Transitioning routine follow-up to primary care could potentially increase guideline adherence by improving access to and continuity of care.
Abstract
Purpose:
The purpose of this study was to examine colorectal cancer (CRC) follow-up care in Nova Scotia, Canada. More specifically, the objectives were to describe adherence to two elements of follow-up guidelines (colonoscopies and physician visits) and to identify factors associated with receiving at least guideline-recommended care.
Methods:
All patients with stage II or III CRC undergoing curative-intent surgery in Nova Scotia, Canada, were identified through the provincial cancer registry and anonymously linked to additional administrative health databases. For a 3-year follow-up period, beginning 1 year after the diagnosis date, descriptive statistics were calculated for physician visits and colonoscopies. Factors associated with receiving at least guideline-recommended care were identified using logistic regression.
Results:
Most patients received follow-up care from multiple physician specialties. In year 3, 58.1% of patients received oncologist follow-up care. Guideline adherence for colonoscopies was 52.4%, whereas guideline adherence for physician visits decreased from 41.9% to 25.4%. Receipt of at least guideline-recommended care was inversely associated with age and comorbidity for colonoscopy and inversely associated with age for physician visits.
Conclusion:
Receipt of follow-up care from oncologists and primary care physicians, prolonged oncologist care, and receipt of care inconsistent with guideline recommendations suggest there may be potential issues with inefficient use of cancer system resources and integration of guidelines into follow-up care practices in Nova Scotia. Transitioning routine follow-up to primary care could potentially increase guideline adherence by improving access to and continuity of care. CRC may be well suited to targeted knowledge translation strategies to improve guideline adherence.
Introduction
Follow-up for patients after definitive, and potentially curative, treatment for cancer has become an established component of medical care.1 The Institute of Medicine in the United States has identified four essential areas of survivorship—or follow-up—care: one, prevention of new cancers and recurrences; two, surveillance for recurrences, metastases, and new cancers; three, prevention and treatment of morbidity (eg, late effects of cancer and/or its treatment); and four, addressing all other health needs (eg, health promotion, psychosocial support).2 In colorectal cancer (CRC), the most important phase of follow-up is arguably the first 2 to 3 years after surgical resection, because up to 80% of recurrences occur in the first 3 years.3 Given the tendency of colon and rectal cancer to metastasize to the liver and lung,4–6 a main goal of follow-up care is to identify local recurrences, distant metastases, or new primary cancers at a point when subsequent curative resection is possible.7–10
Randomized controlled trials of CRC follow-up have highlighted considerable variation in the follow-up strategies that are employed.11–14 Although specific recommendations for follow-up care vary, clinical practice guidelines typically recommend a combination of physician visits, carcinoembryonic antigen (CEA) testing, colonoscopies, and imaging investigations.15–23 Several North American studies have found follow-up care practices that deviate from guideline recommendations, with patients receiving both insufficient and excessive follow-up care.24–27 Such studies have noted variations in follow-up care, highlighting issues related to the quality of care being delivered, and inappropriate use of health system resources.
Internationally, the prevalence of CRC survivors continues to grow as a result of high incidence coupled with declining mortality.27–30 A higher number of survivors requiring follow-up care places increased demands on the cancer care system.31 These increased demands, in combination with evidence that follow-up provided by the patient's primary care physician (PCP) is a safe and effective alternative to oncologist-led follow-up,32–34 has led some cancer centers to transfer routine follow-up to PCPs.35–38 Thus, examining follow-up care practices is important to ensure that resources are being optimized to provide the highest quality of care possible. However, relatively little is known about population-based follow-up care practices for CRC, particularly in Canada. This study examined CRC follow-up care in Nova Scotia, Canada. The objectives were to describe physician use during the follow-up period, examine adherence to two elements of follow-up guidelines (colonoscopies and physician visits), and identify factors associated with receiving at least guideline-recommended care.
Methods
Cohort
All patients who received a CRC diagnosis in Nova Scotia between January 1, 2001, and December 31, 2005, were identified through the Nova Scotia Cancer Registry (NSCR) and staged using the TNM-based Collaborative Stage Data Collection System.39 To allow for a sufficient period of follow-up, patients with stage II or III CRC who underwent curative-intent surgery on or before April 1, 2004, were selected for inclusion in the current study. Exclusion criteria included: death within 1 year of diagnosis; evidence of distant metastases (ie, to liver or lung), new primary cancer, or disease recurrence within 1 year of diagnosis; previous primary cancer diagnosis; and receipt of treatment or follow-up care outside of Nova Scotia. Patients were censored on death, loss of Nova Scotia Medicare eligibility (ie, the publicly-funded health insurance program available to all Nova Scotia residents; this would primarily be the result of emigration), or enrollment in a palliative care program. Consistent with methods employed by Grunfeld et al,40 patients were also censored 90 days before evidence of a new primary cancer or CRC recurrence to ensure patients included in the final cohort were receiving routine well follow-up care.
Data Sources and Measures
From the NSCR, patient demographics, disease history/recurrence, cancer center visits, and treatment data were obtained. Using encrypted anonymized health card numbers, the identified records were linked to several administrative health databases, including the provincial physician billings database (Nova Scotia Medicare), the Canadian Institute for Health Information (CIHI) Discharge Abstracts Database (DAD; contains hospital admissions data), and palliative care program databases (available for two of nine Nova Scotia health districts). The NSCR records were also linked to Canadian Census data using the Postal Code Conversional File (PCCF+ Version 4J; Statistics Canada, Ottawa, Ontario, Canada).
Colonoscopy receipt was identified in the physician billings database and the CIHI DAD (all endoscopic procedures including colonoscopy and sigmoidoscopy). Physician visit data were obtained from the physician billings database and examined by physician type (PCPs, medical oncologists, radiation oncologists, surgeons, gastroenterologists [GIs], and other [ie, all other physician types combined]) and number of visits per year. Both general practitioners and community medicine practitioners, as identified in the database, were considered PCPs. Primary care visits were further separated into CRC related or other based on diagnostic codes. Multiple same-day physician billings by the same physician were counted as one visit. Emergency department and in-patient visits were excluded, because they would be unlikely to represent routine follow-up care.
Social and material deprivation indices were used as measures of socioeconomic status and calculated for each dissemination area (smallest geographic unit defined by Statistics Canada)41 using patient postal codes at time of diagnosis, contained within NSCR records. Postal codes were also used to define patient residence as rural or urban, using classifications developed by Statistics Canada.42 Porter et al43 provide detailed descriptions of these measures. Comorbidity was quantified using a comorbidity score (or count) based on the list of comorbid conditions developed by Elixhauser et al44 (excluding all cancer-related comorbidities) and using International Classification of Diseases, Ninth Revision, Clinical Modification, and 10th Revision coding45 (potential score, 0 to 28). Continuity of care was measured by the usual provider continuity (UPC) index.46 The index is calculated by dividing the number of visits to the physician seen most often by the total number of visits (perfect continuity, UPC = 1; low continuity, UPC ≤ 0.75). For PCPs, the index includes visits within 2 years before CRC diagnosis. For oncologists (medical, radiation, and surgical), the index includes visits during the follow-up period. An index value was not calculated for patients with fewer than three visits for the given period, and emergency department and in-patient visits were excluded. Physician specialty information was obtained from the physicians billing database.
Analysis
The follow-up period was defined as the 3-year period beginning 1 year after the diagnosis date (as determined from NSCR) and ending 4 years after diagnosis. Consistent with previous studies examining cancer follow-up practices using administrative data,40,47,48 the follow-up period commenced 1 year postdiagnosis to exclude tests and visits associated with postoperative complications, routine postoperative visits, and adjuvant therapy (ie, chemotherapy and/or radiotherapy). On the basis of discussions with oncology practitioners, guidelines developed by the American Society of Clinical Oncology16 were identified as those guiding local practice and thus used to evaluate guideline adherence in this study. Two elements of guideline adherence were examined: colonoscopies and physician visits. Because of data limitations, guideline adherence for imaging and CEA testing could not be investigated. For the current study, guideline adherence was defined as follows:
Colonoscopy.
One to two visits over the entire 3-year follow-up period.
Physician visits.
Two to four visits per year of the 3-year follow-up period (including all visits to medical and radiation oncologists, surgeons, and gastroenterologists and all CRC-related visits to PCPs).
Guideline adherence was defined as one to two colonoscopies over the 3-year period, because: one, administrative data do not provide information on indications for colonoscopy, and interval colonoscopies may be legitimately performed for some patients; and two, the follow-up period commenced 12 months after diagnosis, and therefore, some patients may have received two surveillance colonoscopies over the study period (1 to 4 years postdiagnosis).
Descriptive statistics were calculated for colonoscopies and physician visits for each of 3 years of follow-up care for the entire population. A generalized linear model was used to compare the mean number of physician visits across years. Guideline adherence was classified as below recommended (colonoscopies, none; physician visits, < two per year), recommended (colonoscopies, one to two; physician visits, two to four per year), or above recommended (colonoscopies, > two; physician visits, > four per year). Logistic regression was used to identify factors independently associated with receipt of at least guideline-recommended care (ie, recommended plus above recommended care). Covariates for univariate regression analysis included age, sex, social and material deprivation, rural or urban residence, comorbidity, cancer stage and location, and continuity of care. Covariates with P < .1 were retained for multivariate analysis. For all analyses, P < .05 was considered statistically significant. Statistical analysis was performed using SAS version 8.2 (SAS Institute, Cary, NC).
Results
Cohort Characteristics
The final study cohort consisted of 731 patients. Cohort characteristics are listed in Table 1.
Table 1.
Cohort Demographics and Clinical Characteristics
| Characteristic | No. | % |
|---|---|---|
| Total | 731 | 100 |
| Age at diagnosis | ||
| Mean | 68.6 | |
| SD | 12.3 | |
| Age group, years | ||
| < 50 | 47 | 6.4 |
| 50-64 | 219 | 30.0 |
| 65-74 | 202 | 27.6 |
| ≥ 75 | 263 | 36.0 |
| Sex | ||
| Female | 359 | 49.1 |
| Male | 372 | 50.9 |
| Collaborative stage at diagnosis | ||
| II | 411 | 56.2 |
| III | 320 | 43.8 |
| Tumor site | ||
| Colon | 510 | 69.8 |
| Rectum | 221 | 30.2 |
| Residential location | ||
| Urban | 450 | 61.6 |
| Rural | 275 | 37.6 |
| Social deprivation index, quintiles | ||
| Q0* | 125 | 17.1 |
| Q1 | 164 | 22.4 |
| Q2 | 145 | 19.8 |
| Q3 | 160 | 21.9 |
| Q4 | 134 | 18.3 |
| Material deprivation index, quintiles | ||
| Q0 | 157 | 21.5 |
| Q1 | 153 | 20.9 |
| Q2 | 166 | 22.7 |
| Q3 | 134 | 18.3 |
| Q4 | 118 | 16.1 |
| No. of comorbidities | ||
| 0 | 436 | 59.6 |
| 1 | 149 | 20.4 |
| ≥ 2 | 146 | 20.0 |
| PCP continuity of care | ||
| Index | ||
| Mean | 0.84 | |
| SD | 0.18 | |
| High UPC† | 493 | 67.4 |
| Low UPC‡ | 177 | 24.2 |
| Not evaluated | 61 | 8.3 |
| Oncologist continuity of care | ||
| Index | ||
| Mean | 0.76 | |
| SD | 0.22 | |
| High UPC | 194 | 26.5 |
| Low UPC | 202 | 27.6 |
| Not evaluated | 335 | 45.8 |
Abbreviations: PCP, primary care physician; SD, standard deviation; UPC, usual provider continuity index.
Q0 represents the most deprived quintile; Q4 represents the least deprived.
High UPC = 1.
Low UPC ≤ 0.75.
Physician Visits
Table 2 lists the mean number of patient visits to each physician specialty for each year of follow-up. When all patients in the cohort were considered, the mean number of patient visits was greatest for PCPs for each year of follow-up, although fewer than one visit per year was specifically identified as CRC related. The greatest number of CRC-related visits were made to surgeons; however, this number decreased significantly over the 3-year follow-up period (from 1.36 to 0.81 visits; P < .001). In the third year of follow-up, 58.1% of patients continued to receive oncology follow-up (at least one annual visit), compared with 30.0% of patients who visited a PCP for CRC-related care. Importantly, Table 2 demonstrates that most patients visited multiple types of oncologists and PCPs for follow-up care.
Table 2.
Mean No. of Visits to Each Physician Type per Patient per Follow-Up Year
| Physician Specialty | Follow-Up Year 1* (n = 731) |
Follow-Up Year 2 (n = 570) |
Follow-Up Year 3 (n = 477) |
P‖ | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All† |
≥ One Visit‡ |
All |
≥ One Visit |
All |
≥ One Visit |
|||||||||||
| Mean | SE | %§ | Mean | SE | Mean | SE | % | Mean | SE | Mean | SE | % | Mean | SE | ||
| Primary Care (any) | 6.84 | 0.21 | 93.7 | 7.29 | 0.22 | 6.72 | 0.24 | 93.7 | 7.17 | 0.24 | 7.15 | 0.28 | 92.9 | 7.70 | 0.28 | .1314 |
| PCP (CRC related) | 0.69 | 0.05 | 34.2 | 2.02 | 0.10 | 0.63 | 0.05 | 34.7 | 1.82 | 0.11 | 0.53 | 0.05 | 30.0 | 1.76 | 0.12 | .0253 |
| PCP (other) | 6.15 | 0.20 | 91.0 | 6.76 | 0.21 | 6.08 | 0.23 | 91.6 | 6.64 | 0.23 | 6.62 | 0.27 | 90.8 | 7.30 | 0.27 | .0350 |
| Oncology (any) | 2.05 | 0.13 | 70.9 | 2.90 | 0.17 | 1.51 | 0.11 | 62.5 | 2.42 | 0.16 | 1.39 | 0.12 | 58.1 | 2.39 | 0.18 | < .001 |
| Medical oncology | 0.65 | 0.12 | 30.5 | 2.14 | 0.36 | 0.55 | 0.10 | 25.3 | 2.17 | 0.34 | 0.55 | 0.11 | 23.9 | 2.31 | 0.40 | .0617 |
| Radiation oncology | 0.04 | 0.01 | 3.1 | 1.26 | 0.14 | 0.02 | 0.01 | 1.8 | 1.00 | 0.00 | 0.02 | 0.01 | 2.1 | 1.10 | 0.10 | .0562 |
| Surgeon | 1.36 | 0.06 | 60.5 | 2.25 | 0.07 | 0.95 | 0.05 | 50.5 | 1.88 | 0.07 | 0.81 | 0.05 | 47.0 | 1.73 | 0.07 | < .001 |
| Gastroenterology | 0.01 | 0.00 | 1.1 | 1.00 | 0.00 | 0.02 | 0.01 | 1.2 | 1.43 | 0.20 | 0.02 | 0.02 | 1.0 | 2.20 | 1.20 | .6165 |
| Other¶ | 1.82 | 0.14 | 57.5 | 3.17 | 0.22 | 1.74 | 0.12 | 55.6 | 3.14 | 0.19 | 1.68 | 0.13 | 56.0 | 3.00 | 0.21 | .5182 |
Abbreviations: CRC, colorectal cancer; PCP, primary care physician.
First 12 months from diagnosis are treatment period; year 1 of follow-up is 13 to 24 months after diagnosis.
Denominator used for this mean: all uncensored patients in this year.
Denominator used for this mean: all uncensored patients with at least one visit to a given physician type in this year.
Percentage of uncensored cohort with at least one visit to a given physician type.
P values correspond to the difference across years for the mean number of visits for all patients (ie, including patients who had no physician visits).
Other category includes all physician types except PCPs, medical oncologists, radiation oncologists, general surgeons, and gastroenterologists.
Appendix Table A1 (online only) lists the percentage of patients who visited each physician type (or combination of types). Overall, the majority of patients (67.3% in year 1, 59.5% in year 2, and 54.5% in year 3) received follow-up care from a combination of PCPs and oncologists. When specific physician types or combinations of types were considered, most patients visited either a PCP only or a PCP in conjunction with a surgeon. The percentage of patients who received annual visits from both a PCP and surgeon decreased from 36.5% in year 1 to 31.2% by year 3. Meanwhile, the percentage of patients receiving follow-up from a PCP only increased from 25.3% in year 1 to 37.3% in year 3.
Receipt of Guideline-Recommended Care
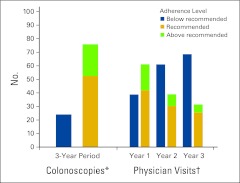
As shown in Appendix Figure A1 (online only), follow-up care practices in Nova Scotia deviated from accepted guidelines. More than half of patients (52.4%) received the recommended number of colonoscopies, with nearly an equal percentage of patients receiving above and below guideline recommendations (23.5% and 24.1%, respectively). The percentage of patients who met the guideline for recommended number of physician visits decreased annually from 41.9% in year 1 to 25.4% in year 3. The percentage of patients who received fewer than the recommended number of physician visits increased from 38.9% in year 1 to 68.5% in year 3.
Receipt of at least guideline-recommended colonoscopy (ie, recommended plus above recommended) was independently associated with age and comorbidity (Table 3). Patients older than age 75 years were less likely to receive a colonoscopy than all other age groups, and those with two comorbidities were less likely to receive a colonoscopy than those with none. Patients older than age 75 years were also less likely to receive at least two visits per year during the follow-up period (Table 3).
Table 3.
Factors Affecting Receipt of at Least Guideline-Recommended Care*
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Colonoscopy | ||||||
| Age group, years | ||||||
| ≥ 75 | 1.00 | — | — | 1.00 | — | — |
| 65-74 | 2.87 | 1.88 to 4.37 | < .001 | 2.7 | 1.80 to 4.20 | < .001 |
| 50-64 | 6.89 | 4.13 to 11.49 | < .001 | 5.6 | 3.30 to 9.50 | < .001 |
| < 50 | 10.71 | 3.24 to 35.38 | < .001 | 9.0 | 2.70 to 29.90 | < .001 |
| Sex | ||||||
| Male | 1.00 | — | — | 1.00 | — | — |
| Female | 0.75 | 0.53 to 1.06 | .0984 | 0.90 | 0.60 to 1.40 | .7153 |
| No. of comorbidities | ||||||
| 0 | 1.00 | — | — | 1.00 | — | — |
| 1 | 0.66 | 0.43 to 1.04 | .0710 | 0.90 | 0.60 to 1.50 | .8205 |
| ≥ 2 | 0.36 | 0.24 to 0.54 | < .001 | 0.60 | 0.40 to 0.90 | .020 |
| Stage | ||||||
| II | 1.00 | — | — | 1.00 | — | — |
| III | 1.50 | 1.06 to 2.13 | .0234 | 1.30 | 0.90 to 1.90 | .1635 |
| Location | ||||||
| Rectum | 1.00 | — | — | 1.00 | — | — |
| Colon | 0.58 | 0.9 to 0.87 | .0079 | 0.70 | 0.50 to 1.20 | .1935 |
| Physician visits† | ||||||
| Age group, years | ||||||
| ≥ 75 | 1.00 | — | — | 1.00 | — | — |
| 65-74 | 1.75 | 1.08 to 2.83 | .0223 | 1.70 | 1.10 to 2.80 | .0311 |
| 50-64 | 2.10 | 1.32 to 3.33 | .0016 | 2.30 | 1.40 to 3.70 | < .001 |
| < 50 | 2.09 | 0.10 to 4.40 | .0509 | 2.20 | 1.00 to 4.70 | .0512 |
| Social deprivation index‡ | ||||||
| Q0 | 1.00 | — | — | 1.00 | — | — |
| Q1 | 1.02 | 0.55 to 1.90 | .9512 | 0.90 | 0.50 to 1.70 | .7660 |
| Q2 | 1.70 | 0.93 to 3.09 | .0838 | 1.60 | 0.80 to 2.90 | .1555 |
| Q3 | 1.65 | 0.92 to 2.98 | .0958 | 1.40 | 0.80 to 2.60 | .2510 |
| Q4 | 0.97 | 0.51 to 1.87 | .9343 | 0.90 | 0.50 to 1.80 | .7731 |
| Material deprivation index‡ | ||||||
| Q0 | 1.00 | — | — | 1.00 | — | — |
| Q1 | 0.95 | 0.54 to 1.67 | .8601 | 0.90 | 0.50 to 1.70 | .7982 |
| Q2 | 1.42 | 0.84 to 2.40 | .1892 | 1.50 | 0.8 to 2.50 | .1736 |
| Q3 | 1.28 | 0.73 to 2.23 | .3938 | 1.20 | 0.70 to 2.10 | .5540 |
| Q4 | 0.50 | 0.25 to 1.01 | .0537 | 0.50 | 0.30 to 1.10 | .0962 |
| Continuity of care: PCP | ||||||
| 0 | 1.00 | — | — | 1.00 | — | — |
| 1 | 0.70 | 0.39 to 1.27 | .2442 | 0.80 | 0.40 to 1.40 | .4110 |
| 2 | 1.39 | 0.82 to 2.35 | .2235 | 1.60 | 0.90 to 2.70 | .1172 |
| 3 | 0.34 | 0.08 to 1.50 | .1547 | 0.40 | 0.10 to 1.90 | .2737 |
| 4 | 1.23 | 0.77 to 1.95 | .3853 | 1.30 | 0.80 to 2.20 | .2576 |
Abbreviations: CRC, colorectal cancer; OR, odds ratio; PCP, primary care physician.
Recommended plus above recommended.
Includes all oncologist, surgeon, and gastroenterologist visits, along with primary care visits with CRC-related diagnosis codes.
Deprivation index values were divided in quintiles such that Q0 = most deprived and Q4 = least deprived.
Discussion
This study provides important population-based data on the provision of follow-up care in Nova Scotia. With respect to which physicians patients are seeing for their routine follow-up care, we found that a large percentage of CRC patients received oncologist follow-up care within the first 4 years after diagnosis. Prolonged oncologist follow-up care may represent inefficient use of specialist cancer resources, particularly because the growing survivor population is not necessarily being met with an increased number of available cancer care providers. This may result in an increased workload for current physicians and potentially delay access to oncologists for patients with suspicious or confirmed cancer. Several trials have shown that PCPs can safely and effectively manage follow-up care for patients with breast cancer,32,33 and one trial demonstrated that PCP-led follow-up care led to similar patient quality of life and clinical outcomes in colon cancer.34 In Canada, most PCPs are willing to provide exclusive follow-up care.49 Nonetheless, reducing demand on specialist cancer resources is particularly challenging, given that oncologists develop relationships with their patients during the treatment period, which can make it difficult to transfer responsibility for care back to PCPs in the community.50 That the majority of patients visited both multiple types of oncologists and PCPs throughout the follow-up period was also observed in a cohort of breast cancer survivors in Ontario, Canada.40 Although visits to multiple physicians for cancer follow-up care are expected for some patients (eg, those who receive follow-up from a PCP may continue to require surgeon visits for periodic colonoscopies or postsurgical management), there is potential for overuse of health system resources (eg, duplication of tests/investigations). Together, these issues underscore the need for informed, multidisciplinary dialogue on how best to manage the growing cancer survivor population.
This study examined receipt of guideline-recommended care for two guideline elements: colonoscopies and physician visits. Together, these two elements represent important aspects of follow-up care. As reported in other North American studies, a considerable proportion of patients in the current study received care below guideline recommendations, whereas others received care in excess of recommendations.24–26 More than half of patients met guideline recommendations for colonoscopies, whereas approximately one quarter of patients received care that exceeded recommendations. These findings are consistent with those of Cooper et al,24 who reported that 73.6% of patients received at least one colonoscopy over 3 years of follow-up. Similarly, Cheung et al25 found that than 85.2% of patients received at least one colonoscopy in 5 years, although the use of a 5-year follow-up period may have captured an increased number of colonoscopies. However, not all patients can undergo colonoscopy after CRC surgery; therefore, they may undergo suboptimal investigations, such as barium enema (BE), accounting for a proportion of the below recommended care. For a subpopulation of the cohort for whom BE data were available (one health district; n = 344), we found little difference in results when the guideline adherence criterion was changed from receipt of colonoscopy to receipt of colonoscopy or BE (70% and 72.9%, respectively, received at least guideline-recommended care; data not shown).
Older age (> 75 years) and greater number of comorbidities were independently associated with not receiving a surveillance colonoscopy, which is also consistent with the literature.24,27 This may be related to difficulties encountered by older populations in navigating or accessing the health care system, or it may reflect physician or patient opinion that a colonoscopy is not worthwhile, because advanced age and/or comorbidities could substantially complicate (or preclude, in some circumstances) surgical resection or adjuvant treatment if a new primary CRC were detected. It is also possible that the competing health care needs of older patients with comorbid illnesses may in part explain the lower receipt of colonoscopy surveillance.
In the current study, the percentage of patients receiving guideline-recommended physician visits decreased each year. Over the 3-year follow-up period, the number of patients who had at least the guideline-recommended number of visits decreased by half (from 61.2% to 31.5%). These rates are substantially lower than those reported in the literature, with previous studies reporting 77% and 92.3% of patients with a minimum of two visits per year,24,25 whereas another study reported 77.6% of patients with two visits within 18 months.26 These differences across studies may result from differences in how physician visits were defined and/or in the guidelines against which adherence was assessed. Increasing age was independently related to fewer physician visits, similar to others' findings.24 As with colonoscopy, this may be related to issues in navigating and/or accessing the health care system, specifically in terms of the patients' ability to drive or commute to and from appointments.
There are several limitations specific to the administrative data used in this study. First, diagnosis codes related to PCP visits may not reliably measure the number of CRC-related PCP visits. We classified PCP visits as CRC related by including a broad range of diagnosis codes related to the prior CRC diagnosis, signs/symptoms suggestive of possible recurrence, and other survivorship issues/concerns (eg, fatigue). Still, the number of CRC-related PCP visits may have been underestimated, given that CRC-related discussions may have occurred during a visit for another medical concern without an associated billing. Second, because CIHI DAD codes do not differentiate between colon/rectum endoscopy procedures, our colonoscopy rates may have been overestimated, because they include sigmoidoscopies that were also performed in the time period. On examining data from the one database that separates colonoscopy and sigmoidoscopy (physician billings), we found that 32% of the procedures were sigmoidoscopies. A proportion of these would have represented routine surveillance (ie, guideline adherence) after colonic resection, because only a small quantity of bowel remained postsurgery (eg, subtotal colectomy). However, the remaining proportion may have been performed for non–routine surveillance purposes (eg, inspection of rectal anastomosis). Unfortunately, the databases preclude our ability to determine reasons for colonoscopy/sigmoidoscopy. Another issue with the grouping of procedures in the CIHI DAD coding system is that it prevented the examination of the American Society of Clinical Oncology 200516 protosigmoidoscopy recommendation for patients with rectal cancer. Third, we were unable to examine all elements of the guidelines. For example, we could not examine CEA blood tests, because patient-level laboratory data were unavailable for the study period. In addition, imaging data were not available for the entire province, and therefore, adherence to imaging recommendations was not examined in this study.
In conclusion, our findings highlight potential issues with inefficient use of cancer system resources and integration of clinical practice guidelines into survivorship care practices in Nova Scotia. Although practice guidelines are meant to guide the delivery of optimal care and are open to clinical judgment in the context of individual patients,51 when a considerable proportion of patients receive care inconsistent with guidelines, both access to and quality of care are questioned; for example, some patients may not be accessing or receiving care adequate to monitor potential recurrences or to manage important survivorship issues (eg, psychosocial concerns, late effects of treatment). Transitioning routine follow-up care to PCPs could reduce burden on the cancer care system and potentially increase guideline adherence by improving access to and continuity of care. Further study is warranted to better understand the reasons for low guideline adherence to develop targeted knowledge translation strategies to improve adherence.52
Acknowledgment
Written on behalf of Team ACCESS (Access to Colorectal Cancer Services in Nova Scotia) investigators. Supported by Canadian Institutes of Health Research, Cancer Care Nova Scotia, Nova Scotia Department of Health and Wellness, Capital Health, Dalhousie Faculty of Medicine, and Dalhousie Medical Research Foundation.
Appendix
Table A1.
Percentage of Unique Patients Who Saw Each Physician Type or Combination of Physician Types for CRC Follow-Up Care
| Physician Specialty* | Year 1 (n = 731)† | Year 2 (n = 570) | Year 3 (n = 477) |
|---|---|---|---|
| PCP only | 25.3 | 33.0 | 37.3 |
| Oncology only | |||
| Medical | 0.1 | 0.2 | 0.4 |
| Radiation | 0.0 | 0.2 | 0.0 |
| Surgical | 1.9 | 1.1 | 1.3 |
| Multiple | 0.8 | 0.4 | 1.0 |
| Total | 2.8 | 1.9 | 2.7 |
| PCP and oncology | |||
| PCP and medical | 8.8 | 10.9 | 9.9 |
| PCP and radiation | 0.8 | 0.2 | 0.4 |
| PCP and surgical | 36.5 | 34.2 | 31.2 |
| PCP and multiple | 21.2 | 14.2 | 13.0 |
| Total | 67.3 | 59.5 | 54.5 |
| Gastroenterology | |||
| GI only | 0.0 | 0.0 | 0.0 |
| PCP and GI only | 0.4 | 0.0 | 0.2 |
| PCP, GI, and any oncologist | 0.7 | 1.2 | 0.8 |
| Total | 1.1 | 1.2 | 1.0 |
| Other physicians only‡ | 0.1 | 0.5 | 0.4 |
| No physician | 3.3 | 4.0 | 4.0 |
Abbreviations: CRC, colorectal cancer; GI, gastroenterologist; PCP, primary care physician.
Each patient is represented in only one category.
First 12 months from diagnosis are treatment period; year 1 of follow-up is 13 to 24 months after diagnosis.
Other category includes all physician types except PCPs, medical oncologists, radiation oncologists, general surgeons, and GIs.
Figure A1.
Rates of adherence. (*) Guideline recommends only one colonoscopy within 3 years after surgery. (†) Includes all oncologist, surgeon, and gastroenterologist visits, plus primary care physician visits with colorectal cancer–related diagnosis.
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Robin Urquhart, Amy Folkes, Geoffrey Porter, Ron Dewar, Eva Grunfeld
Collection and assembly of data: Robin Urquhart, Amy Folkes, Martha Cox, Eva Grunfeld
Data analysis and interpretation: Robin Urquhart, Geoffrey Porter, Cynthia Kendell, Martha Cox, Ron Dewar, Eva Grunfeld
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Edelman MJ, Meyers FJ, Siegel D. The utility of follow-up testing after curative cancer therapy: A critical review and economic analysis. J Gen Intern Med. 1997;12:318–331. doi: 10.1046/j.1525-1497.1997.012005318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hewitt M, Greenfield S, Stovall E, editors. Washington, DC: National Academies Press; 2005. From Cancer Patient to Survivor: Lost in Transition. [Google Scholar]
- 3.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664–8670. doi: 10.1200/JCO.2005.01.6071. [DOI] [PubMed] [Google Scholar]
- 4.Galandiuk S, Wieand HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32. [PubMed] [Google Scholar]
- 5.Kjeldsen BJ, Kronborg O, Fenger C, et al. The pattern of recurrent colorectal cancer in a prospective randomized study and the characteristics of diagnostic tests. Int J Colorectal Dis. 1997;12:329–334. doi: 10.1007/s003840050118. [DOI] [PubMed] [Google Scholar]
- 6.Tepper JE, O'Connell M, Niedzwiecki D, et al. Adjuvant therapy in rectal cancer: Analysis of stage, sex, and local control—Final report of Intergroup 0114. J Clin Oncol. 2002;20:1744–1750. doi: 10.1200/JCO.2002.07.132. [DOI] [PubMed] [Google Scholar]
- 7.Viganò L, Ferrero A, Lo Tesoriere R, et al. Liver surgery for colorectal metastases: Results after 10 years of follow-up—Long-term survivors, late recurrences, and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg RM, Fleming TR, Tangen CM, et al. Surgery for recurrent colon cancer: Strategies for identifying resectable recurrence and success rates after resection—Eastern Cooperative Oncology Group, the North Central Cancer Treatment Group, and the Southwest Oncology Group. Ann Intern Med. 1998;129:27–35. doi: 10.7326/0003-4819-129-1-199807010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 10.McAfee MK, Allen MS, Trastek VF, et al. Colorectal lung metastases: Results of surgical excision. Ann Thorac Surg. 1992;57:780–786. doi: 10.1016/0003-4975(92)91435-c. [DOI] [PubMed] [Google Scholar]
- 11.Pietra N, Sarli L, Costi R, et al. Role of follow-up in management of local recurrences of colorectal cancer: A prospective, randomized study. Dis Colon Rectum. 1998;41:1127–1133. doi: 10.1007/BF02239434. [DOI] [PubMed] [Google Scholar]
- 12.Kjeldsen BJ, Kronborg O, Fenger C, et al. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666–669. [PubMed] [Google Scholar]
- 13.Makela JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer: Results of a prospective randomized trial. Arch Surg. 1995;130:1062–1067. doi: 10.1001/archsurg.1995.01430100040009. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Moranta F, Saló J, Arcusa A, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: A prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386–393. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 15.Desch CE, Benson AB, 3rd, Smith TJ, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999;17:1312–1321. doi: 10.1200/JCO.1999.17.4.1312. [DOI] [PubMed] [Google Scholar]
- 16.Desch CE, Benson AB, 3rd, Somerfield MR, et al. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology Practice Guideline. J Clin Oncol. 2005;23:8512–8519. doi: 10.1200/JCO.2005.04.0063. [DOI] [PubMed] [Google Scholar]
- 17.Fleischer DE, Goldberg SB, Browning TH, et al. Detection and surveillance of colorectal cancer. JAMA. 1989;261:580–585. [PubMed] [Google Scholar]
- 18.Benson AB, 3rd, Desch CE, Flynn PJ, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000;18:3586–3588. doi: 10.1200/JCO.2000.18.20.3586. [DOI] [PubMed] [Google Scholar]
- 19.Figueredo A, Rumble RB, Maroun J, et al. Follow-up of patients with curatively resected colorectal cancer: A practice guideline. BMC Cancer. 2003;3:26. doi: 10.1186/1471-2407-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthony T, Simmang C, Hyman N, et al. Practice parameters for the surveillance and follow-up of patients with colon and rectal cancer. Dis Colon Rectum. 2004;47:807–817. doi: 10.1007/s10350-004-0519-x. [DOI] [PubMed] [Google Scholar]
- 21.Engstrom PF, Arnoletti JP, Benson AB, et al. NCCN clinical practice guidelines in oncology. www.nccn.org/professionals/physician_gls/PDF/colon.pdf.
- 22.Engstrom PF, Arnoletti JP, Benson AB, et al. NCCN clinical practice guidelines in oncology. www.nccn.org/professionals/physician_gls/PDF/rectum.pdf.
- 23.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Cooper GS, Kou TD, Reynolds HL., Jr Receipt of guideline-recommended follow-up in older colorectal cancer survivors: A population-based analysis. Cancer. 2008;113:2029–2037. doi: 10.1002/cncr.23823. [DOI] [PubMed] [Google Scholar]
- 25.Cheung WY, Pond GR, Rother M, et al. Adherence to surveillance guidelines after curative resection for stage II/III colorectal cancer. Clin Colorectal Cancer. 2008;7:191–196. doi: 10.3816/CCC.2008.n.025. [DOI] [PubMed] [Google Scholar]
- 26.Lafata JE, Simpkins J, Schultz L, et al. Routine surveillance care after cancer treatment with curative intent. Med Care. 2005;43:592–599. doi: 10.1097/01.mlr.0000163656.62562.c4. [DOI] [PubMed] [Google Scholar]
- 27.Salz T, Weinberger M, Ayanian JZ, et al. Variation in the use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canadian Cancer Statistics 2010. Toronto, Ontario, Canada: Canadian Cancer Society; 2010. [Google Scholar]
- 29.Berrino F, De Angelis R, Sant M, et al. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: Results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773–783. doi: 10.1016/S1470-2045(07)70245-0. [DOI] [PubMed] [Google Scholar]
- 30.Verdecchia A, Francisci S, Brenner H, et al. Recent cancer survival in Europe: A 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 31.Erikson C, Salsberg E, Forte G, et al. Future supply and demand for oncologists: Challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grunfeld E, Levine MN, Julian JA, et al. Randomized trial of long-term follow-up for early stage breast cancer: A comparison of family physician versus specialist care. J Clin Oncol. 2006;24:848–855. doi: 10.1200/JCO.2005.03.2235. [DOI] [PubMed] [Google Scholar]
- 33.Grunfeld E, Mant D, Yudkin P, et al. Routine follow-up of breast cancer in primary care: A randomised trial. BMJ. 1996;313:665–669. doi: 10.1136/bmj.313.7058.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wattchow DA, Weller DP, Esterman A, et al. General practice vs surgical-based follow-up for patients with colon cancer: Randomised controlled trial. Br J Cancer. 2006;94:1116–1121. doi: 10.1038/sj.bjc.6603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies NJ, Bateup L. London, United Kingdom: Macmillan Cancer Support; 2011. Cancer Follow-Up: Towards a Personalised Approach to Aftercare Services—A Review of Current Practice and Selected Initiatives. [Google Scholar]
- 36.Grunfeld E. Looking beyond survival: How are we looking at survivorship? J Clin Oncol. 2006;24:5166–5169. doi: 10.1200/JCO.2006.06.5953. [DOI] [PubMed] [Google Scholar]
- 37.National Health Service. Leicester, United Kingdom: NHS Improvement; 2009. Rapid Review of Current Service Provision Following Cancer Treatment. [Google Scholar]
- 38.Ristovski-Slijepcevic S. Vancouver, British Columbia, Canada: British Columbia Cancer Agency; 2008. Environmental Scan of Cancer Survivorship in Canada: Conceptualization, Practice and Research. [Google Scholar]
- 39.Collaborative Staging Task Force of the American Joint Committee on Cancer. Bethesda, MD: National Cancer Institute; 2004. Collaborative Staging Manual and Coding Instructions, Version 01.04.00. NIH publication 04-5496. [Google Scholar]
- 40.Grunfeld E, Hodgson DC, Del Giudice, et al. Population-based longitudinal study of follow-up care for breast cancer survivors. J Oncol Pract. 2010;6:174–181. doi: 10.1200/JOP.200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pampalon R, Raymond G. A deprivation index for health and welfare planning in Quebec. Chronic Dis Can. 2000;21:104–113. [PubMed] [Google Scholar]
- 42.McNiven C, Puderer H, Janes D. Ottawa, Ontario, Canada: Statistics Canada; 2000. Census Metropolitan Area and Census Agglomeration Influenced Zones (MIZ): A Description of the Methodology. [Google Scholar]
- 43.Porter G, Urquhart R, Bu J, et al. A team approach to improving colorectal cancer services using administrative health data. Health Res Policy Syst. 2012;10:4. doi: 10.1186/1478-4505-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 46.Ionescu-Ittu R, McCusker J, Ciampi A, et al. Continuity of primary care and emergency department utilization among elderly people. CMAJ. 2007;177:1362–1368. doi: 10.1503/cmaj.061615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder CF, Frick KD, Kantsiper ME, et al. Prevention, screening, and surveillance care for breast cancer survivors compared with controls: Changes from 1998 to 2002. J Clin Oncol. 2009;27:1054–1061. doi: 10.1200/JCO.2008.18.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snyder CF, Earle CC, Herbert RJ, et al. Trends in follow-up and preventive care for colorectal cancer survivors. J Gen Intern Med. 2008;23:254–259. doi: 10.1007/s11606-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Del Guidice ME, Grunfeld E, Harvey BJ, et al. Primary care physicians' views of routine follow-up care cancer survivors. J Clin Oncol. 2009;27:3338–3345. doi: 10.1200/JCO.2008.20.4883. [DOI] [PubMed] [Google Scholar]
- 50.Audisio RA, Robertson C. Colorectal cancer follow-up: Perspectives for future studies. Euro J Surg Oncol. 2000;26:329–337. doi: 10.1053/ejso.1999.0894. [DOI] [PubMed] [Google Scholar]
- 51.O'Malley AS, Clancy C, Thompson J, et al. Clinical practice guidelines and performance indicators as related—but often misunderstood—tools. Jt Comm J Qual Saf. 2004;30:163–171. doi: 10.1016/s1549-3741(04)30018-3. [DOI] [PubMed] [Google Scholar]
- 52.Gagliardi AR, Brouwers MC, Palda VA, et al. How can we improve guideline use? A conceptual framework of implementability. Implement Sci. 2011;6:26. doi: 10.1186/1748-5908-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]