread recognition of the importance of this relationship to public health.(44) The strength of association of FGF23 with these outcomes in individuals with CKD reported here and elsewhere,(3,6) suggests that further research focused on FGF23 mechanisms may provide important insights into CVD risk among persons with CKD.
To our knowledge, only one prior study has examined the association of FGF23 with CVD in community-living individuals not selected on the basis of pre-existing CKD or CVD. In the Health Professionals Follow-up Study (HPFS), Taylor and colleagues reported no statistically significant association of FGF23 with the composite outcome of non-fatal MI and fatal CHD.(8) These findings are consistent with our own. In combined analysis among persons with and without CKD, we observed strong associations of FGF23 with death and HF, but observed null findings with CVD events. Moreover, in the HPFS, among the 422 CHD cases, 333 (79%) were non-fatal MI events, and in our study the association of FGF23 with MI was particularly modest. Participants in HPFS had significantly higher eGFR at baseline compared to those in CHS. The authors reported that results were similar when restricting to persons with eGFR ≥ 60ml/min/1.73m2, they did not report interactions by CKD, nor did they show associations separately in those with lower eGFR, perhaps because power was low to do so within the CKD strata. The HPFS did not evaluate associations with HF or all-cause death. Thus, the present study considerably extends the findings of FGF23 in the general population by evaluating long term follow-up and considerably greater number of events, provides the first comparison of strengths of association between death, HF, and CVD in the general population, and examines associations separately in those with and without CKD.
Strengths of our study include the external validity of a large community-based population, long duration and complete follow-up, large number of events, adjudication of HF and CVD events by a panel of experts, and availability of cystatin C and urine ACR measurements concurrent with FGF23. The study also has important limitations. Sophisticated measures of subclinical CVD and other measures of mineral metabolism complementary to FGF23 (serum phosphorus, calcitriol, and klotho) were not available to provide additional insights to underlying mechanisms. All participants were recruited in US cities and were older and of black or white race. Results may differ in other settings. Results are observational in nature, so residual confounding may persist and causal effects of FGF23 cannot be determined.
In conclusion, in community-living older persons, very modest decrements in eGFR and elevations in urine ACR are each independently associated with higher FGF23. High FGF23, in turn, is associated with all-cause mortality and incident HF; associations that are particularly strong in persons with CKD. The association of FGF23 with incident CVD events was modest in comparison. Measurement of FGF23 may provide new insights to kidney function above and beyond eGFR and ACR, and to the elusive mechanisms linking CKD to CVD events.
Supplementary Material
Acknowledgments
FUNDING SOURCES
Ronit Katz DrPhil had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This study was sponsored by grants from the National Heart Lung and Blood Institute (R01HL096851) and American Heart Association (0575021N) to Dr. Ix. Additionally this paper was supported in part with resources of the VA San Diego Healthcare System. The Cardiovascular Health Study was supported by NHLBI contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086; N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133 and NHLBI grant HL080295, with additional contribution from NINDS. Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-028058 from the NIA.
Footnotes
Disclosures: The authors have no financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jan de Beur SM. Tumor-induced osteomalacia. JAMA. 2005;294:1260–7. doi: 10.1001/jama.294.10.1260. [DOI] [PubMed] [Google Scholar]
- 2.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 doi: 10.1172/JCI46122. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendrick J, Cheung AK, Kaufman JS, et al. FGF-23 Associates with Death, Cardiovascular Events, and Initiation of Chronic Dialysis. J Am Soc Nephrol. 2011;22:1913–1922. doi: 10.1681/ASN.2010121224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker BD, Schurgers LJ, Brandenburg VM, et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Ann Intern Med. 2010;152:640–8. doi: 10.1059/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolf M, Molnar MZ, Amaral AP, et al. Elevated Fibroblast Growth Factor 23 is a Risk Factor for Kidney Transplant Loss and Mortality. J Am Soc Nephrol. 2011;22:956–66. doi: 10.1681/ASN.2010080894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor EN, Rimm EB, Stampfer MJ, Curhan GC. Plasma fibroblast growth factor 23, parathyroid hormone, phosphorus, and risk of coronary heart disease. Am Heart J. 2011;161:956–62. doi: 10.1016/j.ahj.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–60. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 11.Rifkin DE, Katz R, Chonchol M, et al. Albuminuria, impaired kidney function and cardiovascular outcomes or mortality in the elderly. Nephrol Dial Transplant. 2010;25:1560–7. doi: 10.1093/ndt/gfp646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ix JH, Shlipak MG, Wassel CL, Whooley MA. Fibroblast growth factor-23 and early decrements in kidney function: the Heart and Soul Study. Nephrol Dial Transplant. 2010;25:993–7. doi: 10.1093/ndt/gfp699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 14.Tell GS, Fried LP, Hermanson B, Manolio TA, Newman AB, Borhani NO. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson KB, Zahradnik R, Larsson T, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–63. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 16.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 18.Price TR, Psaty B, O’Leary D, Burke G, Gardin J. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:504–7. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 19.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 20.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 21.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ix JH, Chonchol M, Laughlin GA, Shlipak MG, Whooley MA. Relation of sex and estrogen therapy to serum fibroblast growth factor 23, serum phosphorus, and urine phosphrus: the heart and soul study. Am J Kidney Dis. 2011;58:737–45. doi: 10.1053/j.ajkd.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mirza MA, Larsson A, Melhus H, Lind L, Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–51. doi: 10.1016/j.atherosclerosis.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Ix JH. New insights to fibroblast growth factor 23 in kidney transplant. J Am Soc Nephrol. 2011;22:799–801. doi: 10.1681/ASN.2011020190. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Quarles LD. How fibroblast growth factor 23 works. J Am Soc Nephrol. 2007;18:1637–47. doi: 10.1681/ASN.2007010068. [DOI] [PubMed] [Google Scholar]
- 27.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–9. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 28.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 29.Foley RN, Collins AJ, Ishani A, Kalra PA. Calcium-phosphate levels and cardiovascular disease in community-dwelling adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:556–63. doi: 10.1016/j.ahj.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 30.de Boer IH, Kestenbaum B, Shoben AB, Michos ED, Sarnak MJ, Siscovick DS. 25-hydroxyvitamin D levels inversely associate with risk for developing coronary artery calcification. J Am Soc Nephrol. 2009;20:1805–12. doi: 10.1681/ASN.2008111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng J, Wassel CL, Kestenbaum BR, et al. Serum phosphorus levels and the spectrum of ankle-brachial index in older men: the Osteoporotic Fractures in Men (MrOS) study. Am J Epidemiol. 2010;171:909–16. doi: 10.1093/aje/kwq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peralta CA, Katz R, Sarnak MJ, et al. Cystatin C identifies chronic kidney disease patients at higher risk for complications. J Am Soc Nephrol. 2011;22:147–55. doi: 10.1681/ASN.2010050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peralta CA, Shlipak MG, Judd S, et al. Detection of chronic kidney disease with creatinine, cystatin C, and urine albumin-to-creatinine ratio and association with progression to end-stage renal disease and mortality. JAMA. 2011;305:1545–52. doi: 10.1001/jama.2011.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlipak MG, Katz R, Sarnak MJ, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–46. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Ix JH, Shlipak MG, Chertow GM, Whooley MA. Association of cystatin C with mortality, cardiovascular events, and incident heart failure among persons with coronary heart disease: data from the Heart and Soul Study. Circulation. 2007;115:173–9. doi: 10.1161/CIRCULATIONAHA.106.644286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bibbins-Domingo K, Lin F, Vittinghoff E, et al. Predictors of heart failure among women with coronary disease. Circulation. 2004;110:1424–30. doi: 10.1161/01.CIR.0000141726.01302.83. [DOI] [PubMed] [Google Scholar]
- 38.Shlipak MG, Simon JA, Grady D, Lin F, Wenger NK, Furberg CD. Renal insufficiency and cardiovascular events in postmenopausal women with coronary heart disease. J Am Coll Cardiol. 2001;38:705–11. doi: 10.1016/s0735-1097(01)01450-4. [DOI] [PubMed] [Google Scholar]
- 39.Bibbins-Domingo K, Chertow GM, Fried LF, et al. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166:1396–402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 40.Shlipak MG, Wassel Fyr CL, Chertow GM, et al. Cystatin C and mortality risk in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2006;17:254–61. doi: 10.1681/ASN.2005050545. [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez O, Isakova T, Rhee E, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–15. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–53. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 44.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.