Abstract
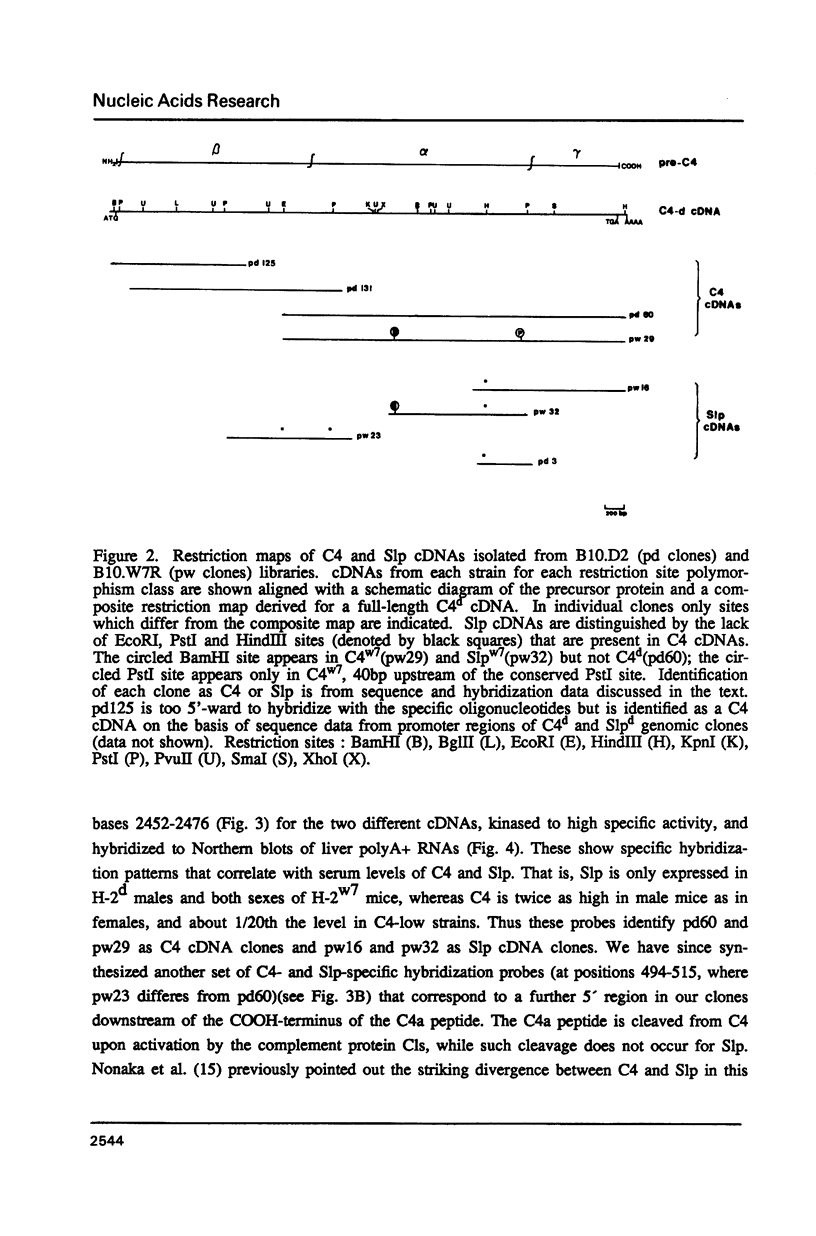
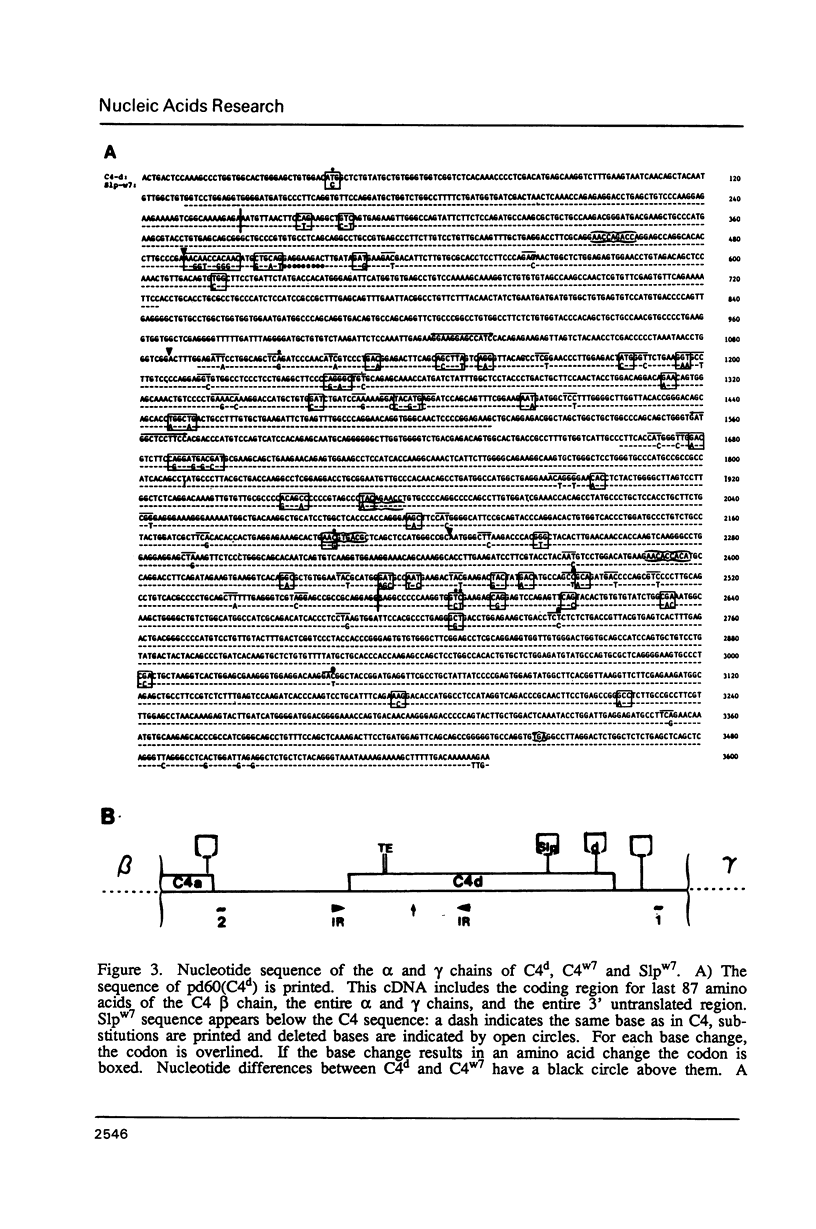
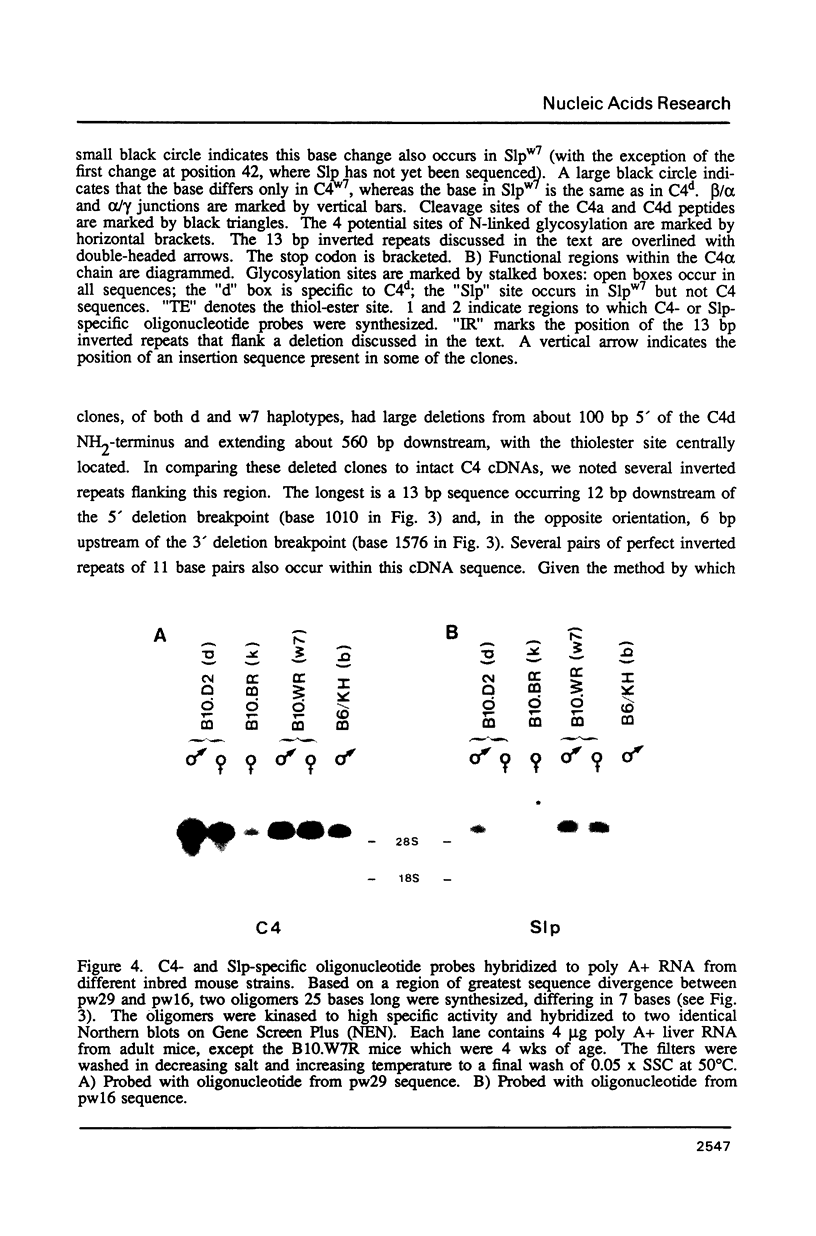
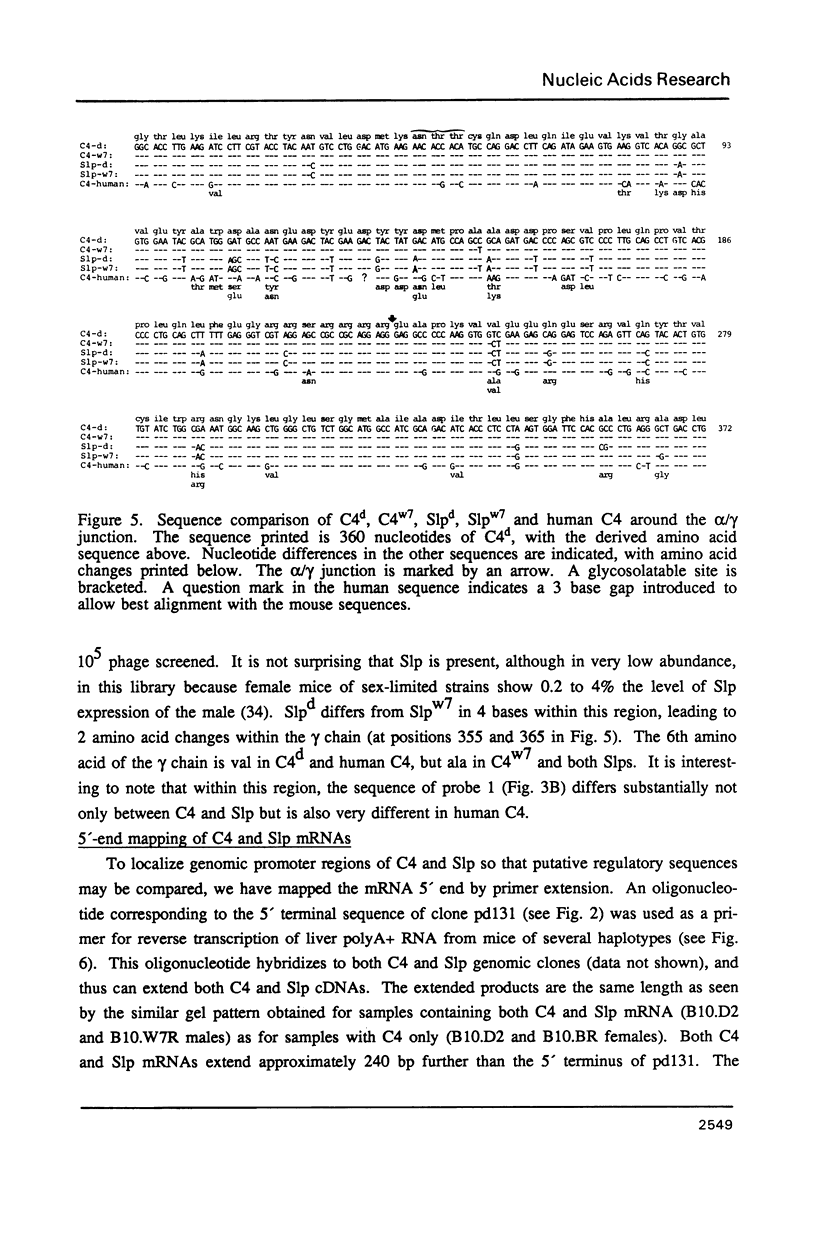
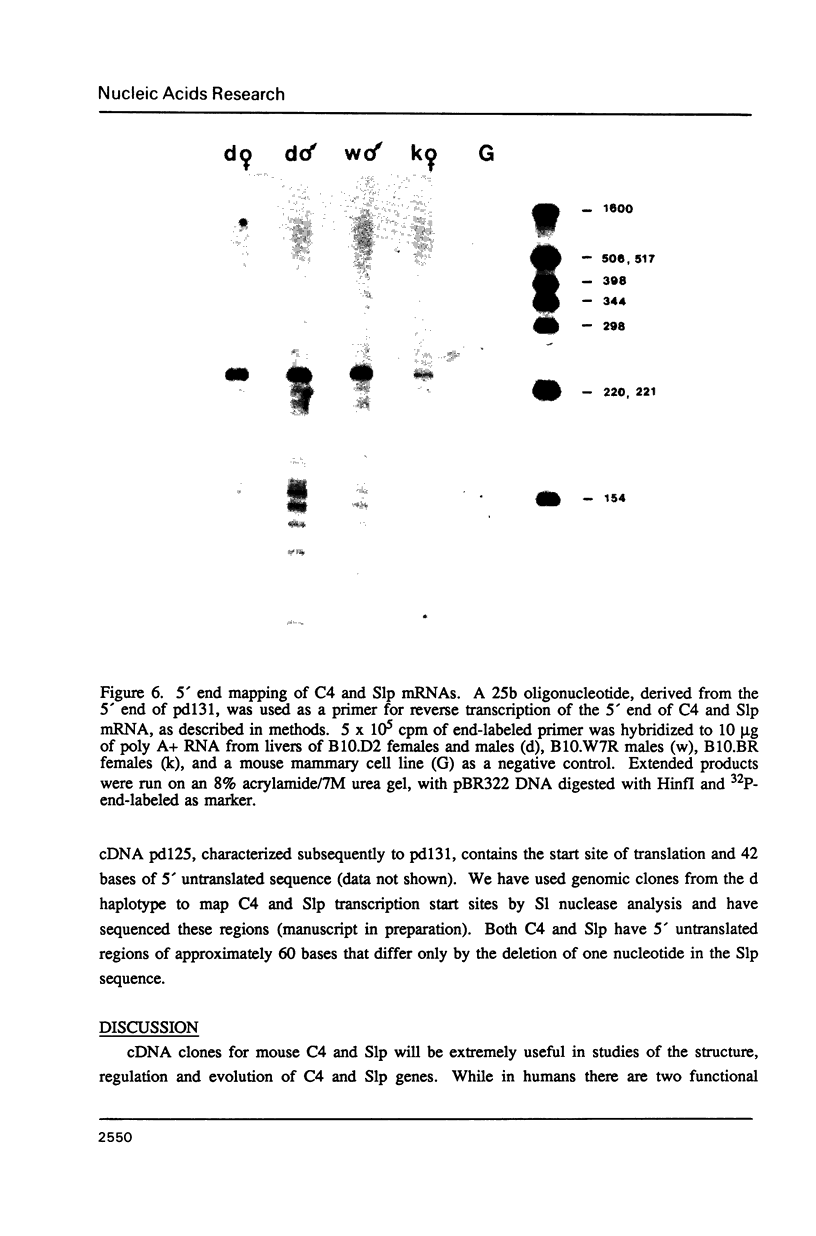
cDNA clones specific for the fourth component of complement (C4) and its androgen-regulated isotype, sex-limited protein (Slp), have been isolated from two mouse haplotypes (H-2d and H-2w7) that show differential C4 activity and differential regulation of Slp. Clones were first isolated using a cDNA probe enriched by subtractive hybridization. Subsequent screening has resulted in cDNAs spanning the entire C4d mRNA, as well as much of C4w7, Slpw7 and a short region of Slpd. The cDNAs for C4 and Slp show extensive sequence homology, but can be distinguished using oligonucleotide probes synthesized to regions of greatest sequence divergence. Sequence differences between C4 and Slp indicate structurally important features of C4 that have been altered in Slp such that Slp is unable to participate in the complement pathway. Of the few nucleotide differences between C4d and C4w7, a single base change resulting in one less glycosylation site in the C4w7 alpha chain could account for its 4-fold reduced hemolytic efficiency. Sequence comparison of multiple alleles of C4 and Slp indicates that possible gene conversion events occurred in the H-2w7 strain that has multiple Slp genes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson J. P., McGinnis K., Brown L., Peterein J., Shreffler D. A murine C4 molecule with reduced hemolytic efficiency. J Exp Med. 1980 Feb 1;151(2):492–497. doi: 10.1084/jem.151.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belt K. T., Carroll M. C., Porter R. R. The structural basis of the multiple forms of human complement component C4. Cell. 1984 Apr;36(4):907–914. doi: 10.1016/0092-8674(84)90040-0. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Brown L. J., Shreffler D. C. Female expression of the H-2-linked sex-limited protein (Slp) due to non-H-2 genes. Immunogenetics. 1980;10(1):19–29. doi: 10.1007/BF01561549. [DOI] [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Chan A. C., Mitchell K. R., Munns T. W., Karp D. R., Atkinson J. P. Identification and partial characterization of the secreted form of the fourth component of human complement: evidence that it is different from major plasma form. Proc Natl Acad Sci U S A. 1983 Jan;80(1):268–272. doi: 10.1073/pnas.80.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Domdey H., Wiebauer K., Kazmaier M., Müller V., Odink K., Fey G. Characterization of the mRNA and cloned cDNA specifying the third component of mouse complement. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7619–7623. doi: 10.1073/pnas.79.24.7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A., Eichinger D., Nussenzweig V. The murine sex-limited protein (Slp): reassessment of its sex limitation. J Immunol. 1982 Oct;129(4):1506–1508. [PubMed] [Google Scholar]
- Ferreira A., Michaelson J., Nussenzweig V. H-2-controlled polymorphism of the gamma chain of Slp (sex-limited protein). Immunogenetics. 1980;11(5):491–497. doi: 10.1007/BF01567817. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Nussenzweig V., Gigli I. Structural and functional differences between the H-2 controlled Ss and Slp proteins. J Exp Med. 1978 Nov 1;148(5):1186–1197. doi: 10.1084/jem.148.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall R. E., Colten H. R. Cell-free synthesis of the fourth component of guinea pig complement (C4): identification of a precursor of serum C4 (pro-C4). Proc Natl Acad Sci U S A. 1977 Apr;74(4):1707–1710. doi: 10.1073/pnas.74.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen T. H., Shreffler D. C. Characterization of a constitutive variant of the murine serum protein allotype, Slp. J Immunol. 1976 Nov;117(5 Pt 1):1507–1513. [PubMed] [Google Scholar]
- Karp D. R., Parker K. L., Shreffler D. C., Slaughter C., Capra J. D. Amino acid sequence homologies and glycosylation differences between the fourth component of murine complement and sex-limited protein. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6347–6349. doi: 10.1073/pnas.79.20.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp D. R. Variation in the glycosylation of the murine sex-limited protein: comparison with the fourth component of murine complement. J Immunol. 1983 Sep;131(3):1405–1410. [PubMed] [Google Scholar]
- Levi-Strauss M., Tosi M., Steinmetz M., Klein J., Meo T. Multiple duplications of complement C4 gene correlate with H-2-controlled testosterone-independent expression of its sex-limited isoform, C4-Slp. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1746–1750. doi: 10.1073/pnas.82.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nonaka M., Nakayama K., Yeul Y. D., Takahashi M. Complete nucleotide and derived amino acid sequences of the fourth component of mouse complement (C4). Evolutionary aspects. J Biol Chem. 1985 Sep 15;260(20):10936–10943. [PubMed] [Google Scholar]
- Nonaka M., Takahashi M., Natsuume-Sakai S., Nonaka M., Tanaka S., Shimizu A., Honjo T. Isolation of cDNA clones specifying the fourth component of mouse complement and its isotype, sex-limited protein. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6822–6826. doi: 10.1073/pnas.81.21.6822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Sepich D. S. Genes for murine fourth complement component (C4) and sex-limited protein (Slp) identified by hybridization to C4- and Slp-specific cDNA. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4908–4911. doi: 10.1073/pnas.81.15.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata R. T., Sepich D. S. Murine sex-limited protein: complete cDNA sequence and comparison with murine fourth complement component. J Immunol. 1985 Dec;135(6):4239–4244. [PubMed] [Google Scholar]
- Ogata R. T., Shreffler D. C., Sepich D. S., Lilly S. P. cDNA clone spanning the alpha-gamma subunit junction in the precursor of the murine fourth complement component (C4). Proc Natl Acad Sci U S A. 1983 Aug;80(16):5061–5065. doi: 10.1073/pnas.80.16.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore H. C., Shreffler D. C. A sex-limited serum protein variant in the mouse: hormonal control of phenotypic expression. Biochem Genet. 1971 Apr;5(2):201–209. doi: 10.1007/BF00485645. [DOI] [PubMed] [Google Scholar]
- Passmore H. C., Shreffler D. C. A sex-limited serum protein variant in the mouse: inheritance and association with the H-2 region. Biochem Genet. 1970 Jun;4(3):351–365. doi: 10.1007/BF00485752. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. R. Complement polymorphism, the major histocompatibility complex and associated diseases: a speculation. Mol Biol Med. 1983 Jul;1(1):161–168. [PubMed] [Google Scholar]
- Porter R. R., Reid K. B. Activation of the complement system by antibody-antigen complexes: the classical pathway. Adv Protein Chem. 1979;33:1–71. doi: 10.1016/s0065-3233(08)60458-1. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D. M., Malissen M., Hood L., Ferreira A., Walthall D., Mitchell M. Multiple C4/Slp genes distinguished by expression after transfection. Mol Cell Biol. 1986 Jan;6(1):134–141. doi: 10.1128/mcb.6.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos M. H., Atkinson J. P., Shreffler D. C. Molecular characterization of the Ss and Slp (C4) proteins of the mouse H-2 complex: subunit composition, chain size polymorphism, and an intracellular (PRO-Ss) precursor. J Immunol. 1978 Sep;121(3):1106–1115. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich D. S., Noonan D. J., Ogata R. T. Complete cDNA sequence of the fourth component of murine complement. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5895–5899. doi: 10.1073/pnas.82.17.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreffler D. C. The S region of the mouse major histocompatibility complex (H-2): genetic variation and functional role in complement system. Transplant Rev. 1976;32:140–167. doi: 10.1111/j.1600-065x.1976.tb00232.x. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]