The authors conclude that intravaginal estradiol treatment, regardless of type, results in elevated circulating E2 levels in this population and should be used with caution.
Abstract
Background:
Intravaginal estradiols (VE) have been proposed as safe alternatives to systemic estrogen therapy in breast cancer survivors.
Patients and Methods:
Postmenopausal women with estrogen receptor–positive breast cancer or at high risk for breast cancer (n = 24) who were taking an aromatase inhibitor (AI) or a selective estrogen receptor modulator (SERM) and VE for ≥ 90 days for atrophic vaginitis and 24 controls taking AI only participated in the study. Serum samples were drawn from VE ring patients before insertion and 30 and 60 days postinsertion, from VE tablet patients the morning before insertion and approximately 12 hours postinsertion, and once from controls. Samples were assayed for E2 concentrations by using highly sensitive radioimmunoassay after ether extraction.
Results:
Mean E2 levels in controls were 3.72 pmol/L (range, < 3.0-7.7 pmol/L); mean E2 levels preinsertion and 12 weeks postinsertion in the VE ring patients were significantly greater than controls (P < .001 for each comparison). Mean preinsertion E2 levels in patients using VE tablets were not significantly different than those of controls (P = .48), and postinsertion levels were 76 pmol/L higher than preinsertion (P < .001).
Conclusion:
VE treatment increased E2 levels. Preinsertion levels for patients receiving VE tablets were not elevated compared with those of controls, suggesting that E2 elevations with this preparation may not be continuously sustained. We conclude that VE treatment, regardless of type, results in elevated circulating E2 levels in this population and should be used with caution.
Introduction
Antiestrogen therapy to suppress or block circulating estrogens, such as aromatase inhibitors (AIs; eg, anastrozole, exemestane, and letrozole) and selective estrogen receptor modulators (SERMs; eg, tamoxifen, raloxifene), are widely used to prevent and treat hormone receptor–positive (estrogen-sensitive) breast cancer.1–3 Atrophic vaginitis is increased in women taking AIs and SERMs, leading to a significant decrease in the overall quality of life, including vaginal dryness, dyspareunia, urinary incontinence and sexual dysfunction.4–10 For example, in the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial, 18.5% of women in the AI arm and 9.1% of women in the tamoxifen arm experienced vaginal dryness.11 Pain or discomfort with intercourse was reported in 17.3% of patients receiving the AI and 8.1% of patients receiving tamoxifen.11
Vaginal estradiols (VEs), such as the 17 beta-estradiol vaginal tablets (Vagifem; Novo Nordisk, Princeton, NJ) and the 17 beta-estradiol ring (Estring; Pharmacia & Upjohn, Kalamazoo, MI), have been used to alleviate atrophic vaginitis in postmenopausal women without breast cancer.4,12–17 However, the use of any form of estrogens in breast cancer survivors or patients at high risk for breast cancer development is controversial.18 In general, the benefit of tamoxifen appears to be independent of circulating endogenous estrogen levels, but the efficacy of AIs is directly related to maintenance of profoundly low estrogen levels. Regardless, a prospective randomized clinical trial comparing systemic estrogen replacement therapy with nil in breast cancer survivors was discontinued prematurely as a result of excess cancer recurrences.19 Twenty-one percent of these patients were receiving tamoxifen. Furthermore, in the ATAC trial, the outcomes for patients randomly assigned to the combined use of anastrozole and tamoxifen were no better than those of patients who received tamoxifen alone, and significantly worse than those of patients who received anastrozole alone, possibly as a result of the weak estrogen agonist effect of tamoxifen in the absence of endogenous estrogen.20
The use of VEs in women being treated with adjuvant AI or SERM therapy for estrogen-sensitive breast cancer has not been well studied, although its use is recommended anecdotally. In one of the few reported studies, circulating estrogen levels appeared to decrease over time, with minimal if any absorption after 3 months.8 Nonetheless, because absorption was observed; the authors concluded that using VEs in women taking an AI is contraindicated.
It has been proposed that over time the initial absorption of VEs is decreased as a result of estrogen-driven vaginal maturation.16,17,21,22 If so, then it has been reasoned that VEs might be safe for the use of alleviating atrophic vaginitis in some patients taking an AI or SERM. In a previously reported study with a similar design to this study but with a less sensitive serum estradiol (E2) assay,23 we did not detect a statistically significant difference in sustained estrogen levels between postmenopausal women taking AIs who were using VE tablets versus those who were not. However, we did observe significantly elevated transient postinsertion circulating estrogen levels, suggesting effective trans-vaginal absorption. We now report an extension of that study in a similar population of women taking an AI or a SERM, with an untreated control group. Patients using VE rings and those using VE tablets are included. A more sensitive radioimmunoassay assay for determination of serum E2 levels was used.
Patients and Methods
Patient Recruitment and Eligibility
This is a prospective clinical study approved by the institutional review board and Human Investigation Committee at William Beaumont Hospital (Royal Oak, MI). Cases and controls were identified and recruited from Cancer Care Associates (CCA), William Beaumont Hospital by one of the authors (D.A.D.) over a 6-month period. Controls were identified from the population of breast cancer patients seen at CCA during a routine follow-up for their breast cancer. Cases receiving VEs were identified by a search of the CCA electronic medical record. Both cases and controls provided consent at the time of a routine follow-up visit.
To be eligible for this study, both cases and controls had to be postmenopausal, as defined by no menstrual period for 1 year or prior bilateral oophorectomy. Patients had a diagnosis of either hormone receptor–positive breast cancer or were at high risk of breast cancer. Both cases and controls who had breast cancer must have successfully completed surgery, radiation therapy, or adjuvant chemotherapy and must have been clinically disease free at the time of enrollment. Both cases and controls were required to have been taking an AI or SERM for ≥ 14 days. The controls were not matched to cases for body mass index, prior chemotherapy, time since diagnosis, or characteristics other than those stated above.
Eligible patients were required to have been taking an AI or SERM as adjuvant therapy or for breast cancer risk reduction and to have had the additional diagnosis of atrophic vaginitis, as determined by symptoms such as vaginal dryness, dyspareunia, and urinary or sexual dysfunction. Atrophic vaginitis was diagnosed by the treating physician. Patients taking VEs had all achieved a clinical benefit and wished to continue treatment for symptom relief. Patients were assumed to be compliant with their VE because they achieved a clinical benefit and were asked if they were compliant during the study period. No formal evaluation of compliance was performed.
VE Tablet Cohort
Patients were eligible for the VE tablet cohort if they had been regularly using a standard dose of 25 μg, 17 beta-estradiol vaginal tablets for ≥ 3 months. Therefore, all these patients were previous users of VE tablets and would be expected to have a mature vaginal epithelium and consistent absorption of the tablets. The 25 μg tablet has a loading dose of every night for 14 days, followed by the standard 25 μg 17 beta-estradiol vaginal tablets two times per week thereafter. Blood samples were collected approximately 12 hours before VE insertion and approximately 12 hours postinsertion to detect peak levels, consistent with the tablets' known absorption profile. Longer term follow-up levels were not determined because the levels fall rapidly to zero after the peak level.
VE Ring Cohort
Patients were eligible for the VE ring cohort if they had been continuously using the VE ring for ≥ 3 months. The VE ring is replaced and inserted once every 90 days. It has been reported to have an initial estradiol peak immediately after insertion of a new ring, with reduced systemic absorption over the 12-week period.16,17,21,22 Blood samples were collected approximately 24 hours before new VE ring insertion and at 30 and 60 days postinsertion, consistent with the rings known absorption profile.
Controls
Controls were required to have been taking daily AI therapy (letrozole, exemestane, or anastrozole) for ≥ 14 days and to have not been using VE of any type. The controls were not questioned about symptoms of vaginal atrophy. Blood samples were collected during a routine clinical visit.
Specimen Collection and Shipment
Blood was drawn and collected in the laboratory at CCA. Samples were stored in an untreated collection tube in a freezer at −70 °C until shipment on dry ice to the assay laboratory for E2 assay.
E2 Assay
E2 concentration was measured by radioimmunoassay after an organic extraction using diethyl ether. The within- and between-batch coefficients of variation were 5.6% and 9.4%, respectively, at a concentration of 33 pmol/L. The sensitivity of the assay was defined as 3 pmol/L by calculation from the 95% CIs of the zero standard.24
Statistical Analyses
Statistical analyses were performed by using the t test for comparison between paired sample sets, as well as the Wilcoxon signed rank test. Statistical analyses were carried out using the SAS statistical software program (version 9; SAS Institute, Cary, NC).
Results
We recruited 48 postmenopausal women (24 control participants receiving AI only, 14 participants using the VE tablet, and 10 participants using the VE ring) between November 2006 and April 2007 (Appendix Table A1, online only). The average age of controls was 68 years (range, 53 to 79 years). The average age of cases using the VE tablet and VE ring was 60 years (range, 49 to 67 years). Cases had been using VEs for an average of 20 months (range, 3 to 73 months) before enrollment in this study.
Controls
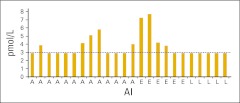
Of the controls, 13 were taking anastrozole, five were taking letrozole, and six were taking exemestane as adjuvant therapy for hormone-sensitive breast cancer (Figure 1). All controls were taking the AIs for no less than 14 days, and none were using a VE. All five controls taking letrozole had E2 levels of < 3.0 pmol/L, eight of 13 taking anastrozole had levels < 3.0 pmol/L (range, < 3.0-5.8 pmol/L), and two of six taking exemestane had levels < 3.0 pmol/L (range, < 3.0-7.7 pmol/L; Figure 1). The mean E2 levels for all controls taking an AI was 3.72 pmol/L (range, < 3.0-7.7 pmol/L).
Figure 1.
Controls taking an aromatase inhibitor (AI) only. The detection limit was 3 pmol/L. Dotted line indicates those values less than 3 pmol/L. A, anastrozole; E, exemestane; L, letrozole.
VE Tablet Cohort
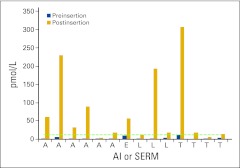
Of the cases receiving the VE tablet, six were taking anastrozole, three were taking letrozole, one was taking exemestane, and four were taking tamoxifen as adjuvant therapy for estrogen-sensitive breast cancer, or breast cancer risk reduction. Figure 2 displays the absolute E2 levels for each patient, preinsertion and postinsertion. To compare all 15 preinsertion and postinsertion levels, mean E2 levels were compared. The mean E2 level preinsertion, inclusive of AIs and SERMs, in patients using the VE tablet was 4.7 pmol/L (standard deviation [SD]: 3.2; 95% CI, 2.9 to 4.9), which was not significantly different than that for control patients (P = .48). The mean E2 level at approximately 12 hours post insertion in patients using the VE tablet was 76 pmol/L (SD = 97 pmol/L; 95% CI, 14 to 89 pmol/L), which was significantly higher than preinsertion levels (P < .001).
Figure 2.
Cases using an aromatase inhibitor (AI) or selective estrogen receptor modulator (SERM) and the intravaginal estrogen tablet. The detection limit was 3 pmol/L. Dotted line indicates those values less than 3 pmol/L. A, anastrozole; E, exemestane; L, letrozole; T, tamoxifen.
A comparison of the median E2 levels of patients taking AIs and VE tablets as compared with controls preinsertion was not significant (2.9 pmol/L; 95% CI, 2.9 to 4.9; P = .93); however, when comparing the postinsertion E2 levels, we found a statistically significant difference (45 pmol/L; 95% CI, 19 to 89; P < .001). One VE tablet patient had serum E2 levels drawn at baseline and approximately 12 hours, but also at 24 hours. At 24 hours her E2 level had returned to baseline (data not shown).
VE Ring Cohort
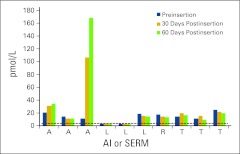
Of the VE ring cases, six were taking an AI (three anastrozole, three letrozole), and four were taking an SERM (one raloxifene, and three tamoxifen) as adjuvant therapy for estrogen-sensitive breast cancer or breast cancer risk reduction. Figure 3 displays the absolute E2 levels for each patient, preinsertion, 30 days postinsertion, and 60 days postinsertion. To compare the three time points in all 10 patients, mean E2 levels were compared. Mean E2 level preinsertion in patients using the VE ring was 14.2 pmol/L (SD = 7.2 pmol/L; 95% CI, 11 to 19 pmol/L), which was significantly higher than E2 levels in controls (P < .001). Mean E2 level at 30 days postinsertion in patients using the VE ring was 10.2 pmol/L (SD = 30.2 pmol/L; 95% CI, 4 to 95 pmol/L), which was not different than preinsertion levels (P = .31). However, the mean E2 level at 60 days postinsertion in patients using the VE ring was 30 pmol/L (SD = 50 pmol/L; 95% CI, 10 to 20 pmol/L) which was statistically significantly elevated compared with preinsertion levels (P = .001).
Figure 3.
Cases using an aromatase inhibitor (AI) or selective estrogen receptor modulator (SERM) and the intravaginal estrogen ring. The detection limit was 3 pmol/L. Dotted line indicates those values less than 3 pmol/L. A, anastrozole; L, letrozole; R, raloxifene; T, tamoxifen.
A comparison of the medians in controls and patients taking AIs only and using the VE ring preinsertion was significant (15.0 pmol/L; 95% CI, 2.9 to 19 pmol/L; P < .014). Again, when comparing median serum E2 levels 60 days postinsertion in ring patients with those of controls, we found a statistically significant difference (15 pmol/L; 95% CI, 1.9 to 35 pmol/L; P < .014).
Discussion
In this study, we have prospectively observed that intravaginal estrogen therapy for women taking either an AI or an SERM for treatment or prevention of breast cancer results in significantly elevated circulating E2 levels. Our data suggest that these elevations occur regardless of whether the VE preparation is by tablet or slow-release ring. We detected elevated E2 levels despite long-term VE use, which has previously been hypothesized to prevent vaginal estrogen absorption due to cornification.4,13,22 E2 levels may not be persistently elevated after VE tablet insertion, as levels appeared to return to baseline within 24 hours for one patient, and because preinsertion levels in all patients were no different than those of women not using VE. However, for women using the VE ring, we detected elevated preinsertion E2 levels (> 10 pmol/L) in all four patients taking an SERM and in two thirds of patients taking an AI, suggesting that absorption is persistent in the VE user. Tamoxifen and raloxifene are not known to have any effect on circulating levels of estrogens in postmenopausal women.25–28
Other studies have shown that estrogen is readily absorbed from the vagina. Randomized trials in postmenopausal women without breast cancer have demonstrated marginally increased, but still postmenopausal, estradiol and estrone levels in women using the estrogenic vaginal ring.17 However, there is indirect evidence of a systemic estrogenic effect in these women, which might be deleterious with regard to breast cancer recurrence.29 Our data are consistent with those from Kendall et al,8 who demonstrated an initial absorption in six of seven postmenopausal women with breast cancer using a VE (either the VE used in the current study (Vagifem) or VE cream (Premarin; Wyeth, New York, NY). However, as in our study, not all patients exhibited an increased level. In the Kendall et al study, one patient did not have any increase in E2, and in one half of the remaining six patients, E2 levels returned to baseline at 12 weeks.
The sample size of both cases and controls was small. The cases sample was limited to the number of patients receiving VEs in our practice. The number of controls was selected to roughly match the number cases. Patients were accepted as controls if they were postmenopausal, receiving an AI in the adjuvant setting without known recurrence, and completed all their local therapy. They were not matched for other possible variables such as compliance, body mass index, prior chemotherapy, or others that might influence the outcome. It is unlikely that matching such a small number of patients would give any further insight. Furthermore, our control patients' E2 levels are consistent with the literature, and each case served as their own control with a baseline E2 level.8
Taken together, these findings have immediate clinical implications. They indicate that VE therapy, although effective in treating atrophic vaginitis, may have adverse effects with regard to breast cancer treatment, as previously reported, large prospective studies have demonstrated worse cancer outcomes in the setting of estrogenic effect in women taking tamoxifen with an AI.20 It is possible that exposure of occult breast cancer to intermittent estrogens may not be detrimental. However, there is no research to substantiate this view.
Unfortunately, antiestrogenic cancer treatments do cause considerable vaginal toxicity, for which, short of estrogen treatment, there are few good alternatives. Several studies have documented that lack of adherence and/or persistence to SERMs and AI is quite high, despite their life-saving benefits, as a result of postmenopausal symptomatology, including atrophic vaginitis.30–33 Over-the-counter nonhormonal vaginal moisturizers and lubricants appear to be only modestly helpful.34–36 There is little research support for vaginal testosterone, dehydroepiandrosterone, or other hormonal interventions.37 Furthermore, these hormones serve as precursors for estrogenic compounds via aromatization. Although it is not known whether pharmacologic use of precursors increases estradiol levels, one must have some concern about this possibility.
A lower dose estradiol vaginal tablet has recently replaced the higher dose. A 17 beta-estradiol tablet formulation is now available in a 10-μg tablet. When compared with the 25-μg 17 beta-estradiol vaginal tablet used in this study, there is similar symptom relief. There is less systemic absorption after both initial and chronic use.38,39 However, the assays used in these studies may be less sensitive than the extraction radioimmunoassay in this study. This formulation has not been reported in breast cancer survivors on an AI or tamoxifen. A prospective trial of the 10-μg tablet is underway.40
In summary, our data suggest that intravaginal estrogen preparations, although attractive for the treatment of postmenopausal atrophic vaginitis, should be used with caution for women with hormone receptor–positive breast cancer taking antiestrogen therapy. For women with symptomatic vaginal atrophy, nonhormonal vaginal moisturizers and lubricants should be tried. For those without symptomatic relief who request intervention to improve their quality of life or compliance, the known risks and benefits should be discussed with their physician.
Appendix
Table A1.
Demographic and Clinical Characteristics of the Study Population
| Characteristic | Controls (n = 24) | VE Cases (n = 24) |
|
|---|---|---|---|
| VE Tablet (n = 14) | VE Ring (n = 10) | ||
| Age, years | |||
| Mean | 68 | 60 | |
| Range | 53-69 | 49-67 | |
| Duration of prior VE use, months | |||
| Mean | 20 | ||
| Range | 3-73 | ||
| Postmenopausal >1 yr | 24 | 14 | 10 |
| Receiving an AI ≥ 14 days | 24 | 10 | 6 |
| Receiving an SERM ≥ 14 days | 0 | 4 | 4 |
| Completed local therapy/adjuvant chemotherapy | 24 | 14 | 10 |
| NED breast cancer | 24 | 14 | 10 |
| Vaginal atrophy | Unknown | 14 | 10 |
| E2 serum draw | At routine physician visit | ∼12 hours before insertion and ∼12 hours after tablet insertion | Before ring insertion, then 30 and 60 days after |
Abbreviations: AI, aromatase inhibitor; E2, estradiol; NED, no evidence of disease; SERM, selective estrogen receptor modulator; VE, vaginal estradiol.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Mitch Dowsett, AstraZeneca (C); Daniel F. Hayes, OncImmune (C) Stock Ownership: Daniel F. Hayes, OncImmune Honoraria: Mitch Dowsett, AstraZeneca Research Funding: Mitch Dowsett, AstraZeneca, Novartis; Daniel F. Hayes, Veridex, Novartis, Pfizer Expert Testimony: Mitch Dowsett, AstraZeneca (C) Other Remuneration: None
Author Contributions
Conception and design: Padmaja Venuturumilli, Daniel F. Hayes, David A. Decker
Financial support: David A. Decker
Administrative support: David A. Decker
Provision of study materials or patients: Anita Ravipati, Padmaja Venuturumilli, Cynthia Kresge, David A. Decker
Collection and assembly of data: Shannon Wills, Anita Ravipati, Padmaja Venuturumilli, Elizabeth Folkerd, Mitch Dowsett, David A. Decker
Data analysis and interpretation: Shannon Wills, Padmaja Venuturumilli, Elizabeth Folkerd, Mitch Dowsett, Daniel F. Hayes, David A. Decker
Manuscript writing: Shannon Wills, Padmaja Venuturumilli, Cynthia Kresge, Elizabeth Folkerd, Mitch Dowsett, Daniel F. Hayes, David A. Decker
Final approval of manuscript: All authors
References
- 1.Early Breast Cancer Trialists Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 3.Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–2442. doi: 10.1056/NEJMra023246. [DOI] [PubMed] [Google Scholar]
- 4.Dugal R, Hesla K, Sørdal T, et al. Comparison of usefulness of estradiol vaginal tablets and estriol vagitories for treatment of vaginal atrophy. Acta Obstet Gynecol Scand. 2000;79:293–297. [PubMed] [Google Scholar]
- 5.Fallowfield L, Cella D, Cuzick J, et al. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol. 2004;22:4261–4271. doi: 10.1200/JCO.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Ferretti G, Bria E, Giannarelli D, et al. Second- and third-generation aromatase inhibitors as first-line endocrine therapy in postmenopausal metastatic breast cancer patients: A pooled analysis of the randomised trials. Br J Cancer. 2006;94:1789–1796. doi: 10.1038/sj.bjc.6603194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganz PA, Greendale GA, Petersen L, et al. Managing menopausal symptoms in breast cancer survivors: Results of a randomized controlled trial. J Natl Cancer Inst. 2000;92:1054–64. doi: 10.1093/jnci/92.13.1054. [DOI] [PubMed] [Google Scholar]
- 8.Kendall A, Dowsett M, Folkerd E, et al. Caution: Vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol. 2006;17:584–587. doi: 10.1093/annonc/mdj127. [DOI] [PubMed] [Google Scholar]
- 9.Notelovitz M. Urogenital aging: Solutions in clinical practice. Int J Gynaecol Obstet. 1997;59(suppl 1):S35–S39. doi: 10.1016/s0020-7292(97)90197-1. [DOI] [PubMed] [Google Scholar]
- 10.Stenberg A, Heimer G, Ulmsten U. The prevalence of urogenital symptoms in postmenopausal women. Maturitas. 1995;22(suppl):S17–S20. doi: 10.1016/0378-5122(95)00958-2. [DOI] [PubMed] [Google Scholar]
- 11.Cella D, Fallowfield L, Barker P, et al. Quality of life of postmenopausal women in the ATAC (“Arimidex,” tamoxifen, alone or in combination) trial after completion of 5 years' adjuvant treatment for early breast cancer. Breast Cancer Res Treat. 2006;100:273–284. doi: 10.1007/s10549-006-9260-6. [DOI] [PubMed] [Google Scholar]
- 12.Mattsson LA, Cullberg G, Eriksson O, et al. Vaginal administration of low-dose oestradiol–effects on the endometrium and vaginal cytology. Maturitas. 1989;11:217–222. doi: 10.1016/0378-5122(89)90213-2. [DOI] [PubMed] [Google Scholar]
- 13.Akrivis Ch, Varras M, Thodos A, et al. Action of 25 microg 17beta-oestradiol vaginal tablets in the treatment of vaginal atrophy in Greek postmenopausal women; clinical study. Clin Exp Obstet Gynecol. 2003;30:229–234. [PubMed] [Google Scholar]
- 14.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006;4:CD001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005;52(suppl 1):S46–S52. doi: 10.1016/j.maturitas.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Rioux JE, Devlin C, Gelfand MM, et al. 17beta-estradiol vaginal tablet versus conjugated equine estrogen vaginal cream to relieve menopausal atrophic vaginitis. Menopause. 2000;7:156–161. doi: 10.1097/00042192-200007030-00005. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg E, Ayton R, Darling G, et al. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric. 2005;8:83–92. doi: 10.1080/13697130500087016. [DOI] [PubMed] [Google Scholar]
- 18.Estrogen replacement therapy in breast cancer survivors. A time for change. Breast Cancer Committees of the Eastern Cooperative Oncology Group. JAMA. 1994;273:540–545. [PubMed] [Google Scholar]
- 19.Holmberg L, Anderson H. HABITS (hormonal replacement therapy after breast cancer–is it safe?), a randomised comparison: Trial stopped. Lancet. 2004;363:453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 20.Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 21.Lynch C. Vaginal estrogen therapy for the treatment of atrophic vaginitis. J Womens Health. 2009;18:1595–1606. doi: 10.1089/jwh.2008.1281. [DOI] [PubMed] [Google Scholar]
- 22.Ballagh SA. Vaginal rings for menopausal symptom relief. Drugs Aging. 2004;21:757–766. doi: 10.2165/00002512-200421120-00001. [DOI] [PubMed] [Google Scholar]
- 23.Howard G, Wills S, Kresge C, et al. The effects of vaginal estrogens on plasma estradiol levels in women taking aromatase inhibitors. Breast Cancer Res Treat. 2007;106(suppl 1):3085a. [Google Scholar]
- 24.Dowsett M, Goss PE, Powles TJ, et al. Use of the aromatase inhibitor 4-hydroxyandrostenedione in postmenopausal breast cancer: Optimization of therapeutic dose and route. Cancer Res. 1987;47:1957–61. [PubMed] [Google Scholar]
- 25.Beattie MS, Costantino JP, Cummings SR, et al. Endogenous sex hormones, breast cancer risk, and tamoxifen response: An ancillary study in the NSABP breast cancer prevention trial (P-1) J Natl Cancer Inst. 2006;98:110–115. doi: 10.1093/jnci/djj011. [DOI] [PubMed] [Google Scholar]
- 26.Cummings SR, Duong T, Kenyon E. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- 27.Kostoglou-Athanassiou I, Ntalles K, Gogas J, et al. Sex hormones in postmenopausal women with breast cancer on tamoxifen. Horm Res. 1997;47:116–120. doi: 10.1159/000185445. [DOI] [PubMed] [Google Scholar]
- 28.Boccardo F, Guarneri D, Rubagotti A, et al. Endocrine effects of tamoxifen in postmenopausal breast cancer patients. Tumori. 1984;70:61–68. doi: 10.1177/030089168407000110. [DOI] [PubMed] [Google Scholar]
- 29.Naessen T, Rodriguez-Macias K, Lithell H. Serum lipid profile improved by ultra-low doses of 17 beta-estradiol in elderly women. J Clin Endocrinol Metab. 2001;86:2757–2762. doi: 10.1210/jcem.86.6.7524. [DOI] [PubMed] [Google Scholar]
- 30.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;73:156–166. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 33.Atkins L, Fallowfield L. Intentional and non-intentional non-adherence to medication amongst breast cancer patients. Eur J Cancer. 2006;42:2271–2276. doi: 10.1016/j.ejca.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Caswell M, Kane M. Comparison of the moisturization efficacy of two vaginal moisturizers: Pectin versus polycarbophil technologies. J Cosmet Sci. 2002;53:81–87. [PubMed] [Google Scholar]
- 35.Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997;15:969–973. doi: 10.1200/JCO.1997.15.3.969. [DOI] [PubMed] [Google Scholar]
- 36.Hayes DF. Clinical practice. Follow-up of patients with early breast cancer. N Engl J Med. 2007;356:2505–2513. doi: 10.1056/NEJMcp067260. [DOI] [PubMed] [Google Scholar]
- 37.Pruthi S, Simon JA, Early AP. Current overview of the management of urogenital atrophy in women with breast cancer. Breast J. 2010;17:403–408. doi: 10.1111/j.1524-4741.2011.01089.x. [DOI] [PubMed] [Google Scholar]
- 38.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17B-estradiol vaginal tablets. Climacteric. 2010;13:219–227. doi: 10.3109/13697137.2010.483297. [DOI] [PubMed] [Google Scholar]
- 39.Bachmann G, Lobo RA, Gut R, et al. Efficacy of low-dose estradiol vaginal tablets. Obstet Gynecol. 2008;111:67–76. doi: 10.1097/01.AOG.0000296714.12226.0f. [DOI] [PubMed] [Google Scholar]
- 40.Memorial Sloan-Kettering Cancer Center Clinical Trials. Serum estradiol levels in postmenopausal women with breast cancer receiving adjuvant aromatase inhibitors and vaginal estrogens. NCT00984300. http://www.ClinicalTrials.gov.