Formulary access and safety warnings had significant impacts on the new use of ESAs in patients with cancer, suggesting that both are effective means of influencing the use of these drugs.
Abstract
Purpose:
To characterize the effects of formulary changes and governmental safety warnings on use of erythropoiesis-stimulating agents (ESAs) in patients with cancer.
Patients and Methods:
We conducted a cross-sectional time-series analysis using health administrative data from Ontario, Canada. From January 1997 to December 2009 we identified all ESA initiations among patients diagnosed with cancer. We explored the effects of two formulary changes that progressively liberalized coverage for ESAs, first by rescinding the requirement for blood transfusion in 2003 and then by removing all restrictions in 2007. We also explored the effect of US Food and Drug Administration and Health Canada warnings issued in the second quarter of 2007. To assess regional variability in ESA use, we determined prescription rates for each of Ontario's 14 regional cancer centers.
Results:
After the first formulary change, the ESA initiation rate increased to 1.66 new users per 1,000 patients with cancer, 374% more than predicted (P < .001). After the second formulary change, the initiation rate increased to 3.97 new users per 1,000 patients with cancer, 73% more than predicted (P < .001). After the safety warnings, this rate declined 81% by study end (P < .001). We found significant regional variation in ESA use.
Conclusion:
Formulary access and safety warnings had significant impacts on the new use of ESA drugs in patients with cancer. This suggests that both are effective means of influencing the use of these drugs. Variable ESA prescription rates across our region may reflect a lack of consensus regarding their utility.
Introduction
Among patients with cancer, anemia is a common and debilitating problem.1 Before the introduction of erythropoiesis-stimulating agents (ESA), treatment of symptomatic malignancy-related anemia consisted primarily of RBC transfusion.2 The scarcity and potential hazards of blood products, along with aggressive promotion by the pharmaceutical industry, contributed to the enthusiasm with which ESAs were initially embraced.3,4
The Ontario Drug Benefits (ODB) program began covering ESA drugs for the treatment of malignancy-related anemia in 1998. Initially, coverage was provided only through a burdensome program in which a case-by-case assessment permitted coverage only for patients who had a hemoglobin concentration less than 10 g/dL, a mean cell volume between 75 fL and 120 fL and had already required blood transfusion. In 2003, access to ESA drug coverage was liberalized when the blood transfusion requirement was lifted; in 2007, these drugs became full benefits under the provincial formulary, with no restrictions governing their use.
We speculated that the progressive liberalization of ESA coverage would have a marked effect on the rate of treatment initiation among patients with cancer. Although the effectiveness of US Food and Drug Administration (FDA) warnings has been questioned,5–7 we hypothesized that ESA initiation rates would be curtailed by the 2007 FDA and Health Canada warnings regarding the risks of thromboembolism, tumor progression, and mortality associated with ESA use in patients with cancer.8,9
To test these hypotheses, we conducted a cross-sectional time series analysis to explore the impacts of formulary listing changes and governmental warnings on ESA treatment initiation trends among patients with cancer. In an additional analysis, we examined regional variability in ESA use among Ontario's 14 regional cancer centers (RCCs).
Patients and Methods
Study Design
We conducted a cross-sectional time-series analysis examining changes in quarterly prescription claims for ESA drugs by the Ontario Public Drug Program between January 1997 and December 2009. Ontario residents are eligible for drug coverage if they are unemployed or disabled, have high prescription drug costs relative to their net household income, receive home care, reside in a long-term care facility, or are aged 65 years or more.10
Data Sources
We identified patients treated with an ESA drug using the Ontario Public Drug Benefit Program database, which contains comprehensive records of prescription medications dispensed to Ontario residents eligible for public drug coverage. We identified those with a prior cancer diagnosis using the Ontario Cancer Registry, a computerized database of information on all Ontario residents newly diagnosed with cancer. We gathered demographic information from the Ontario Registered Persons Database, which contains a unique entry for each resident who has ever received insured health services, and physician characteristics from the Institute for Clinical Evaluative Sciences Physician Database, which provides annual physician data including main specialty and location of practice. These databases are anonymously linked using 10-digit health card numbers and are routinely used to investigate drug safety in Ontario.11–14 This study was approved by the ethics review board of Sunnybrook Health Sciences Centre, Toronto.
Identification of Patients
We studied all Ontarians with a diagnosis of cancer. We identified those with prescriptions for recombinant human erythropoietin (rHuEPO), darbepoetin alfa, and epoetin alfa between the years 1998 and 2009. Individuals were assigned to one of three exposure groups on the basis of their prescription history in each quarter: rHuEPO or darbepoetin alfa only, epoetin alfa only, or multiple ESA drug types. In each quarter, we restricted our analyses to new users of ESA drugs by excluding patients with a prescription for any ESA in the past year.
ESA Treatment Initiation Rates
For each of the three ESA exposure groups (rHuEPO or darbepoetin alfa, epoetin alfa, or multiple ESA drug types) we determined the quarterly treatment initiation rate. We defined this rate as the number of new ESA users in each quarter divided by the total number of Ontarians with a pre-existing diagnosis of cancer who were alive at the beginning of that quarter. We further stratified these rates by age, into people aged less than 65 years, and people aged 65 years of age or more.
ESA Prescribing in RCCs
In an exploratory analysis, we linked prescriptions for ESA drugs from two time intervals (January 1, 2005 to December 31, 2006, and January 1, 2008 to December 31, 2009) to one of the 14 RCCs if the primary practice location of the prescribing physician was in the same dissemination area as one of the RCCs. Dissemination areas are small, stable geographic units with a population size targeted to be between 400 and 700 individuals,15 and therefore it is reasonable to assume that these physicians are associated with the RCC in their area. For each 2-year interval, we calculated the rate of ESA treatment initiation in each RCC as the number of new users of ESA drugs per 1,000 patients with cancer treated in the RCC. Similarly, the prescription rate was calculated as the total number of ESA prescriptions dispensed per 1,000 patients with cancer treated in the RCC.
Statistical Analyses
We used segmented linear regression to estimate changes in levels and trends of the rate of ESA use per 1,000 patients.16 This type of model accounts for baseline level and trend in ESA use while estimating changes in level and trend resulting from the interventions. We included a first-order autoregressive parameter in the model to account for the correlated nature of the time series data. The regression model included a variable reflecting the number of intervals after the first quarter (X1(t)). To estimate the change in level of ESA prescription rate after each intervention, we also included in the regression model two indicator variables as covariates: one that was equal to 1 starting the first quarter of 2003 (X2(t)), and the other that was equal to 1 starting the first quarter of 2007 (X3(t)). Finally, to estimate the change in trend after each intervention, we included two other variables reflecting the number of time intervals after the interventions (X4(t) and X5(t)).
We used slope and trend coefficients to estimate the average difference between the rate of ESA use with the interventions (the observed rate) and without the interventions (the expected rate) for the following quarters: the first quarter after the first intervention (January to March 2003), the last quarter after the first intervention and before the second intervention (October to December 2006), the first quarter after the second intervention (January to March 2007) and the last quarter after the second intervention (October to December 2009). To assess linear regression model assumptions, we tested for residual autocorrelation by using the Durbin-Watson test for autocorrelation at different lags, and the partial autocorrelation and inverse autocorrelation plots were visually assessed for residual (seasonal) autocorrelation.
We reported P values for coefficient estimate and 95% confidence intervals for average differences between observed and expected ESA use.16 All P values were two-sided and used a threshold of 0.05 as the cutoff for statistical significance. All analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
Results
Primary Analysis
During the 12-year assessment period, we identified an average of 369,403 individuals with a diagnosis of cancer in each quarter. Within this group, we identified 13,145 patients who were initiated on an ESA drug, including 9,517 (72.4%) who commenced epoetin alfa, 3,615 (27.5%) who commenced darbepoetin alfa, and 13 (< 1%) with prescriptions for both.
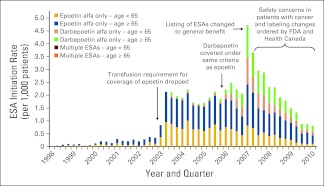
The rate of treatment initiation with an ESA did not change significantly from 1998 to 2002 (P = .10, Figure 1). After the requirement for blood transfusion was lifted in the first quarter of 2003, we observed a marked increase in use, with an ESA treatment initiation rate that was 374% greater than expected (observed = 1.66 new users per 1,000 patients, expected = 0.35 new users per 1,000 patients, P < .001). Similarly, when ESAs became available without restriction in the first quarter of 2007, we observed a treatment initiation rate that was 73% greater than expected (observed = 3.97 prescriptions per 1,000 patients, expected = 2.30 new users per 1,000 patients, P < .001). After warnings were issued by the FDA and Health Canada in March of 2007, we observed a steady decline in the rate ESA treatment initiation. Compared with the first quarter of 2007, we observed a rate in the second quarter that was 8% lower (first quarter = 3.97 new users per 1,000 patients, second quarter = 3.67 new users per 1,000 patients, P < .001), and by the end of our study period, the ESA initiation rate had declined by 81% (average quarterly decline of 0.34 prescriptions per 1,000 patients, P < .001).
Figure 1.
Erythropoiesis-stimulating agent (ESA) initiation rates among patients with cancer. FDA, US Food and Drug Administration.
When considered individually, the overall trends in epoetin alfa and darbepoetin alfa initiation were similar to that observed for ESAs as a whole. When ESAs became available without restriction in the first quarter of 2007, we observed an epoetin alfa treatment initiation rate that was 26% greater than expected (observed = 2.06 prescriptions per 1,000 patients, expected = 1.63 new users per 1,000 patients, P = .0083). In the same quarter, we observed a darbepoetin alfa treatment initiation rate that was 203% greater than expected (observed = 1.89 new users per 1,000 patients, expected = 0.62 new users per 1,000 patients, P < .001).
Additional Analysis
As an additional analysis, we assessed geographic variation in the prescribing of ESAs within RCCs in Ontario by using cross-sectional data from the first 2-year interval (January 2005 to December 2006) and the most recent 2-year interval (January 2008 to December 2009). At both time points, we observed substantial variability in ESA treatment initiation rates between the 14 RCCs, ranging from zero to 18.4 (95% CI, 16.6 to 20.3) per 1,000 patients in the first interval (Appendix Table A1, online only) and from 1.7 (95% CI, 1.0 to 2.4) to 17.7 (95% CI, 14.7 to 20.7) per 1,000 patients in the second interval (Table 1). In both intervals, we observed highly variable overall ESA prescription rates (from zero to 97.6 prescriptions per 1,000 patients in the first interval and from 3.7 to 147.2 prescriptions per 1,000 patients in the second interval).
Table 1.
Rates of New ESA Treatment and Overall ESA Prescription by Ontario Regional Cancer Center, January 1, 2008 to December 31, 2009
| Regional Cancer Center | No. Patients | New ESA Users (N = 1,221) |
All ESA Prescriptions (N = 7,870) |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. per 1,000 Patients | 95% CI | No. | % | No. per 1,000 Patients | ||
| 1 | 7,271 | 129 | 10.6 | 17.7 | 14.7 to 20.7 | 1,070 | 13.6 | 147.2 |
| 2 | 11,439 | 192 | 15.7 | 16.8 | 14.4 to 19.2 | 763 | 9.7 | 66.7 |
| 3 | 19,794 | 200 | 16.4 | 10.1 | 8.7 to 11.5 | 1,402 | 17.8 | 70.8 |
| 4 | 4,658 | 44 | 3.6 | 9.4 | 6.6 to 12.2 | 178 | 2.3 | 38.2 |
| 5 | 10,980 | 90 | 7.4 | 8.2 | 6.5 to 9.9 | 580 | 7.4 | 52.8 |
| 6 | 6,740 | 50 | 4.1 | 7.4 | 5.4 to 9.4 | 138 | 1.8 | 20.5 |
| 7 | 38,845 | 254 | 20.8 | 6.5 | 5.7 to 7.3 | 2,295 | 29.2 | 59.1 |
| 8 | 21,366 | 133 | 10.9 | 6.2 | 5.1 to 7.3 | 850 | 10.8 | 39.8 |
| 9 | 5,236 | 28 | 2.3 | 5.3 | 3.3 to 7.3 | 105 | 1.3 | 20.1 |
| 10 | 2,186 | 7 | 0.6 | 3.2 | 0.8 to 5.6 | 8 | 0.1 | 3.7 |
| 11 | 7,302 | 21 | 1.7 | 2.9 | 1.7 to 4.1 | 91 | 1.2 | 12.5 |
| 12 | 8,098 | 19 | 1.6 | 2.3 | 1.3 to 3.3 | 84 | 1.1 | 10.4 |
| 13 | 13,651 | 30 | 2.5 | 2.2 | 1.4 to 3.0 | 163 | 2.1 | 11.9 |
| 14 | 14,401 | 24 | 2.0 | 1.7 | 1.0 to 2.4 | 143 | 1.8 | 9.9 |
| Total | 171,967 | 1,221 | 100.0 | 7.1 | 6.7 to 7.5 | 7,870 | 100.0 | 45.8 |
Abbreviation: ESA, erythropoiesis-stimulating agent.
Discussion
In this cross-sectional time-series analysis we found increased ESA treatment initiation rates associated with formulary listing changes that liberalized access to these drugs. We also observed a subsequent decrease in new use associated with safety warnings issued by the FDA and Health Canada. We also found that at the beginning and end of the analysis period, the rates of ESA treatment initiation and overall prescription utilization varied significantly across the 14 RCCs in Ontario.
Our study illustrates the effects of formulary changes on pharmaceutical use. Despite aggressive marketing efforts,17 the extent of formulary coverage appeared to be the dominant factor in the initiation of an ESA drug. At the population level, restricted access to these drugs before 2007 likely mitigated the impact of the adverse events now known to be associated with ESA use.18–21 Our study also suggests a responsiveness of physicians to warnings from the FDA and Health Canada. Although some studies have indicated that FDA warnings have little influence on prescribing practices,5–7 our study showed that safety warnings issued by regulatory agencies were associated with a significant reduction in the ESA prescription rate. Health Canada warnings do not require consent before treatment; therefore, administrative barriers are not a likely explanation for our findings. The marked variability in the use of ESA drugs among Ontario's RCCs present at both the beginning and end of our analysis period may indicate that the lack of consensus regarding the utility of these drugs precedes both the FDA and Health Canada warnings.
This study has many strengths, including its large and population-based nature. We reliably ascertained prescription rates using the ODB database, which has an error rate of less than 1%.22 Ontario's universal health care system allowed our study to be free of the selection biases characteristic of private health insurance cohorts. However, some limitations merit emphasis. Although we did not find any changes in clinical practice guidelines or availability of blood products at the intervention points we assessed, we cannot exclude the possibility that other factors influenced rates of new ESA use. We did not have access to ESA prescriptions reimbursed by private drug plans and therefore cannot determine our province's absolute drug initiation rates. However, it is unlikely that changes in the proportion of publicly and privately funded ESA prescriptions accounted for the changes we observed in our intervention analyses. With respect to the different RCCs, we were not able to determine each center's case mix and therefore cannot rule this out as a cause of the variability in the treatment choices we observed.
In the setting of a publicly funded prescription drug plan, ESA initiation rates were strongly influenced by formulary restrictions and warnings issued by national health agencies. These are effective tools by which to regulate prescription drug use.
Acknowledgment
We thank Brogan for providing access to the drug identification numbers used to identify eligible drugs. Supported by the Lawson Health Research Institute and the Ontario Drug Policy Research Network which receives funding from the Ontario Ministry of Health and Long-term Care (MOHLTC) Drug Innovation Fund. M.A.W. was supported by the Clinical Investigator Program Award at the University of Western Ontario and funding from MOHLTC. The Institute of Clinical Evaluative Sciences receives funding from MOHLTC. The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources.
Appendix
Table A1.
Rates of New ESA Treatment and Overall ESA Prescription by Ontario Regional Cancer Center, January 1, 2005 to December 31, 2006
| Regional Cancer Center* | No. Patients | New ESA Users (N = 1,327) |
All ESA Prescriptions (N = 6,794) |
|||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. New Users per 1,000 Patients | 95% CI | No. | % | No. Prescriptions per 1,000 Patients | ||
| 3 | 19,679 | 363 | 10.5 | 18.4 | 16.6 to 20.3 | 1696 | 25.0 | 86.2 |
| 2 | 13,597 | 235 | 6.8 | 17.3 | 15.1 to 19.5 | 947 | 13.9 | 69.6 |
| 1 | 6,835 | 90 | 2.6 | 13.2 | 10.5 to 15.9 | 669 | 9.8 | 97.9 |
| 6 | 7,804 | 89 | 2.6 | 11.4 | 9.0 to 13.8 | 275 | 4.0 | 35.2 |
| 9 | 3,764 | 24 | 0.7 | 6.4 | 3.8 to 8.9 | 126 | 1.9 | 33.5 |
| 4 | 4,845 | 30 | 0.9 | 6.2 | 4.0 to 8.4 | 144 | 2.1 | 29.7 |
| 12 | 10,040 | 58 | 1.7 | 5.8 | 4.3 to 7.3 | 349 | 5.1 | 34.8 |
| 7 | 39,736 | 218 | 6.3 | 5.5 | 4.8 to 6.2 | 1515 | 22.3 | 38.1 |
| 5 | 10,597 | 57 | 1.6 | 5.4 | 4.0 to 6.8 | 321 | 4.7 | 30.3 |
| 8 | 23,228 | 89 | 2.6 | 3.8 | 3.0 to 4.6 | 485 | 7.1 | 20.9 |
| 11 | 3,906 | 15 | 0.4 | 3.8 | 1.9 to 5.8 | 47 | 0.7 | 12.0 |
| 13 | 9,786 | 35 | 1.0 | 3.6 | 2.4 to 4.8 | 129 | 1.9 | 13.2 |
| 14 | 15,431 | 24 | 0.7 | 1.6 | 0.9 to 2.2 | 91 | 1.3 | 5.9 |
| 10 | 1,689 | 0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | |
| Total | 170,937 | 1,327 | 100.0 | 7.1 | 6.7 to 7.5 | 6,794 | 100.0 | 39.7 |
Abbreviation: ESA, erythropoiesis-stimulating agent.
Nos. correspond to those of Regional Cancer Centers listed in Table 1.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Muhammad Mamdani, Amgen Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Matthew A. Weir, Tara Gomes, Eric Winquist, David Juurlink, Muhammad Mamdani
Financial support: Muhammad Mamdani
Collection and assembly of data: Matthew A. Weir, Tara Gomes, Muhammad Mamdani
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: Incidence and treatment. J Natl Cancer Inst. 1999;91:1616–1634. doi: 10.1093/jnci/91.19.1616. [DOI] [PubMed] [Google Scholar]
- 2.Skillings JR, Sridhar FG, Wong C, et al. The frequency of red cell transfusion for anemia in patients receiving chemotherapy. A retrospective cohort study. Am J Clin Oncol. 1993;16:22–25. doi: 10.1097/00000421-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Goodnough LT. Risks of blood transfusion. Anesthesiol Clin North America. 2005;23:241–252. v. doi: 10.1016/j.atc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Steinbrook R. Erythropoietin, the FDA, and oncology. N Engl J Med. 2007;356(24):2448–2451. doi: 10.1056/NEJMp078100. [DOI] [PubMed] [Google Scholar]
- 5.Horlen C, Malone R, Bryant B, et al. Frequency of inappropriate metformin prescriptions. JAMA. 2002;287:2504–2505. doi: 10.1001/jama.287.19.2504-a. [DOI] [PubMed] [Google Scholar]
- 6.Masoudi FA, Wang Y, Inzucchi SE, et al. Metformin and thiazolidinedione use in Medicare patients with heart failure. JAMA. 2003;290:81–85. doi: 10.1001/jama.290.1.81. [DOI] [PubMed] [Google Scholar]
- 7.Smalley W, Shatin D, Wysowski DK, et al. Contraindicated use of cisapride: Impact of Food and Drug Administration regulatory action. JAMA. 2000;284:3036–3039. doi: 10.1001/jama.284.23.3036. [DOI] [PubMed] [Google Scholar]
- 8.Health Canada. http://www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/prof/_2007/aranesp_eprex_hpc-cps-eng.php.
- 9.US Food and Drug Administration. Public Health Advisory: Erythropoiesis-Stimulating Agents. http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124262.htm. 6-18-2009.
- 10.Ontario Ministry of Health and Long-Term Care. http://www.health.gov.on.ca/en/public/programs/drugs/programs/odb/odb.aspx.
- 11.Juurlink DN, Mamdani MM, Lee DS, et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med. 2004;351:543–551. doi: 10.1056/NEJMoa040135. [DOI] [PubMed] [Google Scholar]
- 12.Weir MA, Juurlink DN, Gomes T, et al. Beta-blockers, trimethoprim-sulfamethoxazole, and the risk of hyperkalemia requiring hospitalization in the elderly: A nested case-control study. Clin J Am Soc Nephrol. 2010;5:1544–1551. doi: 10.2215/CJN.01970310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park-Wyllie LY, Juurlink DN, Kopp A, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 14.Juurlink DN, Gomes T, Lipscombe LL, et al. Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: Population based cohort study. BMJ. 2009;339:b2942. doi: 10.1136/bmj.b2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics Canada. More Information on Dissemination Area (DA) http://www12.statcan.ca/census-recensement/2006/ref/dict/geo021a-eng.cfm.
- 16.NorthEast SAS Users Group. Estimating Confidence Intervals Around Relative Changes in Outcomes in Segmented Regression Analyses of Time Series Data. Presented at the 15th Annual NorthEast SAS Users Group Conference; September 29 to October 2, 2002; Buffalo, NY. [Google Scholar]
- 17.Frosch DL, Krueger PM, Hornik RC, et al. Creating demand for prescription drugs: A content analysis of television direct-to-consumer advertising. Ann Fam Med. 2007;5:6–13. doi: 10.1370/afm.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299:914–924. doi: 10.1001/jama.299.8.914. [DOI] [PubMed] [Google Scholar]
- 19.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 20.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: A survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 21.Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: A meta-analysis. CMAJ. 2009;180:E62–E71. doi: 10.1503/cmaj.090470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy AR, O'Brien BJ, Sellors C, et al. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol. 2003;10:67–71. [PubMed] [Google Scholar]