Hospitalizations for neutropenic complications increased from 1989 to 1997 and then stabilized, whereas the number of hospitalizations with cancer diagnoses has remained steady since 1989.
Abstract
Purpose:
Neutropenic complications (NCs) after myelosuppressive chemotherapy are associated with significant morbidity and mortality. We described NC rates by using US hospital discharge data.
Materials and Methods:
This cross-sectional analysis used data from the US National Inpatient Sample database. Hospital discharges with cancer diagnoses (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code) from 1989 to 2007 were analyzed for the ICD-9-CM neutropenia code. NC rates per 10,000 discharges were calculated for all adult discharges without radiation therapy (study population, all cancers); lung cancer, breast cancer, and non-Hodgkin's lymphoma (NHL); and all three combined. The use of growth factors and myelosuppressive chemotherapy from 1994 to 2008 was estimated by using the IMS Health Drug Distribution Database.
Results:
Estimated lung cancer and breast cancer discharges remained relatively steady, whereas NHL discharges increased. NC rates for each study cancer increased two-fold until the late 1990s before stabilizing and/or declining. The average hospital stay for all three cancers decreased from 10.4 days to 7.1 days. The mortality rates for NCs for the three cancers combined decreased at a fairly constant rate from 10% in 1989 to 5.4% in 2007. Estimated discharges for NCs from 1989 to 2007 ranged from 111,000 to 169,000 for the study population, from 57,000 to 103,000 for all cancers, and from 21,000 to 40,000 for the three study cancers. The use of growth factors and myelosuppressive chemotherapy increased from 1994 to 2008.
Conclusion:
Whereas the number of hospitalizations with cancer diagnoses has remained steady since 1989, hospitalizations for NCs increased approximately two-fold from 1989 to 1997 and then stabilized.
Introduction
Although chemotherapy offers improved survival for many cancers, numerous chemotherapeutic regimens have narrow therapeutic indexes, which may result in serious and often life-threatening events, such as infection as manifested by febrile neutropenia (fever and grade 3/4 neutropenia).1 The risk of neutropenic complications (NCs) is related to the specific chemotherapeutic regimen, individual patient factors (eg, age and comorbidities), and the use of supportive care therapies which mitigate that risk (eg, granulocyte colony-stimulating factors [G-CSFs]).2 There are few published data that report national trends for NCs, despite their relative frequency and the efforts made to prevent them.
The objective of this study was to estimate NCs on the basis of the number of hospital discharges for which cancer and neutropenia diagnoses occurred together, by using data from 1989 to 2007 from the US Agency for Healthcare Research and Quality Healthcare Cost and Utilization Project Nationwide Inpatient Sample (NIS) database. NC rates were examined for the study population (ie, all discharges minus study exclusions); all cancer discharges (ie, not only the study cancers); separately for lung cancer, breast cancer in women, and non-Hodgkin's lymphoma (NHL); and for the three cancers combined (ie, study cancers). Because changes in NC trends may reflect changes in the use of G-CSFs and myelosuppressive chemotherapy, the numbers of doses of selected G-CSFs and common chemotherapeutic agents administered in the United States from 1994 to 2008 were estimated.
Materials and Methods
Data Sources
Data for the primary analyses were from the Agency for Healthcare Research and Quality NIS all-payer inpatient care database,3 a probability-weighted sample designed to allow estimation of the total number of US hospitalization discharges. The NIS database is composed of approximately 1,000 randomly selected community hospitals drawn from eight to 42 states depending on the survey year to provide an approximately 20% stratified sample of US community hospitals (defined as nonfederal, short-term hospitals, including academic medical centers). The number of hospitals in the sample ranged from 758 (1988) to 1,044 (2007). The sample contained data from 5 to 8 million hospital discharges each year, which represented approximately 90% of the US population in each study year. Data for the secondary analysis of chemotherapy trends (ie, the number of doses of chemotherapeutic agents and G-CSFs) were estimated by using the IMS Health Drug Distribution Database, which reflects 94% of all US sales of these agents.
Study Population
The total study population included all hospital discharge records for patients aged ≥ 18 years who had no evidence of radiation therapy (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]: procedure codes 92.2, 92.3). Radiation-related hospitalizations were excluded to avoid inclusion of radiation-induced neutropenia that could occur with radiation monotherapy. Because radiation monotherapy (as opposed to combined chemoradiotherapy) could not be separately excluded, all hospital discharges of patients who received radiation therapy were excluded. The study population was further narrowed to hospitalizations that contained ICD-9-CM codes for any malignant cancer (ICD-9-CM: 140-208). Finally, cohorts evaluated within the cancer population included lung cancer (ICD-9-CM: 162), breast cancer in women (ICD-9-CM: 174), and NHL (ICD-9-CM: 200, 202).
Variable Definitions
Neutropenic complication was defined by a broad definition of neutropenia (ICD-9-CM: 288.0) and a narrow definition of neutropenia (ICD-9-CM: 288.0 and selective infection codes possibly associated with febrile neutropenia, as listed in Appendix Table A1, online only). Hospital discharge-level variables in the NIS included demographics (eg, age and sex) and hospital discharge-specific criteria (eg, type of admission, diagnosis codes, procedure codes, hospital discharge status, expected primary and secondary payers, and total charges). Hospital-related variables included location and whether a hospital was a teaching institution.
Statistical Analyses
We reported the estimated absolute number of national hospitalization discharges and the annual number and rate of hospital discharges with NCs per 10,000 hospital discharges represented in the NIS database by year from 1989 through 2007 for the study population, all cancers; NHL, lung, and female breast cancer separately; and for all three cancers combined (the study cancers). Estimation was done using SAS/STAT software, Version 9.2 of the SAS System for Windows, specifically PROC SURVEYMEANS (continuous variables) and PROC SURVEYFREQ (categorical variables) with appropriate weights and sample design variables included in the NIS database, including hospital (or cluster) and stratum variables identified (ie, as defined by region, ownership, location/teaching function, and bed size) to ensure design consistency of variance estimators. Given the goals of the analysis, the cross-sectional annualized data (ie, multiple time points), and the large sample sizes at each time point, we constructed CIs rather than performing statistical tests.
The number of doses of G-CSFs and myelosuppressive chemotherapeutic agents used from 1994 to 2008 was estimated by using the IMS Health Drug Distribution Database and dosing data. Dosing for chemotherapeutic agents was obtained from drug package insert dosing recommendations. For filgrastim and pegfilgrastim, the WHO-defined daily dose (pegfilgrastim, 0.30 mg; filgrastim, 0.35 mg)4 was used to obtain an estimate of the number of doses used for each product. The numbers of defined daily doses of therapy divided by the average number of doses sold in a given year were plotted for G-CSFs and selected myelosuppressive chemotherapeutic agents to provide a common scale for the average percentage of doses that were sold in any given year during the 1994 to 2008 analysis period (ie, an estimate of growth rate).
Results
Study Attrition
The total number of estimated discharges in the United States during the analysis period ranged from approximately 35 million to 39 million. Using only discharges for patients who were aged ≥ 18 years and had no evidence of radiation therapy (ie, study population), the estimates ranged from 28 to 32 million discharges, with 111,000 to 169,000 discharges for NCs. When further restricted to all cancer discharges, the estimates were approximately 2.5 million discharges per year, with approximately 57,000 to 103,000 discharges for NCs per year. Subsequently, the cancer discharges were analyzed by the three study cancers. The remaining analyses focus on the rates of NCs within these subgroups.
Hospital Discharges for Cancer
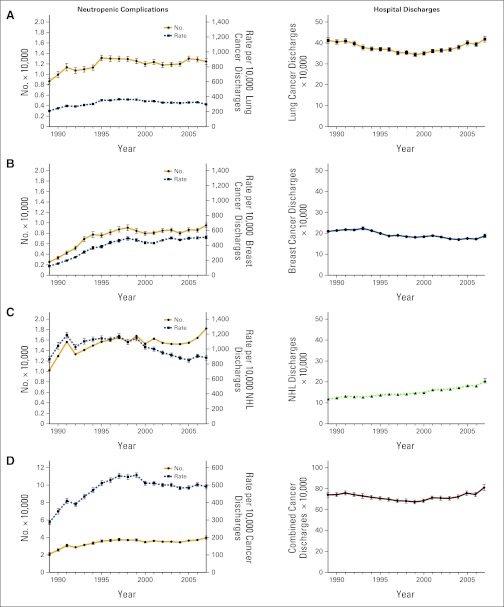
The estimated numbers of lung cancer and breast cancer hospital discharges per year in the United States were approximately 400,000 for lung cancer (Figure 1A, right panel; ranging from 346,000 to 419,000 per year) and approximately 200,000 for breast cancer (Figure 1B, right panel; ranging from 170,000 to 223,000 per year). NHL hospital discharges nearly doubled from 1989 to 2007, increasing from 119,000 to 206,000 per year (Figure 1C, right panel). For the three study cancers combined, the number of hospital discharges was relatively stable (Figure 1D, right panel; ranging from 671,000 to 808,000 per year). All cancer hospital discharges (including but not limited to the three cancer types) remained fairly steady over time at approximately 2.5 million per year from 1989 to 2007 (ranging from 2.3 to 2.7 million/yr). For all cancer hospital discharges, the mean (SE) patient age ranged from 65.2 (0.41) years to 66.4 (0.25) years, whereas the proportion of females changed from 50.8% (1993) to 48.6% (2007).
Figure 1.
Absolute number and rate per 10,000 hospital discharges with neutropenic complications (left panels) and absolute number of hospital discharges (International Classification of Diseases, Ninth Revision, Clinical Modification code: 288; right panels) for (A) lung cancer, (B) breast cancer, (C) non-Hodgkin's lymphoma (NHL), and (D) the three cancers combined, from the Nationwide Inpatient Sample (1989-2007).
Demographics Among Study Cancer Hospital Discharges With NCs
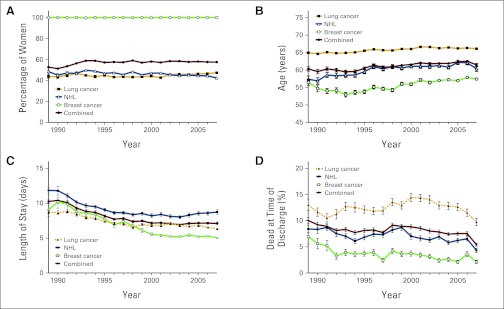
We further examined the three study cancers with NCs separately and combined. Over the course of the study (1989-2007), roughly half of the discharged patients were female (ranging from 51.3% to 59.0%; Figure 2A), with mean age in the early 60s (ranging from mean [SE] 59.5 [0.46] years to 62.5 [0.31] years; Figure 2B). For lung cancer hospital discharges, less than half of patients discharged were female (ranging from 42.7%–47.4%), with mean age in the mid-60s (ranging from mean [SE] 64.6 [0.31] to 66.6 [0.26] years]), whereas for female breast cancer hospital discharges, the mean (SE) age was lower, ranging from 52.9 (0.63) years to 57.9 (0.33) years. For NHL, the proportion of females was less than half (ranging from 42.4% to 49.8%), and mean (SE) age increased over time, ranging from mean 56.9 (0.91) years to 62.2 (0.64) years. For lung cancer, breast cancer, and NHL, 83% to 88% of hospital discharges with NCs occurred in urban settings.
Figure 2.
(A) Sex, (B) age, (C) length of stay, and (D) mortality as a discharge status among hospital discharges with neutropenic complications (International Classification of Diseases, Ninth Revision, Clinical Modification code: 288) for lung cancer, breast cancer, and non-Hodgkin's lymphoma (NHL), by individual cancer type and for all three types combined, from the Nationwide Inpatient Sample (1989-2007).
Study Cancer NCs
We examined the annual number of NC-related discharges and the rate of hospital discharges with NCs (ie, number of NCs per 10,000 hospital discharges) for the study cancers. The estimate of the absolute number of hospital discharges with NCs for lung cancer rose until 1995, after which the results remained fairly constant, whereas the adjusted rate of lung cancer NC discharges per 10,000 declined after 1995 (Figure 1A, left panel). For breast cancer, the absolute number of NCs hospital discharges and rate per 10,000 breast cancer hospital discharges rose steeply until 1998, after which both appeared to stabilize (Figure 1B, left panel). For NHL, there was a fairly consistent rise in the absolute number of NC hospital discharges, whereas the adjusted rate of NC discharges per 10,000 peaked in 1997 and declined thereafter (Figure 1C, left panel). For the three study cancers combined, the absolute number of hospitalization discharges with NCs increased almost two-fold until 1997 and remained relatively stable thereafter (Figure 1D, left panel). After increasing almost two-fold until 1997, the rate per 10,000 discharges for the three study cancers decreased in 1999 and remained relatively stable thereafter (Figure 1D, left panel). When the hospital discharge records were examined by a narrower definition for NCs (ie, containing ICD-9-CM codes for both neutropenia and infection), similar results were seen for the individual specific cancer types and the three combined (data not shown).
Length of Stay and Outcome for Study Cancer Hospital Discharges With NCs
Regarding the length of stay for hospital discharges, a similar decrease over time was seen (Figure 2C), with longer hospital stays for NHL and shorter hospital stays for lung cancer and breast cancer. Breast cancer hospital discharge length of stay showed the most dramatic change, ranging from a peak of 10.2 days in 1990 to a low of 5.0 days in 2007. Hospitalization length of stay for NCs decreased from 10.4 days in 1990 and steadily improved to 7.1 days in 2007.
The mortality rates for NCs associated with lung cancer admissions had some variation, rather than a steady decline, although the rates did decrease over this time span (13% to 9.7%; Figure 2D). The mortality rates for NCs decreased more steadily over the study period for breast cancer (6.9% to 2.1%) and NHL (from 8.4% to 4.3%). Overall, the mortality rates for NCs in the three cancers combined decreased from 10% in 1989 to 5.4% in 2007 at a fairly constant rate (Figure 2D).
Use of G-CSF and Myelosuppressive Chemotherapy Over Time
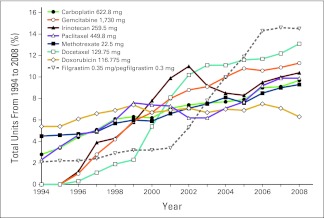
Using annual drug sales data obtained from the IMS Health Drug Distribution Database, package insert dosing data, and WHO-defined daily doses, we estimated the annual number of medication doses and standardized them by the average number of doses sold over the analysis period (1994-2008). G-CSF use increased after the approval of filgrastim in 1991 and again after the approval of pegfilgrastim in 2002 (Figure 3). This trend in growth factor use paralleled the increased use of several commonly prescribed myelosuppressive chemotherapeutic agents associated with febrile neutropenia, such as taxanes (eg, docetaxel, paclitaxel), topoisomerase I inhibitors (eg, irinotecan), platinum-based agents (eg, carboplatin), and antimetabolites (eg, methotrexate; Figure 3). However, the estimated proportion of doxorubicin doses administered was stable.
Figure 3.
Estimated relative changes in the use of filgrastim/pegfilgrastim and myelosuppressive chemotherapy. The IMS Health Drug Distribution Database (1994-2008) and dosing data were used to calculate the number of days receiving filgrastim/pegfilgrastim on the basis of WHO-defined daily doses. The numbers of doses of chemotherapeutic agents were calculated by using recommended dosing data from the package inserts.
Discussion
To our knowledge, NC rates thus far have been reported for single clinical studies or large observational studies but not longitudinally or at the national level in the United States.5,6 NC rates are of special interest, as key clinical studies and guidelines have indicated that treatment patterns, particularly for lung cancer and breast cancer, have evolved to include increasingly myelosuppressive chemotherapy over the past 20 years. Current estimates of NC rates in the United States are thus likely underestimated as a result of the use of radiation therapy in combination with myelosuppressive chemotherapy in lung cancer. Treatment for NHL has been primarily based on cyclophosphamide, doxorubicin, vincristine, and prednisolone or cyclophosphamide, doxorubicin, vincristine, and prednisolone plus rituximab over this time span.7 During the study period, there was the important clinical development of dose-dense chemotherapy for breast cancer that incorporates the use of G-CSF prophylaxis.8 To examine these trends, we performed a descriptive cross-sectional analysis of NCs using NIS hospital discharge data from 1989 to 2007. Key findings showed that after excluding patients who received radiation therapy, on average, the estimated number of NCs in the total study population increased by approximately 30,000 cases per year. Furthermore, although the rate of discharges for the three study cancers combined remained relatively steady over time from 1989 to 2007, the rate of hospital discharges with NCs increased almost two-fold from 1989 to 1997. The use of G-CSF, especially after 2002, paralleled the clinical use of increasingly myelosuppressive chemotherapy regimens (dose-dense regimens, the use of taxanes, etc)9–11 and may well have mitigated the steeply rising annual incidence of hospitalizations from NCs from 1990 to 2000. However, the results of this analysis were derived from a combination of disparate data sources, which is a potential limitation of the assessment of the use of these agents.
The length of stay for hospital discharges with NCs decreased over time from 10.4 days in 1990 to 7.1 day in 2007. Of note, the length of stay for lung cancer may have been affected by changes in mortality rates. This change in length of stay may also be related to the advent of the diagnosis-related group payment system in the 1980s, and increased reimbursement restrictions put on the system since its introduction in 1983. In-hospital mortality rates with NCs for all study cancers fell from 10% in 1989 to 5.4% in 2007 at a fairly constant rate. Recent studies have suggested that G-CSF may influence mortality and may be one factor in reducing in-hospital mortality,12–14 although better inpatient treatment of patients with neutropenic infections could have contributed to this result.
There are several caveats that must be considered in interpreting these study findings. One fundamental source of bias is that this study relies on coded data and, hence, incorporates any coding errors that may have occurred. In a previous study, the sensitivity for defining neutropenia from ICD-9-CM coding was reported to be 80% when compared with other data sources.15 These data also reflect billing decisions, which may not always completely correlate with clinical assessments. In addition, the unit of analysis was the hospital discharge, not the patient; therefore, patient-level conclusions are out of scope for this study. Given the nature of the database, no adjustments could be made for cancer stage, treatment intent (curative v palliative), or chemotherapy regimen. Also of note, the NC data were not adjusted for changes in chemotherapy use; shifts in care from one treatment modality to another (surgery, radiation, chemotherapy, or targeted therapy); or changes in population growth, cancer incidence, prevalence, or survival. These data are not easily accessible because the majority of NCs still require hospitalization,16,17 and large hospital discharge databases are often are not linkable to outpatient databases that would contain many of these treatment-related and patient-related data.
We sought to examine outpatient data using both the National Ambulatory Care Survey and National Hospital Ambulatory Care Survey databases to capture NCs observed in freestanding outpatient clinics and in clinics that are part of hospitals, respectively. However, the data obtained from these databases were not reliable because the number of cases was small and the SEs large. It may be that the study design did not permit sampling of an adequate number of oncologists, thus resulting in the number of NC cases being too low to report. Regardless, there was not a detectable trend in outpatient treatment of neutropenia in these data sets. This could be because most treatment of NCs occurs in hospitals.16,17 It is possible that other efforts to ameliorate NCs, such as dose-reduction strategies or other changes in chemotherapy regimens (especially in patients with metastatic disease or other factors predictive of poor outcome), may have affected the incidence of NCs.1,12,18–20 Our results indicate that the increased use of commonly prescribed myelosuppressive chemotherapies during this period is also of interest because increased absolute NC events would not be unexpected with some of these regimens.20 Although the analysis of the IMS Health Drug Distribution Database indicated increased use of most myelosuppressive chemotherapies, it is important to note that methotrexate is often prescribed for nononcology indications such as autoimmune diseases.21,22
To provide a broader context, we calculated 5-year cancer survival and prevalence rates from 1990 to 2001 based on data published in the Surveillance, Epidemiology and End Results Cancer Statistics Review, 1975 to 2006.23 These analyses showed that 5-year survival rates for all cancers, breast cancer, lung cancer, and NHL all increased from 1990 to 2001, with the smallest increase occurring with lung cancer and the greatest increase occurring with NHL. Specifically, survival for all cancers was up by 9.4% from 1990 to 2001, with the largest survival gains for NHL, which had a 16.6% increase, representing a 32.4% increase in survival. We likewise examined 5-year prevalence rates for all three study cancers over the time frame examined. The prevalence of lung cancer was unchanged from 1989 to 2006, whereas breast cancer increased by 18.2% and NHL increased by 50.0% compared with the 1989 rate. These data indicate that any decreases in NC hospital discharges over time were not due to a decrease in the number of patients with cancer.
In conclusion, our analyses demonstrated an increase in the number of hospitalizations for NCs from 1990 to 2000 with subsequent stabilization. This likely reflects the integrated effects of multiple factors considered by oncologists when making treatment decisions regarding neutropenia. These factors include the availability of increasingly myelosuppressive chemotherapy regimens, which may affect survival rates; widespread use of G-CSFs, such as pegfilgrastim and filgrastim, both prophylactically and therapeutically; and consideration of individual patient characteristics such as age and comorbid conditions. The relatively constant rate of NCs over the past several years indicates that it remains a significant clinical problem for patients. Additional research into how to optimize the balance of aggressive chemotherapy and prevention of febrile neutropenia may aid efforts to increase survival while minimizing the effects of febrile neutropenia.
Acknowledgment
Supported by Amgen. We thank Susanna Mac, MD, PhD, (Amgen) and Benjamin Scott, PhD, (funded by Amgen) for assistance in the preparation of this article.
Appendix
Table A1.
International Classification of Diseases, Ninth Edition Clinical Modification Codes for Infections
| Code | Description |
|---|---|
| 001 | Cholera |
| 002 | Typhoid and paratyphoid fevers |
| 003 | Other Salmonella infections |
| 004 | Shigellosis |
| 005 | Other food poisoning |
| 008.0 | Intestinal infections due to E. coli |
| 008.1 | Intestinal infections due to Arizona group of paracolon bacilli |
| 008.2 | Intestinal infections due to Aerobacter aerogenes |
| 008.3 | Intestinal infections due to Proteus (mirabilis) (morganii) |
| 008.4 | Intestinal infections due to other specified bacteria |
| 008.5 | Bacterial enteritis, unspecified |
| 009 | Ill-defined intestinal infections |
| 013 | CNS tuberculosis |
| 018 | Miliary tuberculosis |
| 020 | Plague |
| 021 | Tularemia |
| 022 | Anthrax |
| 023 | Brucellosis |
| 024 | Glanders |
| 025 | Melioidosis |
| 026 | Rat-bite fever |
| 027 | Other bacterial zoonoses |
| 032 | Diphtheria |
| 033 | Whooping cough |
| 034 | Streptococcal throat/scarlet fever |
| 035 | Erysipelas |
| 036 | Meningococcal infection |
| 037 | Tetanus |
| 038 | Septicemia |
| 039 | Actinomycotic infections |
| 040 | Other bacterial diseases |
| 041 | Bacterial infection in other diseases not specified |
| 098 | Gonococcal infections |
| 100 | Leptospirosis |
| 101 | Vincent's angina |
| 112.0 | Candidiasis, of mouth |
| 112.4 | Candidiasis, of lung |
| 112.5 | Candidiasis, disseminated |
| 112.8 | Candidiasis, of other specified sites |
| 114 | Coccidioidomycosis |
| 115 | Histoplasmosis |
| 116 | Blastomycotic infection |
| 117 | Other mycoses |
| 118 | Opportunistic mycoses |
| 320 | Bacterial meningitis |
| 321.0 | Cryptococcal meningitis |
| 321.1 | Meningitis in other fungal diseases |
| 324 | CNS abscess |
| 325 | Phlebitis of intracranial sinus |
| 360.0 | Purulent endophthalmitis |
| 376.0 | Acute inflammation of orbit |
| 380.14 | Malignant otitis externa |
| 383.0 | Acute mastoiditis |
| 420.99 | Acute pericarditis due to other specified organisms |
| 421 | Acute and subacute endocarditis |
| 461 | Acute sinusitis |
| 462 | Acute pharyngitis |
| 463 | Acute tonsillitis |
| 464 | Acute laryngitis and tracheitis |
| 465 | Acute upper respiratory infections of multiple sites/not otherwise specified |
| 475 | Peritonsillar abscess |
| 481 | Pneumococcal pneumonia |
| 482 | Other bacterial pneumonia |
| 485 | Bronchopneumonia, organism unspecified |
| 486 | Pneumonia, organism unspecified |
| 491.21 | Obstructive chronic bronchitis with acute exacerbation |
| 494 | Bronchiectasis |
| 510 | Empyema |
| 513 | Abscess of lung and mediastinum |
| 522.5 | Periapical abscess without sinus |
| 522.7 | Periapical abscess with sinus |
| 526.4 | Inflammatory conditions of the jaw |
| 527.3 | Abscess of the salivary glands |
| 528.3 | Cellulitis and abscess of oral soft tissues |
| 540 | Acute appendicitis |
| 541 | Appendicitis, not otherwise specified |
| 542 | Other appendicitis |
| 562.01 | Diverticulitis of small intestine without hemorrhage |
| 562.03 | Diverticulitis of small intestine with hemorrhage |
| 562.11 | Diverticulitis of colon without hemorrhage |
| 562.13 | Diverticulitis of colon with hemorrhage |
| 566 | Abscess of anal and rectal regions |
| 567 | Peritonitis |
| 569.5 | Intestinal abscess |
| 569.61 | Infection of colostomy or enterostomy |
| 569.83 | Perforation of intestine |
| 572.0 | Abscess of liver |
| 572.1 | Portal pyemia |
| 575.0 | Acute cholecystitis |
| 590 | Kidney infection |
| 599.0 | Urinary tract infection, not otherwise specified |
| 601 | Prostatic inflammation |
| 604 | Orchitis and epididymitis |
| 614 | Female pelvic inflammation disease |
| 615 | Uterine inflammatory disease |
| 616.3 | Abscess of Bartholin's gland |
| 616.4 | Other abscess of vulva |
| 646.6 | Infections of genitourinary tract in pregnancy |
| 658.4 | Infection of amniotic cavity |
| 670 | Major puerperal infection |
| 675.1 | Abscess of breast |
| 681 | Cellulitis, finger/toe |
| 682 | Other cellulitis and abscess |
| 683 | Acute lymphadenitis |
| 685.0 | Pilonidal cyst with abscess |
| 686 | Other local skin infection |
| 711.0 | Pyogenic arthritis |
| 728.86 | Necrotizing fasciitis |
| 730 | Osteomyelitis |
| 790.7 | Bacteremia |
| 958.3 | Posttraumatic wound infection, not elsewhere classified |
| 996.6 | Infection or inflammation of device/graft |
| 998.5 | Postoperative infection |
| 999.3 | Infectious complications of medical care not otherwise specified |
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Victoria Chia, Amgen (C); Jason Legg, Amgen (C); Richard Barron, Amgen (C) Consultant or Advisory Role: None Stock Ownership: Victoria Chia, Amgen; Jason Legg, Amgen; Richard Barron, Amgen Honoraria: None Research Funding: Chris M. Kozma, Amgen; Michael Dickson, Amgen Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: All authors
Administrative support: Richard Barron
Collection and assembly of data: Michael Dickson
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Lyman GH, Lyman CH, Agboola O. Risk models for predicting chemotherapy-induced neutropenia. Oncologist. 2005;10:427–437. doi: 10.1634/theoncologist.10-6-427. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Fort Washington, PA: National Comprehensive Cancer Network; 2011. NCCN Clinical Practice Guidelines in Oncology: Myeloid Growth Factors, Version 1.2011. [DOI] [PubMed] [Google Scholar]
- 3.Agency for Healthcare Research and Quality. Rockville, MD: Agency for Healthcare Research and Quality; 2009. HCUP Nationwide Inpatient Sample: Healthcare Cost and Utilization Project (HCUP). 1989-2007. [Google Scholar]
- 4.WHO Collaborating Centre for Drug Statistics Methodology. International Language for Drug Utilization Research: Anatomical Therapeutic Chemical (ATC) and Define Daily Dose (DDD) Classification System. www.whocc.no/
- 5.Caggiano V, Weiss RV, Rickert TS, et al. Incidence, cost, and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103:1916–1924. doi: 10.1002/cncr.20983. [DOI] [PubMed] [Google Scholar]
- 6.Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106:2258–2266. doi: 10.1002/cncr.21847. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. Fort Washington, PA: National Comprehensive Cancer Network; 2011. NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin's Lymphomas Version 2.2011. [Google Scholar]
- 8.Norton L. Conceptual and practical implications of breast tissue geometry: Toward a more effective, less toxic therapy. Oncologist. 2005;10:370–381. doi: 10.1634/theoncologist.10-6-370. [DOI] [PubMed] [Google Scholar]
- 9.Shayne M, Culakova E, Wolff D, et al. Dose intensity and hematologic toxicity in older breast cancer patients receiving systemic chemotherapy. Cancer. 2009;115:5319–5328. doi: 10.1002/cncr.24560. [DOI] [PubMed] [Google Scholar]
- 10.Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. J Natl Compr Canc Netw. 2009;7:99–108. doi: 10.6004/jnccn.2009.0009. [DOI] [PubMed] [Google Scholar]
- 11.Markman M. Managing taxane toxicities. Support Care Cancer. 2003;11:144–147. doi: 10.1007/s00520-002-0405-9. [DOI] [PubMed] [Google Scholar]
- 12.Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: A systematic review. J Clin Oncol. 2007;25:3158–3167. doi: 10.1200/JCO.2006.08.8823. [DOI] [PubMed] [Google Scholar]
- 13.Lyman GH, Dale DC, Wolff DA, et al. Acute myeloid leukemia or myelodysplastic syndrome in randomized controlled clinical trials of cancer chemotherapy with granulocyte colony-stimulating factor: A systematic review. J Clin Oncol. 2010;28:2914–2924. doi: 10.1200/JCO.2009.25.8723. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Michels SL, Reynolds MW, et al. Risk of mortality in patients with cancer who experience febrile neutropenia. Cancer. 2010;116:5555–5563. doi: 10.1002/cncr.25332. [DOI] [PubMed] [Google Scholar]
- 15.Chen-Hardee S, Chrischilles EA, Voelker MD, et al. Population-based assessment of hospitalizations for neutropenia from chemotherapy in older adults with non-Hodgkin's lymphoma (United States) Cancer Causes Control. 2006;17:647–654. doi: 10.1007/s10552-005-0502-4. [DOI] [PubMed] [Google Scholar]
- 16.Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health. 2008;11:172–179. doi: 10.1111/j.1524-4733.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 17.Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: A multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23:1178–1184. doi: 10.1200/JCO.2005.09.102. [DOI] [PubMed] [Google Scholar]
- 18.Aapro MS, Cameron DA, Pettengell R, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42:2433–2453. doi: 10.1016/j.ejca.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer. 2004;100:228–237. doi: 10.1002/cncr.11882. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH. Prediction and prevention of neutropenic complications in older patients receiving cancer chemotherapy. Am Soc Clin Oncol Ed Book. 2011:191–196. [Google Scholar]
- 21.Kieseier BC, Jeffery DR. Chemotherapeutics in the treatment of multiple sclerosis. Ther Adv Neurol Disord. 2010;3:277–291. doi: 10.1177/1756285610379885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yildirim-Toruner C, Diamond B. Current and novel therapeutics in the treatment of systemic lupus erythematosus. J Allergy Clin Immunol. 2011;127:303–312. doi: 10.1016/j.jaci.2010.12.1087. quiz 313-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horner MJ, Ries LAG, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2009. SEER Cancer Statistics Review, 1975-2006. [Google Scholar]