The authors found that a one-page treatment summary and care plan was accepted and retained by adult survivors of pediatric and young adult cancers, and did not result in undue worry or concern for most participants.
Abstract
Purpose:
Survivors of pediatric and young adult cancer are at increased risk for treatment-related problems. Yet, few survivors receive risk-based care. The treatment summary and care plan are recommended to improve understanding of cancer treatment, potential late effects, and recommended screening. It is unknown whether survivors retain, understand, value, and disseminate the document, and whether it causes worry.
Methods:
We surveyed 111 adult survivors of pediatric and young adult cancer 1 to 6 weeks after receipt of a one-page treatment summary and care plan (response rate, 96%). Participants answered questions regarding retention, understanding, value, dissemination, concern, and preferences.
Results:
Participants were majority female (58%), college-educated (60%), diagnosed with cancer before age 21 (76%), on average 18 years from diagnosis (range, 2 to 50 years), and treated with radiation and chemotherapy (61%). Median age was 30 years (range, 18 to 65 years). A majority of participants stated that they understood the treatment summary (95%), retained the document (95%), and valued it (92%). A minority reported that the document caused concern (14%) or wanted more information than the form provided (20%). Although the time between receipt of the document and survey was brief, many described dissemination of the document to their personal circle (44%) or an outside provider (10 [33%] of 30 who saw an outside doctor).
Conclusion:
A one-page treatment summary and care plan was well-received and did not cause report of undue concern. Additional health-related information was requested by some, and dissemination to outside providers could be improved.
Introduction
As a result of remarkable improvements in cancer detection and therapy, the number of pediatric and young adult cancer survivors in the United States is rapidly rising.1 Nonetheless, survivors of pediatric and young adult cancer face a substantial risk of late effects and early mortality2–8; by 30 years from their initial diagnosis, 73% of pediatric cancer survivors will develop at least one chronic physical health condition, whereas in 42% the condition will be severe, life-threatening, disabling, or result in death.2 Many late effects can be prevented through early diagnosis and treatment if survivors and their caregivers are adequately informed.9–11
Treatment summaries and survivorship care plans have been proposed as one method to improve communication and risk-based care. Pediatric oncologists have been creating cancer treatment summaries at the end of therapy for many decades,12,13 but these documents were often independent of a plan for follow-up care.9 The Institute of Medicine, the Children's Oncology Group, the National Institute for Health and Clinical Excellence, and others have endorsed a combined cancer treatment summary and care plan.9,14–18 Many long-term follow-up programs now include a document with both elements; individual components include cancer diagnosis, treatment, potential late effects, and recommendations for follow-up care and surveillance.
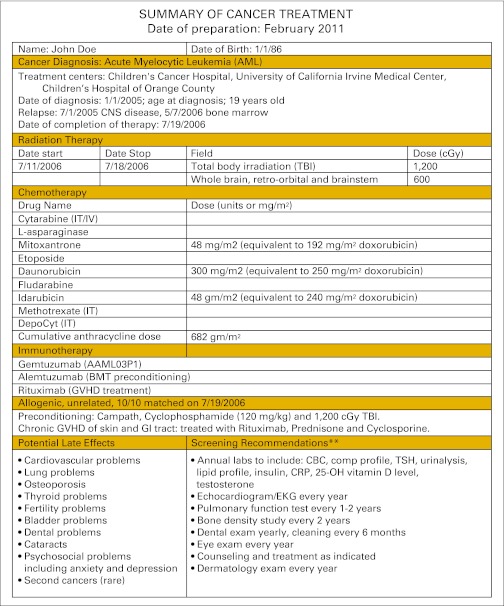
Prior studies suggest that patients and primary care physicians are receptive to the concept of a treatment summary and care plan.11,19–23 A recent study24 found that a mailed personalized treatment summary and care plan resulted in improved adherence to recommended screening. Nonetheless, it is not known how these documents might be received in a real-world clinical setting. We distributed a comprehensive, individualized treatment summary and care plan to patients as part of their routine long-term follow-up care (Figure 1). One to 6 weeks later, we questioned patients regarding whether the document raised worry and concern and whether they retained, understood, valued, and disseminated the treatment summary and care plan.
Figure 1.
Example of the summary of cancer treatment and follow-up care plan. BMT, bone marrow transplantation; CBC, complete blood count; CRP, c-reactive protein; EKG, electrocardiogram; GVHD, graft-versus-host disease; IT, intrathecal; IV, intravenous; TSH, thyroid-stimulating hormone.
Methods
Patients
We conducted a cross-sectional telephone survey of consecutive patients seen in the Memorial Sloan-Kettering Cancer Center (MSKCC) Adult Long-Term Follow-Up Program for a medical visit between May and August 2010. Eligible participants were cancer survivors 18 years or older; fluent in English; currently cancer free; and without significant neurocognitive, visual, or hearing deficits. During the study period, 148 patients were seen in the clinic. There were 24 ineligible survivors (non-English speaking, n = 3; severe neurocognitive deficit, n = 5; blind, n = 2; deaf, n = 2; interim visit for new acute problem, n = 12). There were eight eligible survivors who were not notified of the study (and thus not contacted) because of high clinic volume on the day that they were seen; the providers did not have adequate time to review the study. Of the 116 eligible survivors who were notified of the study and contacted, 111 (96%) completed the telephone survey; one refused to participate when reached by phone, and four could not be reached.
The following data were abstracted from the medical record: age at survey, age at diagnosis, sex, cancer diagnosis, cancer treatment, health insurance status, education level (college graduate or not), and visit status (first-time or return). The MSKCC institutional review board approved all aspects of the study.
Procedures
Telephone survey.
Participants were interviewed 1 to 6 weeks after their medical visit, using an internally developed 29-item survey to ascertain retention, understanding, value, dissemination, concern, worry, and preferences for the treatment summary. The survey was rated an 8.2 on the Flesch-Kincaid reading scale.
Retention was defined as having the treatment summary on hand or at home at the time of the survey. Participants were asked by the interviewer to refer to their treatment summary during the survey. Because many participants were at work during the time of the survey, those who indicated having the document at home in a personal file were counted as having retained the form.
To determine value, we asked participants, “How valuable is the treatment summary to you?” Responses were given on a 4-point Likert scale (not at all, a little bit, moderately, or extremely). In addition, participants were asked whether they found the document to be helpful in understanding the plan for their care and whether they considered it a link to their outside providers.
Dissemination was evaluated in two ways. Participants were asked whether they had seen an outside provider or been to an emergency room since receiving the treatment summary, and if so, whether they had shared the document. In addition, participants were asked whether the document had been shared with a spouse, friend, or other person.
In consideration of reported health-related worry among cancer survivors,24–27 we included questions regarding general health worry in the past week from the Memorial Symptom Assessment Scale. This instrument was developed to assess a wide variety of psychological and physical symptoms among patients with cancer and has been well validated in this population.28 Our survey asked, “In the past week, did you worry about your health?” followed by questions on the frequency and severity of worry. We also asked whether the treatment summary and care plan caused the participant additional worry. Finally, we asked whether receipt of the document, the “Potential Late Effects” section, or the “Screening Recommendations” section made the participant feel concerned or not concerned.
Finally, patient preferences regarding the current paper form and possible alternatives (eg, wallet card, online, e-mail) were assessed. Participant were also asked whether they had a primary care physician.
Treatment Summary and Care Plan
The MSKCC Adult Long-Term Follow-Up team uses a single-page template to enable efficient documentation of treatment history, potential late effects, and recommended follow-up. It typically takes 1 to 4 hours for the medical team to prepare a treatment summary and care plan for a survivor entering the program, depending on the recency and complexity of the cancer history and the availability of electronic records. Subsequent modification to an existing document as a result of updated guidelines or new diagnoses usually requires 15 to 20 minutes. Typically, the document is reviewed in detail during a new patient visit and briefly during follow-up. Although providers are available after the visit, questions about the document are rare.
Statistical Analyses
Data were analyzed with SAS Version 9.2 (SAS Institute, Cary, NC) and STATA Version 8.0 (Stata, College Station, TX). Bivariate analyses with χ2 or exact tests, where appropriate, were performed, and odds ratios with 95% CIs were calculated by logistic regression. Multivariate logistic regression was performed to determine factors independently associated with desiring more information on the treatment summary.
Results
Of 116 eligible subjects who agreed to be contacted, 111 (96%) completed the telephone survey. There were no significant differences between the participants and nonparticipants with regards to cancer history and demographics. Participants were majority female (58%), college-educated (60%), diagnosed with primary cancer before age 21 (76%), on average 18 years from diagnosis (range 2 to 50 years), and treated with both radiation and chemotherapy (61%). Subjects ranged in age from 18 to 65 years, with the large majority (74%) between 18 and 39 years old. Nearly one quarter (23%)of participants were new patients who had never received a treatment summary and care plan before. Although all participants had health insurance, just 57% were found to have a primary care physician outside MSKCC (Table 1). Participants who had survived a lymphoma were significantly older than nonlymphoma survivors in the survey, with a median age of 42 years compared with 27 years (P < .001).
Table 1.
Baseline Characteristics of Survivors of Pediatric and Young Adult Cancers (N = 111)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 30 | |
| Range | 18.0-65.0 | |
| Time since cancer diagnosis, years | ||
| Median | 18 | |
| Range | 2.0-50.0 | |
| Age at cancer diagnosis, years | ||
| Median | 16 | |
| Range | 0.08-41 | |
| Sex | ||
| Female | 64 | 58 |
| Male | 47 | 42 |
| Completed college* | 66 | 60 |
| First-time visit | 25 | 23 |
| Health insurance (including public insurance) | 111 | 100 |
| Primary care provider | 63 | 57 |
| Cancer diagnosis | ||
| Lymphoma | 34 | 31 |
| Leukemia | 26 | 23 |
| Bone tumor | 20 | 18 |
| Soft tissue sarcoma | 15 | 14 |
| Central nervous system | 7 | 6 |
| Neuroblastoma | 4 | 4 |
| Other | 4 | 4 |
| Wilms' tumor | 1 | 1 |
| Treatment modality | ||
| Chemotherapy plus radiation | 68 | 61 |
| Chemotherapy only | 29 | 26 |
| Stem-cell transplantation | 23 | 21 |
| Radiotherapy only | 14 | 13 |
| Chemotherapy | ||
| Anthracyclines plus alkylating agents | 67 | 60 |
| Alkylating agents only | 13 | 12 |
| Anthracylines only | 10 | 9 |
| Other chemotherapy only | 7 | 6 |
Education status was available for 101 of 110 patients.
Retention, Understanding, Value, and Dissemination
Overall response to the treatment summary and care plan was highly positive. One to 6 weeks after receiving a treatment summary and care plan in clinic, 95% of participants had retained the document; 55% had it on hand and 40% had it at home in a personal file.
To the question, “During your visit, did you understand everything on the treatment summary?” 95% subjects responded affirmatively. Of the six individuals who reported not understanding everything, one reported coming to an understanding by calling a nurse practitioner via the phone number on the document. The remaining five reported not understanding part of the cancer history, recommendations, or contact information. Only one, a 37-year-old female survivor, accepted the interviewer's offer for a clinician to call to explain the content in question; the other participants declined, indicating they would wait until their next visit for clarification.
To the question, “How valuable is the treatment summary to you?” 43% of participants selected “moderately” valuable and 50% responded “extremely” valuable. Similarly high proportions found the treatment summary and care plan helpful to them in understanding the plan for their care (95%) and considered it an important link between health care providers (95%).
Of 30 participants who had visited an outside provider since receiving the document, 10 reported giving the provider a copy of the form. No participant had visited the emergency room. Many (44%) had disseminated copies to someone in their personal circle. When compared by retention, understanding, demographics, or cancer history, participants who disseminated a summary were not significantly different from those who did not (results not shown).
A greater proportion of new patients (17 of 25; 68%) indicated learning new information from the treatment summary and care plan compared with returning patients (33 of 86; 39%; P = .009); however, new and returning participants did not differ significantly in retention, understanding, or value.
Worry and Concern
We found a small number of patients who reported frequent (15%) or almost constant (2%) general health worry in the past week. Fewer participants (6% of all subjects) rated the worry as “severe” or “very severe.“ A prior diagnosis of lymphoma (odds ratio [OR] = 2.72; 95% CI, 1.18 to 6.29; P = .02) and older age (OR = 1.06; 95% CI, 1.02 to 1.10; P = .002) increased the odds of general health worry.
Participants were questioned regarding concern and worry specifically in response to the treatment summary and care plan and its individual components. The document as a whole caused concern for 14% of participants; 86% reported that they were not concerned by the overall form. The “Screening Recommendations” section was troubling for some participants; 14% reported that this section caused concern. The “Potential Late Effects” section caused concern for 28% of participants, although moderate or extreme worry about potential late effects was reported by only 13%.
Participants were asked whether receiving the treatment summary and care plan increased the severity of health worry. Few reported moderate or extreme worry about “cancer” (4%), “coping with survivorship” (3%), or “sexual and/or reproductive issues” (6%). The seven participants who reported sexual or reproductive worry were overwhelmingly male (6 of 7 [86%], P = .02) and significantly younger compared with the other 104 participants (median age, 24 to 32 years, P = .02).
Overall, 31% of participants were found to be concerned in response to the treatment summary and care plan or its individual sections. Neither these participants nor those who reported general health worry in the past week differed significantly from others in terms of retention, understanding, value, or dissemination of the document (data not shown).
Additions to the Treatment Summary and Care Plan
A large majority (95%) of participants expressed interest in an online or wallet-card version of the treatment summary and care plan. When asked about the amount of information on the document, 80% indicated that the form provided sufficient content, 20% indicated “too little,” and none indicated “too much.” Participants requested the following additions: (1) current medications and allergies, (2) all current health care providers with contact information, (3) all surgeries (cancer related and noncancer related), (4) current late effects status, (5) tests with dates and results, (6) mental health recommendations and resources, (7) all dosages of cancer therapy.
Bivariate analysis (Table 2) found that participants who sought more information on the treatment summary were significantly older (median age in years, 40 v 29; OR per year increase in age = 1.06; 95% CI, 1.02 to 1.10; P = .005), had more years of survivorship (median time since diagnosis in years, 24 v 16; OR per year increase in time since diagnosis = 1.06; 95% CI, 1.01 to 1.11; P = .01), and were more likely to have reported not fully understanding the treatment summary (OR 19.6, 95% CI: 2.06, 185.4; P = .01), compared with participants who were satisfied with the amount of information. After adjusting for age, sex, and time since diagnosis, not understanding the treatment summary increased the odds of desiring more information on the document (OR = 43.4, 95% CI, 3.43 to 548.1; P = .004) (Table 2).
Table 2.
Odds of Seeking More Information on the Summary of Cancer Treatment and Follow-Up Care Plan Among Survivors of Pediatric and Young Adult Cancer (N = 111)
| Characteristic | OR | 95% CI | P | Multivariate-Adjusted OR* | 95% CI | P |
|---|---|---|---|---|---|---|
| Age, years | 1.06 | 1.02 to 1.10 | .005 | 1.03 | 0.98 to 1.10 | .25 |
| Female sex | 1.75 | 0.65 to 4.70 | .27 | 1.35 | 0.47 to 3.85 | .58 |
| Time since diagnosis, years | 1.06 | 1.01 to 1.11 | .01 | 1.03 | 0.97 to 1.10 | .37 |
| Treatment modality | 1.28 | 0.81 to 2.05 | .29 | 1.43 | 0.84 to 2.45 | .19 |
| First visit to program | 0.29 | 0.06 to 1.32 | .11 | 0.21 | 0.04 to 1.07 | .06 |
| Reported concern on receipt of summary | 2.96 | 0.94 to 9.32 | .06 | 0.76 | 0.27 to 2.19 | .62 |
| Did not understand treatment summary | 19.6 | 2.06 to 185.4 | .01 | 43.4 | 3.43 to 548.1 | .004 |
Abbreviation: OR, odds ratio.
Adjusted for age, sex, and time since diagnosis.
Discussion
In this cross-sectional study of adult survivors of pediatric and young adult cancer, most participants retained, understood, and valued the one-page treatment summary and follow-up care plan. In addition, the majority reported that the treatment summary and care plan did not cause them undue concern or worry. Many participants shared the form within their personal circle and with outside providers, although overall dissemination was modest. Importantly, only 57% of participants had a primary care physician outside MSKCC, which may help to explain this finding. Our findings should lend confidence to providers creating treatment summaries and care plans for their patients.
Approximately one in five participants wanted more information on the document, with much of the desired content falling beyond the scope of the typical treatment summary and care plan. Not surprisingly, we found that older participants and those who did not understand the document were more likely to want more information. Providers could identify these survivors by eliciting a patient's level of comprehension during the visit, and could consider revising individual treatment summaries and care plans as feasible.
Importantly, we found that a small group of patients reported frequent or constant general health worry in the past week. This finding is consistent with prior work in cancer survivors.25–27 A small but significant minority of participants reported that the treatment summary and care plan itself triggered concern and worry. Of note, a recent study of Hodgkin's lymphoma survivors found that a mailed one-page survivorship care plan did not worsen tension and anxiety.24 Nonetheless, providers should be alert to the risk of health-related anxiety in this population and to the possible psychological effects of the follow-up care plan. Our program offers counseling and psychological support to every patient. Notably, worried participants in our study did not differ in their retention or dissemination of the treatment summary.
This study is the first, to our knowledge, to provide evidence that adult survivors of pediatric and young adult cancer retain, understand, and value the treatment summary and care plan in a real-world clinic setting. Only a small number of prior studies have examined patient response to treatment summaries and survivorship care plans. Two prior studies29,30 reported on an Internet-based tool to create survivorship care plans. Jacobs et al29 reported that fewer than half of survivors were satisfied with the level of information generated by the Internet tool; in contrast, 80% of participants in our study were satisfied with the level of information provided by the form. Three other studies,31–33 including a 2007 report on a series of focus groups offering perspectives on post-treatment cancer care, found enthusiastic interest among adult survivors for the prospective receipt of a survivorship care plan but did not distribute patient-specific survivorship care plans or assess their impact.
A recent Cochrane review34 examined the use of recordings or written summaries of medical consultations for patients with active cancer. Sixteen studies including 2,318 adult participants were included. Between 60% and 100% of participants across 12 studies reported that they read or listened to the summary at least once after the initial visit. Among the 10 studies that examined anxiety and depression, no study found a difference between participants who received the summary and those who did not.
Our study has a few limitations. Because of the clinic-based nature of our study population and the unfunded nature of this study, we limited eligibility to patients who were English speaking and did not have significant neurocognitive deficits. Including multiple survey languages or modalities could strengthen future studies of the treatment summary and care plan. Patients in this study were insured, educated, and observed as part of a survivorship program, which may limit generalizability. A more marginalized population may be in greater need of the treatment summary and care plan. In addition, our outcomes were self-reported and measured after receipt of the document. Because the study interview was conducted by a study staff member and not a clinician, and in light of the nature of our study population, we expected participants to answer reliably. Future studies could include outside confirmation of study end points and should consider before-and-after measures of concern and worry. Finally, as a result of the brief nature of our study, we could not examine health care practices or compliance with recommended screening in conjunction with feelings towards the document. Oeffinger et al24 recently reported that a one-page mailed survivorship care plan resulted in improved adherence with recommended screening among Hodgkin's lymphoma survivors; future studies of the clinical impact of the treatment summary and care plan in other groups of cancer survivors are warranted.
Importantly, the survey did permit participants to recommend improvements to the treatment summary template. In light of participant feedback received during the survey, the medical team updated the “Potential Late Effects” section of the treatment summary and care plan to include “psychosocial problems including anxiety and depression” and considered further revisions suggested by survey participants. As Edwards35 reported more than a decade ago, patients treated for cancer need different types of information at different times and for different purposes. Participants in our study also suggested providing up-to-date medical information on the back of the treatment summary and care plan or offering an online version of the form.
In conclusion, we found that a one-page treatment summary and care plan was accepted and retained by adult survivors of pediatric and young adult cancers. Furthermore, although some survivors expressed general health worry, the treatment summary and care plan did not result in undue worry or concern for most participants. Effectiveness of this document may be limited, however, as only half of participants identified a primary care provider and dissemination was modest. Future studies could assess health care practices in light of retention and understanding of the treatment summary.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Peter D. Spain, Kevin C. Oeffinger, Joanne Candela, Mary S. McCabe, Emily S. Tonorezos
Financial support: Kevin C. Oeffinger
Administrative support: Peter D. Spain, Kevin C. Oeffinger
Provision of study materials or patients: Peter D. Spain, Kevin C. Oeffinger, Mary S. McCabe
Collection and assembly of data: Peter D. Spain, Joanne Candela
Data analysis and interpretation: Peter D. Spain, Mary S. McCabe, Xiaomei Ma, Emily S. Tonorezos
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.National Cancer Institute. SEER Cancer Statistics Review, 1975-2003. 2006. http://seer.cancer.gov/csr/1975_2003/
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 4.Yeh JM, Nekhlyudov L, Goldie SJ, et al. A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med. 2010;152:409–417. W131–W138. doi: 10.1059/0003-4819-152-7-201004060-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–179. doi: 10.1001/jama.2010.923. [DOI] [PubMed] [Google Scholar]
- 7.Skinner R, Wallace WH, Levitt GA. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7:489–498. doi: 10.1016/S1470-2045(06)70724-0. [DOI] [PubMed] [Google Scholar]
- 8.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2363–2373. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewitt M, Weiner SL, Simone JV, editors. Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 10.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: The Children's Oncology Group long-term follow-up guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 11.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs LA, Palmer SC, Schwartz LA, et al. Adult cancer survivorship: Evolution, research, and planning care. CA Cancer J Clin. 2009;59:391–410. doi: 10.3322/caac.20040. [DOI] [PubMed] [Google Scholar]
- 13.Stevens MM. “Shuttle sheet”: A patient-held medical record for pediatric oncology families. Med Pediatr Oncol. 1992;20:330–335. doi: 10.1002/mpo.2950200412. [DOI] [PubMed] [Google Scholar]
- 14.Scottish Intercollegiate Guidelines Network. Long-Term Follow-Up of Survivors of Childhood Cancer: A National Clinical Guideline. http://www.sign.ac.uk/pdf/sign76.pdf.
- 15.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 16.American Society of Clinical Oncology. ASCO Cancer Treatment Summaries. http://www.cancer.net/patient/Survivorship/ASCO+Cancer+Treatment+Summaries.
- 17.Kremer L, Jaspers M, van Leeuwen F, et al. Landelijke richtlijnen voor follow-up van overlevenden van kinderkanker. Tijdschrift voor Kindergeneeskund. 2006;74:247–250. [Google Scholar]
- 18.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. http://www.survivorshipguidelines.org.
- 19.Hill-Kayser CE, Vachani C, Hampshire MK, et al. An internet tool for creation of cancer survivorship care plans for survivors and health care providers: Design, implementation, use and user satisfaction. J Med Internet Res. 2009;11:e39. doi: 10.2196/jmir.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewitt ME, Bamundo A, Day R, et al. Perspectives on post-treatment cancer care: Qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25:2270–2273. doi: 10.1200/JCO.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- 21.Cancer NCCf: Guidance on Cancer Services. London, England: National Institute for Health and Clinical Excellence; 2005. Improving Outcomes in Children and Young People With Cancer. [Google Scholar]
- 22.Blaauwbroek R, Tuinier W, Meyboom-de Jong B, et al. Shared care by paediatric oncologists and family doctors for long-term follow-up of adult childhood cancer survivors: A pilot study. Lancet Oncol. 2008;9:232–238. doi: 10.1016/S1470-2045(08)70034-2. [DOI] [PubMed] [Google Scholar]
- 23.Blaauwbroek R, Zwart N, Bouma M, et al. The willingness of general practitioners to be involved in the follow-up of adult survivors of childhood cancer. J Cancer Surviv. 2007;1:292–297. doi: 10.1007/s11764-007-0032-z. [DOI] [PubMed] [Google Scholar]
- 24.Oeffinger KC, Hudson MM, Mertens AC, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2011;56:818–824. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobbie WL, Stuber M, Meeske K, et al. Symptoms of posttraumatic stress in young adult survivors of childhood cancer. J Clin Oncol. 2000;18:4060–4066. doi: 10.1200/JCO.2000.18.24.4060. [DOI] [PubMed] [Google Scholar]
- 26.Odo R, Potter C. Understanding the needs of young adult cancer survivors: A clinical perspective. Oncology (Williston Park) 2009;23(suppl 11):7–33. [PubMed] [Google Scholar]
- 27.Finnegan L, Campbell RT, Ferrans CE, et al. Symptom cluster experience profiles in adult survivors of childhood cancers. J Pain Symptom Manage. 2009;38:258–269. doi: 10.1016/j.jpainsymman.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: An instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs LA, Palmer SC, Schwartz LA, et al. Adult cancer survivorship: Evolution, research, and planning care. CA Cancer J Clin. 2009;59:391–410. doi: 10.3322/caac.20040. [DOI] [PubMed] [Google Scholar]
- 30.Hill-Kayser CE, Vachani C, Hampshire MK, et al. An internet tool for creation of cancer survivorship care plans for survivors and health care providers: Design, implementation, use and user satisfaction. J Med Internet Res. 2009;11:e39. doi: 10.2196/jmir.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewitt ME, Bamundo A, Day R, et al. Perspectives on post-treatment cancer care: Qualitative research with survivors, nurses, and physicians. J Clin Oncol. 2007;25:2270–2273. doi: 10.1200/JCO.2006.10.0826. [DOI] [PubMed] [Google Scholar]
- 32.Burg MA, Lopez EDS, Dailey A, et al. The potential of survivorship care plans in primary care follow-up of minority breast cancer patients. J Gen Int Med. 2009;24(suppl 2):S467–S471. doi: 10.1007/s11606-009-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan ME, Butow P, Marven M, et al. Survivorship care after breast cancer treatment - Experiences and preferences of Australian women. Breast. doi: 10.1016/j.breast.2010.12.006. [epub ahead of print, January 11, 2011] [DOI] [PubMed] [Google Scholar]
- 34.Pitkethly M, Macgillivray S, Ryan R. Recordings or summaries of consultations for people with cancer. Cochrane Database Syst Rev. 2008;3:CD001539. doi: 10.1002/14651858.CD001539.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Edwards D. Head and neck cancer services: Views of patients, their families and professionals. Br J Oral Maxillofac Surg. 1998;36:99–102. doi: 10.1016/s0266-4356(98)90175-9. [DOI] [PubMed] [Google Scholar]