Abstract
OBJECTIVES
Stereotactic body radiation therapy (SBRT) is a technique used to deliver high, ablative doses of radiation in a limited number of fractions to ≥1 extracranial target(s). While recent studies have shown that SBRT provides effective local tumor control in medically inoperable early stage lung cancer patients, its implementation in clinical practice is unknown.
METHODS
A random sample of 1600 American radiation oncologists was surveyed regarding lung SBRT usage, including year adopted, most common prescription, respiratory motion management, and target localization. A biological equivalent dose (BED) was calculated using the linear quadratic model with α/β=10. Spearman’s rank correlation coefficients (rs) were calculated to identify factors associated with BED.
RESULTS
Of 1373 contactable physicians, 551 responses (40%) were received. Of 510 evaluable responses, 275 physicians (54%) reported using lung SBRT, over half of whom adopted it in 2008 or later. The most commonly reported prescriptions were 20 Gy × 3 (22%), 18 Gy × 3 (21%), and 12 Gy × 4 (17%). Three fraction regimens were most common (48%), with nearly all (89%) prescribing ≥18 Gy/fraction. The median BED was 132 Gy, with 95% of reported prescriptions having BED ≥100 Gy. Factors associated with increased BED included use of fiducial markers (rs=0.26, p<0.001), use of planar imaging (rs=0.18, p<0.01), and years experience with lung SBRT (rs=0.13, p=0.04).
CONCLUSIONS
Lung SBRT has rapidly become a widely adopted treatment approach in the United States with a range of varying implementations. Further research and additional prospective trials are necessary to optimize this novel approach.
Keywords: stereotactic body radiotherapy, lung neoplasms, clinical practice patterns, survey
Introduction
Stereotactic body radiation therapy (SBRT) is a technique used to deliver high, ablative doses of radiation in a limited number of fractions to ≥1 extracranial target(s)1, 2. Recent studies have shown that SBRT provides effective local tumor control (91% 3-year primary and local control) with limited treatment-related morbidities (4% grade 4 adverse events) in medically inoperable early stage peripheral lung cancer patients3–8.
Despite encouraging results and growing interest in SBRT, little is known regarding its prevalence and use in the radiation oncology community beyond a previous survey conducted in Japan9. We thus conducted a nationwide survey of practicing radiation oncologists in the United States regarding their overall SBRT usage. An initial report describing overall SBRT adoption trends, different disease sites treated, and motivations for adoption has been published elsewhere10. The study found that SBRT has rapidly become a widely adopted treatment approach among American radiation oncologists, used by 64% of surveyed physicians, of whom nearly half adopted it in 2008 or later. Among SBRT users, the most common disease sites treated were lung (89%), spine (68%), and liver (55%).
We present herein further analysis from the nationwide survey, with a focus on analyzing lung SBRT clinical practice patterns. This report provides important additional information regarding current practices in radiation oncology by identifying factors that are associated with different implementations of lung SBRT in clinical practice.
Materials and Methods
Survey
The methodology for the survey was described previously10. Briefly, a group of 1600 radiation oncologists was randomly selected from the approximately 5000 active members listed in the American Society for Radiation Oncology (ASTRO) directory using a random number generator to select a page number and then a listing number on that page. The sample size was chosen based on the response rate from prior surveys11, 12 and the goal of a 95% confidence level in a margin of error of ±5% for the prevalence of overall SBRT usage. These physicians were surveyed via email and fax between July and September 2010. Responses were considered evaluable if at least partially completed. For the purposes of this survey, SBRT was defined as high-dose conformal radiotherapy delivered in ≤ 5 fractions with steep dose gradients around an extracranial target. All physicians were asked to supply information regarding their practice (type, size, and specialty) and year they graduated from residency. Lung SBRT users were asked about year adopted, number of patients treated, use of fiducial markers, use of a stereotactic body frame, target localization techniques, motion management, and most common prescription.
Statistical Analysis
The survey results are presented as the percentage of evaluable responses. Biological equivalent dose (BED) is a commonly used tool to compare different dose fractionation regimens on a single continuous scale13, 14. BED for the most common prescription was calculated using the linear quadratic model with α/β=10. Chi-square tests were performed to identify differences in proportions of lung SBRT usage and fractionation scheme between various groups. Confidence intervals (CI) for proportions were computed using the modified Wald method. Spearman’s rank correlation coefficients (rs) were calculated to identify associations with BED, motion management techniques, and fiducial marker usage. Significant values were defined as those with a p<0.05. Statistical analysis was performed with SPSS Statistics software, version 19 (Chicago, IL).
Results
Sample
Of the 1600 physicians surveyed, 1373 (85.8%) were contactable. Of these, 551 (40.1%) physicians responded, including 21 who declined to participate and 20 who were retired, leaving 510 evaluable respondents.
Respondents represented physicians from 48 states and the District of Columbia. A total of 319 responses (62.5%) were from physicians in private practice while the remaining 191 (37.5%) were from academic physicians. The median practice size was 5 physicians (range: 1–60 physicians). The median time in practice was 16 years (range: 1–42 years). Of responding radiation oncologists, 68 (13.3%) classified themselves as lung specialists.
Lung SBRT Users
Of the 510 evaluable respondents, 63.9% (95% CI: 60–68%) reported having used SBRT in their practice. The lung was the most common disease site treated with SBRT, reported by 275 respondents (53.9%, 95% CI: 50–58%), over half of whom adopted it in 2008 or later. Lung SBRT was more common among physicians who had been in practice 1 to 10 years compared with those in practice >10 years (63% vs 51%, p=0.02) and academic physicians compared to private practice (57% vs 52%, p=0.046). Its usage was found to be similar across geographic regions of Midwest, South, East, and West at 60%, 56%, 50%, and 48%, respectively (p=0.20). Radiation oncologists that classified themselves as lung specialists were more likely to have used lung SBRT (81% vs 50%, p<0.0001), but with no significant difference in practice patterns (prescription fractionation and BED, motion management techniques, or fiducial marker usage).
Prescription Fractionation and BED
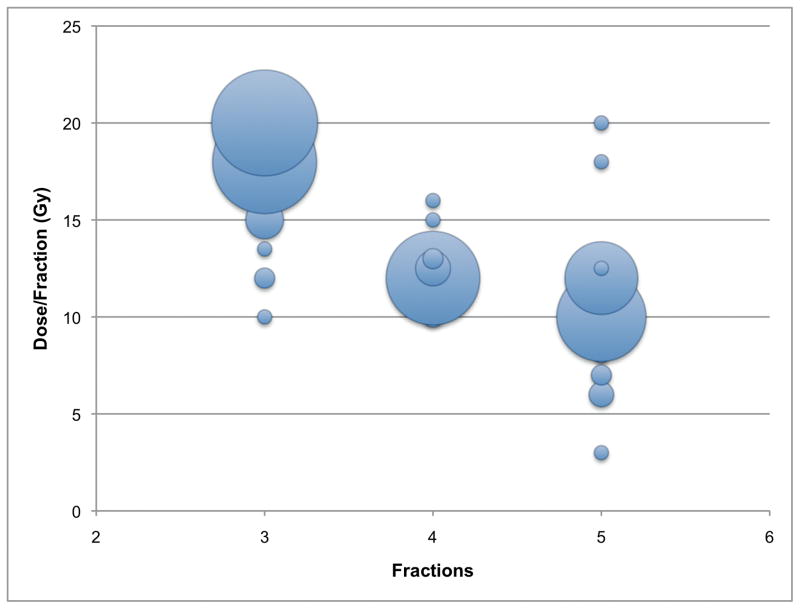
Figure 1 shows the most common lung SBRT prescription as supplied by 255 respondents (92.7% of lung SBRT users). The reported prescriptions were varied, with the most common being 20 Gy × 3 (22%), 18 Gy × 3 (21%), and 12 Gy × 4 (17%). All reported prescriptions were delivered over 3 (48%), 5 (31%), or 4 (22%) fractions.
FIGURE 1.
Bubble chart of the most common lung SBRT prescriptions (n=255). The size of the bubble corresponds to the number of responses. The largest bubbles reflect prescriptions of 20 Gy_3 (22%), 18 Gy_3 (21%), 12 Gy_4 (17%), 10 Gy_5 (15%), and 12 Gy_5 (10%).
Of 3 fraction regimens, 89% of respondents prescribed ≥18 Gy/fraction. Respondents were more likely to deliver treatment in 3 fractions if they used fiducial markers (72% vs 38%, p<0.001), used 2D planar imaging for target localization (58% vs 35%, p<0.001), or adopted lung SBRT earlier (2000–2008) as compared to more recently (2009–2010) (55% vs 35%, p=0.003). Users of CT-based 3D volumetric imaging for target localization were more likely to prescribe using >3 fractions (66% vs 42%, p=0.001). Practice type and size, use of a stereotactic body frame, and respiratory motion management techniques (respiratory gating, abdominal compression, etc) were not associated with significant differences in fractionation.
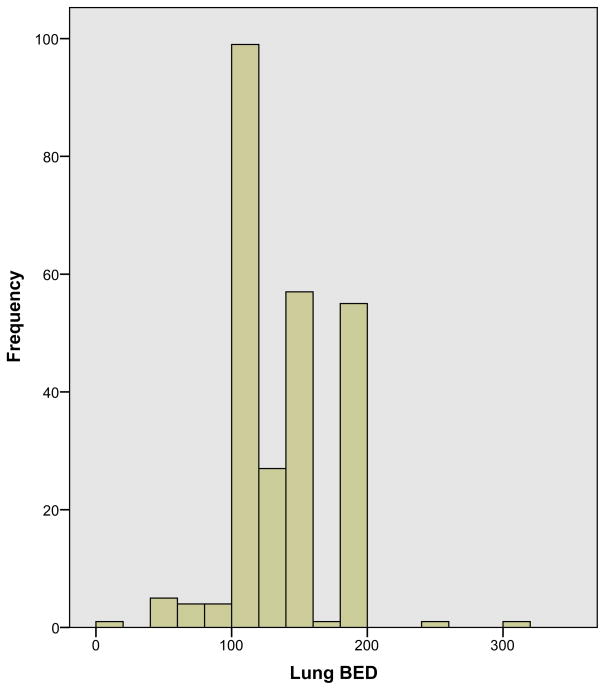
Among the most commonly prescribed lung SBRT regimens, the median BED was 132 Gy (range: 20–300 Gy), with 95% of reported prescriptions having BED ≥100 Gy (Fig. 2). BED had a strong inverse correlation with the number of fractions (rs: −0.73, p<0.001), with shorter treatment regimens associated with higher BED. Thus, all factors associated with 3 fraction regimens (use of fiducial markers, use of planar imaging, and early adoption of lung SBRT) were also significantly correlated with increased BED, while volumetric imaging was associated with decreased BED (Table 1)
FIGURE 2.
Histogram of the biological equivalent dose (BED) of the most common lung SBRT prescriptions (n = 255). BEDs were calculated using the linear quadratic model with a/b = 10. The median reported BED was 132 Gy.
Table 1.
Spearman's rank correlation coefficient between surveyed characteristics and prescription BED* for lung SBRT†
| Characteristic | rs †† | p value |
|---|---|---|
| Private practice | −0.010 | 0.874 |
| Practice size | −0.052 | 0.414 |
| Years in practice | 0.073 | 0.248 |
| Years experience using SBRT | 0.120 | 0.058 |
| Years experience using lung SBRT | 0.129 | 0.042 |
| Number of patients treated | 0.122 | 0.058 |
| Stereotactic body frame | −0.015 | 0.806 |
| Planar imaging | 0.178 | 0.004 |
| Volumetric imaging | −0.192 | 0.002 |
| External coordinate system | −0.015 | 0.807 |
| Fiducial markers | 0.260 | <0.001 |
| Respiratory gating | 0.084 | 0.240 |
| Breath-hold apparatus | −0.135 | 0.057 |
| Abdominal compression | −0.005 | 0.945 |
| Number of fractions | −0.730 | <0.001 |
Bolded rows correspond to statistically significant correlations.
BED: biological equivalent dose.
SBRT: stereotactic body radiation therapy.
rs: Spearman's rank correlation coefficient.
Motion Management
To manage respiratory-induced motion, physicians most commonly reported using abdominal compression (55.6%) or respiratory gating (54.2%) (Table 2). The use of a stereotactic body frame was positively associated with abdominal compression (rs= 0.41, p<0.001) and negatively correlated with respiratory gating (rs: −0.26, p<0.001). Abdominal compression was less common with additional years in practice (rs: −0.16, p=0.02) and the use of planar imaging (rs: −0.14, p=0.03). The use of respiratory gating was found with the use of planar imaging (rs= 0.27, p<0.001), the use of fiducial markers (rs= 0.17, p=0.02), and years experience with SBRT (rs= 0.16, p=0.02). Breath-hold techniques for motion management were less common among respondents (14.4%) and had positive and negative associations with the use of planar (rs= 0.14, p=0.04) and volumetric (rs: −0.14, p=0.04) imaging, respectively.
Table 2.
Characteristics associated with respiratory motion management techniques in lung SBRT*
| Abdominal Compression | Respiratory Gating | Breath-hold | |
|---|---|---|---|
| Overall usage | 55.6% | 54.2% | 14.4% |
| Positive association | SBF† | Planar imaging Fiducial markers SBRT experience |
Planar imaging |
| Negative association | Planar imaging Years in practice | SBF | Volumetric imaging |
Overall usage does not sum to 100 because respondents could select multiple techniques.
SBRT: stereotactic body radiation therapy.
SBF: stereotactic body frame
Fiducial Marker Usage
A minority of physicians (31.3%) reported the regular use of fiducial markers. Positive associations were found with the use of planar imaging (rs=0.33, p<0.001), increase dose per fraction (rs=0.31, p<0.001), private practice (rs=0.17, p=0.006), use of external coordinate system (rs=0.16, p=0.008), use of respiratory gating for motion management (rs=0.17, P=0.02), and years experience with SBRT (rs=0.14, P=0.03). Negative associations were found with the use of volumetric imaging (rs: −0.17, P=0.006) and a stereotactic body frame (rs: −0.15, P=0.01). Practice size and years in practice were not associated with significant differences in fiducial marker usage.
Discussion
The objective of the current study was to describe the clinical practice patterns of lung SBRT in the United States. We found that lung SBRT is a widely adopted treatment approach, currently used by over half of responding radiation oncologists. Its use will most likely increase in the coming years because approximately two-thirds of respondents who reported not currently using SBRT also reported plans to adopt it10.
In contrast to the Japanese survey that reported 88% of surveyed institutions treating lung with SBRT in >3 fractions9, lung SBRT in the United States is most commonly delivered using 3 fractions, as reported by almost half of respondents. The reason for this difference is uncertain, but may be influenced by the relative prominence of 3 fraction regimens reported in studies conducted in the United States3, 4. Interestingly, the recently closed Radiation Therapy Oncology Group (RTOG) 0915 trial investigating adverse events for lung SBRT compared a 4-fraction treatment to a single fraction regimen. There are, however, plans to compare the preferred regimen from the RTOG 0915 trial to a 3-fraction regimen.
While BED using the linear quadratic model may not be the most precise representation of the radiobiology of hypofractionated treatment in SBRT14, it nonetheless provided a helpful metric to compare different dosing schedules. The vast majority of reported prescriptions had BED ≥100 Gy, likely in response to previous studies that have associated improved local control and overall 3-year survival with BEDs above this threshold15. However, 5% of respondents reported most commonly using prescriptions with BEDs below this threshold, the reasons for which are uncertain. The most commonly reported prescription of 20 Gy × 3 was also the most aggressive, with a BED of 180 Gy that was only surpassed by 2 respondents.
A significant technical challenge in lung SBRT is accounting for respiratory-induced target motion16, 17. The majority of respondents addressed this problem through the use of abdominal compression18 and/or respiratory gating19, 20, while breath-hold techniques21 were less common. Abdominal compression was associated with the use of a stereotactic body frame, which is consistent with our previous findings that body frame usage was almost entirely for immobilization rather than target localization purposes10. Respiratory-gating was associated with the use of planar imaging and experience with SBRT. While the prevalence of abdominal compression was similar to findings reported from the Japanese survey9, respiratory gating is much more commonly used in the United States (54% vs 10%). A difficulty in comparing the results, however, is that this survey allowed multiple responses for motion management while the Japanese study required a single response. Additionally, it is possible that respondents interpreted respiratory gating to include assessment of respirator-induced motion using a 4-dimentional computed tomography (4DCT) scan to create an internal target volume (ITV) for treatment planning22 rather than turning the beam on and off based on the phase of the respiratory cycle, which would lead to an overestimation of its true prevalence.
Fiducial marker usage to assist with target localization23 was reported by less than one-third of lung SBRT users. Its use was positively associated with multiple factors, including planar imaging, while negatively associated with volumetric imaging. The association with planar imaging is likely attributed to concomitant usage in the Cyberknife system (Accuray Inc, Sunnyvale, CA), although the brand name of radiation equipment was not surveyed. With the increased adoption of in-room volumetric imaging in the United States11, fiducial marker usage may decline in the future.
The current study has several important limitations. We received responses from 40% of randomly selected, contactable physicians who represented a wide variety of demographics. Despite repeated attempts to collect responses, nonresponse and recall bias likely exist. It is possible that SBRT nonusers were less likely to respond, which would lead to an overestimation of the true prevalence of SBRT use. The survey was conducted as a broad nationwide query of overall SBRT usage and could not address more detailed nuances of lung SBRT usage such as type of equipment, dose constraints to normal tissue, and central vs peripheral lung tumors.
In summary, we found that over half of surveyed radiation oncologists have treated lung tumors with SBRT and the number will most likely grow. There is a wide range of varying lung SBRT implementations, and further research and additional prospective trials are necessary to optimize this novel approach. SBRT presents a promising treatment option for medically inoperable, early stage, peripherally located lung cancer. A Phase I/II trial is currently in progress (RTOG 0813) to determine a safe and effective dose for centrally located lung tumors24. At this time, however, there are no completed randomized experiences comparing outcomes for treatment with radiation therapy to surgery for surgical candidates.
Acknowledgments
Financial Support: Supported in part by NIH grant 5T35HL007491-30.
Footnotes
Conflicts of Interest Disclosure: None
Abstract accepted for presentation at the 53rd Annual Meeting of the American Society of Radiation Oncology, Miami, Florida, October 2–6, 2011.
References
- 1.Chang BK, Timmerman RD. Stereotactic body radiation therapy: a comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 3.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley JD, El Naqa I, Drzymala RE, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung cancer: the pattern of failure is distant. Int J Radiat Oncol Biol Phys. 2010;77:1146–1150. doi: 10.1016/j.ijrobp.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70:685–692. doi: 10.1016/j.ijrobp.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 6.Nagata Y, Negoro Y, Aoki T, et al. Clinical outcomes of 3D conformal hypofractionated single high-dose radiotherapy for one or two lung tumors using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2002;52:1041–1046. doi: 10.1016/s0360-3016(01)02731-6. [DOI] [PubMed] [Google Scholar]
- 7.Takeda A, Sanuki N, Kunieda E, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 Gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys. 2009;73:442–448. doi: 10.1016/j.ijrobp.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys. 2010;77:344–349. doi: 10.1016/j.ijrobp.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Nagata Y, Hiraoka M, Mizowaki T, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys. 2009;75:343–347. doi: 10.1016/j.ijrobp.2009.02.087. [DOI] [PubMed] [Google Scholar]
- 10.Pan H, Simpson DR, Mell LK, et al. A survey of stereotactic body radiotherapy use in the United States. Cancer. 2011 doi: 10.1002/cncr.26067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simpson DR, Lawson JD, Nath SK, et al. A survey of image-guided radiation therapy use in the United States. Cancer. 2010;116:3953–3960. doi: 10.1002/cncr.25129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S 2004. Cancer. 2005;104:1296–1303. doi: 10.1002/cncr.21284. [DOI] [PubMed] [Google Scholar]
- 13.Fowler JF. Linear quadratics is alive and well: in regard to Park et al. (Int J Radiat Oncol Biol Phys 2008;70:847–852) Int J Radiat Oncol Biol Phys. 2008;72:957. doi: 10.1016/j.ijrobp.2008.06.1929. author reply 958. [DOI] [PubMed] [Google Scholar]
- 14.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 15.Hiraoka M, Matsuo Y, Nagata Y. Stereotactic body radiation therapy (SBRT) for early-stage lung cancer. Cancer Radiother. 2007;11:32–35. doi: 10.1016/j.canrad.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Jiang SB, Wolfgang J, Mageras GS. Quality assurance challenges for motion-adaptive radiation therapy: gating, breath holding, and four-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2008;71:S103–107. doi: 10.1016/j.ijrobp.2007.07.2386. [DOI] [PubMed] [Google Scholar]
- 17.Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys. 2006;33:3874–3900. doi: 10.1118/1.2349696. [DOI] [PubMed] [Google Scholar]
- 18.Negoro Y, Nagata Y, Aoki T, et al. The effectiveness of an immobilization device in conformal radiotherapy for lung tumor: reduction of respiratory tumor movement and evaluation of the daily setup accuracy. Int J Radiat Oncol Biol Phys. 2001;50:889–898. doi: 10.1016/s0360-3016(01)01516-4. [DOI] [PubMed] [Google Scholar]
- 19.De La Fuente Herman T, Vlachaki MT, Herman TS, et al. Stereotactic body radiation therapy (SBRT) and respiratory gating in lung cancer: dosimetric and radiobiological considerations. J Appl Clin Med Phys. 2010;11:3133. doi: 10.1120/jacmp.v11i1.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keall PJ, Kini VR, Vedam SS, et al. Potential radiotherapy improvements with respiratory gating. Australas Phys Eng Sci Med. 2002;25:1–6. doi: 10.1007/BF03178368. [DOI] [PubMed] [Google Scholar]
- 21.Gagel B, Demirel C, Kientopf A, et al. Active breathing control (ABC): determination and reduction of breathing-induced organ motion in the chest. Int J Radiat Oncol Biol Phys. 2007;67:742–749. doi: 10.1016/j.ijrobp.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 22.Underberg RW, Lagerwaard FJ, Cuijpers JP, et al. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:1283–1290. doi: 10.1016/j.ijrobp.2004.07.665. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MJ. Fiducial-based targeting accuracy for external-beam radiotherapy. Med Phys. 2002;29:334–344. doi: 10.1118/1.1448823. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]