A number of factors influence adherence to adjuvant chemotherapy clinical practice guidelines for colorectal cancer and should be incorporated in future work as novel regimens enter clinical practice.
Abstract
Purpose:
Clinical practice guidelines (CPGs) recommend adjuvant chemotherapy after curative-intent surgery for colorectal cancer (CRC). Studies have shown variable rates of adherence to adjuvant therapy CPGs. This study sought to determine the proportion of patients in Nova Scotia receiving CPG-concordant adjuvant chemotherapy within 12 weeks of surgery for CRC in 2001 to 2005, and to identify factors associated with chemotherapy receipt beyond 12 weeks from surgery or chemotherapy nonreceipt.
Methods:
Patients with stages IIB or III colon or stages II or III rectal cancer who underwent curative-intent surgery in Nova Scotia were identified through the provincial cancer registry and anonymously linked to 14 administrative health databases. Chart review was conducted to obtain chemotherapy data and reasons for chemotherapy nonreceipt. Logistic regression was used to identify factors independently associated with receipt of chemotherapy and meeting the 12-week benchmark (P < .05).
Results:
A total of 1,151 patients were identified, of whom 59% received chemotherapy. Factors predicting chemotherapy receipt were male sex, age < 75 years, no hospital readmission within 30 days of surgery, stage III disease, no prior cancer diagnosis, and rectal cancer. Of the 679 patients who received chemotherapy, 479 (72%) met the 12-week benchmark, with male sex, urban residence, less social deprivation, colon cancer and increased length of hospital stay as significant factors. Of the 472 patients who did not receive chemotherapy, the most common reason for nonreceipt was no consultation with a medical oncologist (53%).
Conclusion:
A number of factors influence adherence to adjuvant chemotherapy CPGs for CRC and should be incorporated in future work as novel regimens enter clinical practice.
Introduction
An estimated 163,000 cases of colorectal cancer (CRC) are expected to be diagnosed in North America in 2011.1,2 CRC commonly presents as early-stage disease, with the majority of patients being eligible for curative-intent surgery.3,4 Despite surgery, the 5-year risk of systemic recurrence in the absence of any further therapy is approximately 50% for those with lymph node involvement and 20% to 30% if the lymph nodes are negative.5
Adjuvant chemotherapy results in improved disease-free and overall survival for node-positive (stage III) or high-risk, node-negative (stage IIB) colon cancer. Similar benefits are observed for adjuvant chemoradiotherapy for node-positive (stage III) or node-negative (stage II) rectal cancer. As a result, the 1990 National Institutes of Health (NIH) Consensus conference recommended adjuvant chemotherapy for CRC, which was widely adopted.6
Until 2006, standard adjuvant chemotherapy for colon cancer consisted of bolus fluorouracil (FU) and folinic acid delivered daily for 5 days, once monthly for 6 months. For rectal cancer, standard chemoradiotherapy consisted of two cycles of bolus FU before and after 5 to 6 weeks of pelvic radiation administered with concurrent infusional FU.7,8 Previous investigations have demonstrated varied rates of adherence to published adjuvant chemotherapy CPGs for CRC, although there are relatively few population-based studies.9,10
Our objective was to assess the rates of adherence to adjuvant chemotherapy CPGs for a population-based sample of individuals with CRC between 2001 and 2005 treated with curative intent in Nova Scotia. We assessed rates of chemotherapy receipt, meeting a 12-week benchmark timeline for treatment initiation from date of surgery, and factors associated with chemotherapy receipt beyond 12 weeks from surgery or nonreceipt.
Methods
The Nova Scotia Cancer Registry (NSCR) identified all individuals diagnosed with CRC in Nova Scotia between January 1, 2001 and December 31, 2005. All cases were staged using the Collaborative Stage Data Collection System11 and anonymously linked at the patient level to 14 administrative health databases, including hospital discharge and physician billing databases, thus providing comprehensive clinical-demographic, diagnostic, and health service use data for all patients.12
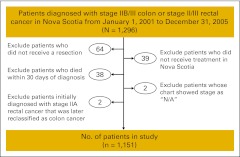
Within the time frame under study, 1,296 patients diagnosed with stages IIB/III colon or stages II/III rectal cancer were identified. Reasons for exclusion are presented in Appendix Figure A1 (online only); the final sample size was 1,151. Patient demographics and tumor characteristics were obtained through the NSCR. We calculated a comorbidity score by using International Classification of Diseases codes in the 2 years before diagnosis and computed length of stay and rates of hospital readmission within 30 days of surgical discharge via linkage to hospital discharge abstracts.13 We linked postal codes at diagnosis to 2001 census data by using the Postal Code Conversion File Plus file to determine rural or urban residence14,15 and socioeconomic status at the dissemination area level (the smallest geographic unit defined by Statistics Canada). Three indicators of material deprivation (proportion of persons with a high school diploma, employment:population ratio, average income) and three indicators of social deprivation (proportion of persons living alone; proportion of single-parent families; and proportion of separated, divorced, and widowed persons) were extracted from census data and used to generate social and material deprivation indices using the Quebec Model.16
Primary outcomes were the proportion of patients who received adjuvant chemotherapy and the proportion who met a 12-week benchmark from surgery date to start of chemotherapy. Chemotherapy data were captured from the NSCR, which includes the Oncology Patient Information System, and physicians' billings. Because of known limitations in chemotherapy data within administrative health databases,17–19 a focused chart review of all patients who received a medical oncology consultation but did not receive chemotherapy according to the linked administrative data was conducted; methods are described in detail elsewhere.20 The chart review also included categorized reasons (defined a priori) for nonreceipt of chemotherapy after medical oncology consultation. When multiple reasons were possible, the dominant one was adjudicated by two investigators (D.R., R.U.), with “patient decision” being assigned when there was no clear evidence of a dominant clinical or oncologic factor or when this factor was stated in the consultation notes.
Descriptive data were computed for all clinical-demographic characteristics and outcomes. Logistic regression was used to identify factors independently associated with receipt of adjuvant chemotherapy and meeting the 12-week benchmark. Covariates for univariate regression models were age, sex, social and material deprivation, residence, comorbidity, cancer stage and location, number of lymph nodes examined, surgical presentation (emergent or elective), hospital length of stay, and 30-day readmission rates. Age, sex, and covariates with P < .1 were retained for the multivariate regression models. All analyses were performed with SAS Version 8.2 (SAS Institute, Cary NC). Statistical significance was set at P < .05.
Ethical approval was obtained from the Capital District Health Authority's Research Ethics Board (CDHA-RS/2008-049).
Results
A total of 1,151 patients with stage IIB or III colon or stage II or III rectal cancer were identified as having undergone curative-intent surgery in the province of Nova Scotia between January 1, 2001 and December 31, 2005. Patient characteristics are presented in Table 1. Of these, 679 (59%) received adjuvant chemotherapy as per CPGs. Significant factors that predicted chemotherapy receipt in the multivariate analysis are presented in Table 2. More advanced disease stage, younger age, and male sex were significantly associated with receipt of adjuvant chemotherapy in both the colon and rectal subgroups.
Table 1.
Demographic and Clinical Characteristics of Study Cohort (N = 1,151)
| Characteristic | Colon (n = 657) |
Rectal (n = 494) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age at diagnosis, years | ||||
| < 50 | 40 | 6.1 | 41 | 8.3 |
| 50-64 | 168 | 25.6 | 170 | 34.4 |
| 65-74 | 183 | 27.9 | 130 | 26.3 |
| ≥ 75 | 266 | 40.5 | 153 | 31.0 |
| Mean | 69.6 | 67.0 | ||
| SD | 12.1 | 12.4 | ||
| Sex | ||||
| Female | 353 | 53.7 | 174 | 35.2 |
| Male | 304 | 46.3 | 320 | 64.8 |
| Collaborative stage at diagnosis | ||||
| IIA | 176 | 35.6 | ||
| IIB | 90 | 13.7 | 41 | 8.3 |
| IIIA | 47 | 7.2 | 37 | 7.5 |
| IIIB | 357 | 54.3 | 146 | 29.6 |
| IIIC | 163 | 24.8 | 94 | 19.0 |
| District health authority | ||||
| With tertiary care center | 359 | 54.6 | 279 | 56.5 |
| Without tertiary care center | 298 | 45.4 | 215 | 43.5 |
| Residential location | ||||
| Urban | 423 | 64.4 | 322 | 65.2 |
| Rural | 226 | 34.4 | 167 | 33.8 |
| Unknown | 8 | 1.2 | 5 | 1.0 |
| Social deprivation index, quintile | ||||
| 0 (most deprived) | 117 | 17.8 | 92 | 18.6 |
| 1 | 148 | 22.5 | 105 | 21.3 |
| 2 | 131 | 19.9 | 96 | 19.4 |
| 3 | 133 | 20.2 | 101 | 20.4 |
| 4 (least deprived) | 124 | 18.9 | 99 | 20.0 |
| Unknown | 4 | 0.6 | 1 | 0.2 |
| Material deprivation index, quintile | ||||
| 0 (most deprived) | 144 | 21.9 | 104 | 21.1 |
| 1 | 140 | 21.3 | 95 | 19.2 |
| 2 | 139 | 21.2 | 120 | 24.3 |
| 3 | 130 | 19.8 | 101 | 20.4 |
| 4 (least deprived) | 100 | 15.2 | 73 | 14.8 |
| Unknown | 4 | 0.6 | 1 | 0.2 |
| Elixhauser comorbidity score | ||||
| 0 | 359 | 54.6 | 351 | 71.1 |
| 1 | 145 | 22.1 | 74 | 15.0 |
| ≥ 2 | 153 | 23.3 | 69 | 14.0 |
| No. of previous primary cancers (any type) | ||||
| 0 | 546 | 83.1 | 457 | 92.5 |
| 1 | 95 | 14.5 | 34 | 6.9 |
| ≥ 2 | 16 | 2.4 | 3 | 0.6 |
| Surgical presentation | ||||
| Emergency | 192 | 29.2 | 29 | 5.9 |
| Elective | 459 | 69.9 | 460 | 93.1 |
| Unknown | 6 | 0.9 | 5 | 1.0 |
| No. of lymph nodes examined | ||||
| < 12 | 373 | 56.8 | 322 | 65.2 |
| ≥ 12 | 260 | 39.6 | 162 | 32.8 |
| Unknown | 24 | 3.7 | 10 | 2.0 |
| Length of stay for hospital admission, days | ||||
| Mean | 17.9 | 14.8 | ||
| SD | 20.7 | 14.5 | ||
| Readmission within 30 days | 63 | 9.6 | 72 | 14.6 |
| Chemotherapy use | ||||
| Receipt of medical oncology consultation | 494 | 75.2 | 398 | 80.6 |
| Receipt of chemotherapy | 363 | 55.3 | 316 | 64.0 |
| Meeting the 12-week benchmark (for those receiving chemotherapy) | 286 | 78.8 | 211 | 66.8 |
Abbreviation: SD, standard deviation.
Table 2.
Factors Associated With Chemotherapy Receipt*
| Variable | Effect | OR | 95% CI | P |
|---|---|---|---|---|
| Colon | ||||
| Age | (per year) | 0.9 | 0.8 to 0.9 | < .001 |
| Sex | Female v male | 0.7 | 0.4 to 1.1 | .0839 |
| Stage at diagnosis | III v II | 8.2 | 4.2 to 15.9 | < .001 |
| Hospital LOS | Quartile 4 (longest) v quartile 1 (shortest) | 0.3 | 0.1 to 0.6 | .0027 |
| Rectal | ||||
| Age | (per year) | 0.9 | 0.9 to 0.9 | < .001 |
| Sex | Female v male | 0.6 | 0.4 to 1.0 | .0347 |
| Stage at diagnosis | III v II | 2.5 | 1.6 to 4.1 | .001 |
| Previous cancer | 1 v none | 0.3 | 0.1 to 0.7 | .0095 |
| Colorectal | ||||
| Age | (per year) | 0.9 | 0.9 to 0.9 | < .001 |
| Sex | Female v male | 0.6 | 0.5 to 0.9 | .0064 |
| Site | Colon v rectum | 0.7 | 0.5 to 0.9 | .0220 |
| Stage at diagnosis | III v II | 4.0 | 2.7 to 5.9 | < .001 |
| No. of positive lymph nodes | ≥ 12 v < 12 | 0.8 | 0.6 to 1.1 | .1630 |
| Unknown v < 12 | 2.7 | 1.1 to 7.1 | .0388 | |
| Previous cancer | 1 v 0 | 0.6 | 0.4 to 1.0 | .0499 |
| Hospital LOS | Quartile 4 (longest) v quartile 1 (shortest) | 0.4 | 0.2 to 0.7 | .0022 |
| Readmission within 30 days | Yes v no | 0.5 | 0.3 to 0.8 | .0070 |
Abbreviations: LOS, length of stay; OR, odds ratio.
Significance set at P < .05.
Of the 679 patients who received chemotherapy, 497 (73.2%) met the 12-week timeline. Significant factors associated with receipt of chemotherapy within benchmark times are presented in Table 3. In total, 497 patients (43%) both received adjuvant chemotherapy and began it within the 12-week benchmark.
Table 3.
Factors Associated With Chemotherapy Receipt Within 12 Weeks of Curative-Intent Surgery*
| Variable | Effect | OR | 95% CI | P |
|---|---|---|---|---|
| Colon | — | |||
| Rectal | ||||
| Patient residence | Urban v rural | 3.9 | 2.1 to 7.2 | < .001 |
| Social deprivation | Quartile 4 (least deprived) v quartile 0 (most deprived) | 3.2 | 1.1 to 9.6 | .0368 |
| Colorectal | ||||
| Patient residence | Urban v rural | 2.2 | 1.4 to 3.4 | < .001 |
| Social deprivation | Quartile 4 (least deprived) v quartile 0 (most deprived) | 2.5 | 1.1 to 5.6 | .0279 |
| Site | Colon v rectum | 2.8 | 1.7 to 4.4 | < .001 |
| Hospital LOS | Quartile 2 v quartile 1 (shortest) | 2.0 | 1.1 to 3.7 | .0221 |
NOTE. Dash indicates no significant effect found.
Abbreviations: LOS, length of stay; OR, odds ratio.
Significance set at P < .05.
Four hundred seventy-two patients (41%) in the cohort did not receive chemotherapy. The primary reason for not receiving chemotherapy was lack of consultation with a medical oncologist (250 patients; 52.8%). A number of factors were significantly different between the group who received a medical oncology consult (n = 892) and those who did not (n = 259). These differences (Student t test) included age > 75 years (P < .001), female sex (P < .001), stage II disease (P < .001), colon cancer (P = .03), greater material deprivation (P = .012), two or more comorbidities (P < .001), prior history of cancer (P = .0014), greater postoperative hospital length of stay (P < .001), and emergency presentation at diagnosis (P < .001).
For those with stage IIB disease, 42.7% of those with fewer than 12 nodes examined received adjuvant chemotherapy compared with 22% of those with 12 or more nodes examined. For all other disease stages, rates of chemotherapy receipt were similar when stratified by fewer than 12 versus 12 or more nodes examined.
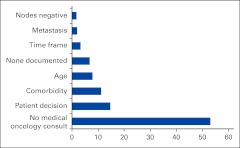
For the 222 patients who did not receive chemotherapy but did have a consultation with a medical oncologist, patient decision was the most common reason for nonreceipt, followed by comorbidity and advanced age (Appendix Figure A2, online only). There were 69 patients for whom patient decision was adjudicated as the primary reason for nonreceipt. Those who declined chemotherapy were significantly older (P < .001) and were more likely to be female (P < .001), have two or more comorbidities, and have had a greater postsurgical hospital length of stay (both P < .001).
Discussion
The adoption of adjuvant chemotherapy has increased significantly since the NIH Consensus conference recommendations were published and has led to demonstrable improvements in survival outcomes.21–23 Despite these observations, a significant minority of patients who would otherwise be candidates for adjuvant chemotherapy do not receive it, with advanced age, nonwhite ethnicity, and female sex being factors associated with nonreceipt.21,24
The proportion of patients who received adjuvant chemotherapy in our cohort was 59%, which is consistent with reports from other Canadian jurisdictions over a similar time period.25–27 Younger age and stage III disease were independently associated with chemotherapy receipt, also consistent with observations from Canada and elsewhere.25–27 Interestingly, advanced age, despite being a commonly observed factor for nonreceipt of chemotherapy, has not been associated with lack of a survival advantage nor independently associated with increases in chemotherapy-induced toxicities, perhaps partly as a result of dose modification.28–31 Comorbid conditions or more significant social or material deprivation were not independently associated with chemotherapy nonreceipt, and only 8% of patients overall and 17% of those who received a medical oncology consultation did not receive chemotherapy, primarily as a result of advanced age.
Female sex was associated with reduced odds of chemotherapy receipt for both the rectal cancer and overall CRC cohorts. This finding was not observed in the British Columbia or combined western provincial data, although data from British Columbia suggested that men were more likely to receive palliative chemotherapy for metastatic disease compared with women.26
A greater postoperative hospital length of stay and a previously diagnosed cancer were associated with decreased odds of receiving adjuvant chemotherapy for the colon and rectal cancer cohorts, respectively. For the entire cohort, both of these factors, as well as 30-day hospital readmission, significantly decreased the odds of chemotherapy receipt. These findings suggest important nonmodifiable patient- and treatment-related factors affecting chemotherapy receipt that should be incorporated into future investigations of access and receipt of adjuvant therapies across all cancer types. To our knowledge, our data are the first to elucidate these specific factors in the CRC patient population.
Four hundred ninety-seven (73.2%) of the 679 patients who received adjuvant chemotherapy met the 12-week timeline. Previous investigation of elapsed times to start of adjuvant therapy for both breast and lung cancer have observed a number of patient, disease, and system factors influencing elapsed times.32–36 We did not observe any significant factors influencing this time for the colon cancer cohort. For the rectal cancer cohort, both rural residence and social deprivation were significantly associated with not meeting this benchmark. These two factors, along with greater postoperative hospital length of stay and rectal disease site, significantly affected the odds of beginning chemotherapy within 12 weeks within the entire cohort.
Adjuvant therapy for rectal cancer includes a course of radiation often “sandwiched ” between two cycles of chemotherapy before and after radiation. Whereas chemotherapy for colon cancer can often be delivered close to home, receipt of adjuvant radiation requires travel to centers with radiation treatment facilities, resulting in out-of-pocket costs as well as home and work schedule disruption. Patients with limited resources and those living in rural areas may be most vulnerable to these factors, and, if they are not able or choose not to receive adjuvant radiation therapy, may decide or be recommended to forego chemotherapy because of the uncertain benefit of adjuvant chemotherapy alone for rectal cancer. Our results suggest that optimization of adjuvant chemotherapy receipt for rectal cancer might be improved by minimization of logistic and supportive factors that potentially prevent receipt of radiation therapy.
Most adjuvant trials specify a time window of 8 weeks from the date of definitive surgery to the initiation of adjuvant chemotherapy as an inclusion criterion for trial participation.37–39 During the time frame under study, a 12-week benchmark was adopted by the Nova Scotia gastrointestinal disease site group for patients in routine clinical practice, consistent with recommendations from other Canadian cancer care jurisdictions.40 Data regarding the impact of adjuvant treatment delays on survival outcomes have only recently been published and suggest that shorter time frames are optimal.41,42 Adherence to more stringent timelines will likely result in a greater proportion of eligible patients falling outside of recommendations in the absence of significant expansions in cancer care system capacities and greater attention to patient-related factors that preclude timely start of treatment.
Of the 472 patients (41%) in our cohort who did not receive adjuvant chemotherapy, the most common reason for nonreceipt was lack of a medical oncology consultation (250 patients; 52.8%). Adjuvant chemotherapy is restricted to being prescribed by a medical oncologist, although administration and follow-up of patients could be supervised at community hospitals province-wide under the care of family physicians or general internists. Referral to a medical oncologist has been identified as a crucial step in the receipt of adjuvant chemotherapy.43–44 Preselection of patients referred to medical oncology on the basis of clinical and demographic factors may result in a patient population most likely to benefit from a discussion of adjuvant chemotherapy, although it is also a potential factor limiting access to adjuvant therapies for some who might otherwise benefit. We identified a number of patient and clinical factors that were significantly different between those who received a medical oncology consult and those who did not. Some of these such as age, sex, comorbidities, and lower disease stage have been previously reported,9,21,23,24 but to our knowledge, ours is the first study to report that greater material deprivation, a previous cancer, and emergency presentation at diagnosis as well as postoperative length of stay were also factors associated with receiving a medical oncology consultation.
High-volume surgical oncologists practicing within multidisciplinary settings have been observed to be more likely to collaborate on decisions regarding adjuvant therapy for CRC than low-volume surgical oncologists or general surgeons.45 In Nova Scotia, surgical oncology expertise over the study time frame was limited to the tertiary care center of Halifax, suggesting that expansion of surgical oncology services and multidisciplinary province-wide tumor boards might help to increase collaborative decision making and referrals to medical oncology.
Data on patient preference in regard to receipt of oncologic intervention are relatively scant.46 We observed 69 patients who received a medical oncology consultation but declined adjuvant chemotherapy. Compared with those who received adjuvant chemotherapy, this group was significantly older, and was more likely to have two or more comorbidities, have had a longer postoperative length of stay, and to be female. Apart from this last factor, patient preferences appeared to be influenced by parameters often incorporated in the oncologic assessment of all patients referred for consideration of adjuvant chemotherapy. We were unable to further define potential discordance between oncology opinion and patient preference, and this remains an area for considerable further research. We were also unable to ascertain possible reasons for women being more likely to decline chemotherapy.
This study was population-based, provincial-registry derived from and linked to 14 relevant health administration databases, allowing us to capture important clinical-demographic and health care use information. An additional strength is the chart review, linked at the patient level to administrative records that captured data for all patients who received a medical oncology consultation.
Our study, however, has a number of limitations inherent in research that relies heavily on secondary information sources. Although the supplemental chart review was performed to optimize chemotherapy ascertainment, an underestimation of chemotherapy receipt is still possible owing to the requirement of medical oncology consultation for the chart review. A small number of patients may have received adjuvant chemotherapy prescribed by a health care provider who did not bill for the service. It is also possible that administrative databases within smaller centers may not specifically capture chemotherapy administration. We were limited in our ability to ascertain critical components of the adequacy of chemotherapy receipt, such as duration, dose reductions, acute and long-term toxicities, and quality of life during treatment. In a small number of cases, we were unable to ascertain whether chemotherapy was administered with adjuvant or palliative intent.
Our results are likely generalizable to other jurisdictions with single-payer, universal health care coverage but may be less so for predominantly free-market medical systems such as that in the United States or those with mixed systems such as exist in most European jurisdictions. It should be noted that in settings where access to medical care is strongly influenced by private insurance coverage, some of the ecologic variables assessed in our population (eg, material and social deprivation indices) may play an even greater role in adherence to CPGs for adjuvant chemotherapy.
We undertook this study to better understand adherence to CPGs for adjuvant colorectal cancer chemotherapy in the province of Nova Scotia. Working through the provincial gastrointestinal disease site group and the cancer outcome research unit, both based in the province's main teaching hospital and linked to the provincial cancer agency, opportunities for ongoing quality assurance and improvement have been identified. In particular, future work delineating exact reasons for nonreferral to medical oncology as well as provincial efforts at ensuring that those from relatively disadvantaged health districts have the same access to tertiary care referrals and expertise as those from more advantaged areas has been identified as a priority for further programmatic and research intervention.
In conclusion, this population-based analysis of adherence to CPGs for adjuvant chemotherapy for patients with CRC identified a number of factors associated with both nonreceipt of chemotherapy and receipt of chemotherapy outside of timeline benchmarks from date of curative-intent surgery in the province of Nova Scotia. Novel adjuvant chemotherapy regimens of increasing complexity and different routes of administration are generally replacing FU-based regimens, and future studies of both receipt and timing of adjuvant chemotherapy for CRC will need to be adapted to better understand adherence to CPGs for these new therapies.38,39,47
Acknowledgment
The data used in this study were compiled as part of the Canadian Institutes of Health Research (CIHR) program Access to Quality Cancer Care: New Emerging Team (NET) CIHR/CCNS Team in Access to Colorectal Cancer Services in Nova Scotia.
Appendix
Figure A1.
Exclusion criteria diagram for study cohort.
Figure A2.
Reasons for nonreceipt of adjuvant chemotherapy (%).
Authors' Disclosures of Potential Conflicts of Interest
The author(s) indicated no potential conflicts of interest.
Author Contributions
Conception and design: Daniel Rayson, Robin Urquhart, Eva Grunfeld, Geoffrey Porter
Administrative support: Martha Cox, Eva Grunfeld, Geoffrey Porter
Collection and assembly of data: Daniel Rayson, Robin Urquhart, Eva Grunfeld
Data analysis and interpretation: Daniel Rayson, Robin Urquhart, Martha Cox, Geoffrey Porter
Manuscript writing: Daniel Rayson, Robin Urquhart, Martha Cox, Geoffrey Porter
Final approval of manuscript: All authors
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society. Toronto, Canada: Canadian Cancer Society; 2010. Canadian Cancer Statistics 2010. [Google Scholar]
- 3.Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 4.Saltz LB, Minsky B. Adjuvant therapy of cancers of the colon and rectum. Surg Clin North Am. 2002;82:1035–1058. doi: 10.1016/s0039-6109(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 5.Jonker DJ, Spithoff K, Maroun J. Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care. Adjuvant systemic chemotherapy for stage II and III colon cancer after complete resection: An updated practice guideline. Clin Oncol (R Coll Radiol) 2011;23:314–322. doi: 10.1016/j.clon.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 6.NIH Consensus Conference. Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1150. [PubMed] [Google Scholar]
- 7.O'Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 8.O'Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med. 1994;331:502–507. doi: 10.1056/NEJM199408253310803. [DOI] [PubMed] [Google Scholar]
- 9.Wirtzfeld DA, Mikula L, Gryfe R, et al. Concordance with clinical practice guidelines for adjuvant chemotherapy in patients with stage I-III colon cancer: Experience in 2 Canadian provinces. Can J Surg. 2009;52:92–97. [PMC free article] [PubMed] [Google Scholar]
- 10.Van Cutsem E, Costa F. Progress in the adjuvant treatment of colon cancer; has it influenced clinical practice? JAMA. 2005;294:2758–2760. doi: 10.1001/jama.294.21.2758. [DOI] [PubMed] [Google Scholar]
- 11.American Joint Committee on Cancer Collaborative Stage Work Group. Chicago, IL: American Joint Committee on Cancer; 2010. Collaborative Stage Data Collection System Coding Instructions, version 02.00.00 [Incorporates updates through January 1, 2010] [Google Scholar]
- 12.Urquhart R, Grunfeld E. Building tools to measure and improve access to and quality of colorectal cancer care in Nova Scotia. Can J Gastroenterol. 2010;24(suppl SA):91A. [Google Scholar]
- 13.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Statistics Canada. Ottawa, Canada: Statistics Canada; 2011. Postal code conversion file plus (PCCF+), version 4J, with postal codes through September 2006. [Google Scholar]
- 15.McNiven C, Puderer H, Janes D. Ottawa, Canada: Geography Division, Statistics Canada; 2000. Census Metropolitan Area and Census Agglomeration Influenced Zones (MIZ): A Description of the Methodology (Geography Working Paper Series No. 2000-2) [Google Scholar]
- 16.Pampalon R, Raymond G. A deprivation index for health and welfare planning in Quebec. Chronic Dis Can. 2000;21:104–113. [PubMed] [Google Scholar]
- 17.Canadian Partnership Against Cancer. Toronto, Canada: Canadian Partnership Against Cancer; 2009. The System Performance Initiative: A First Year Report. [Google Scholar]
- 18.Malin JL, Kahn KL, Adams J, et al. Validity of cancer registry data for measuring quality of breast cancer care. J Natl Cancer Inst. 2002;94:835–844. doi: 10.1093/jnci/94.11.835. [DOI] [PubMed] [Google Scholar]
- 19.Bickell NA, Chassin MR. Determining the quality of breast cancer care: Do tumor registries measure up? Ann Intern Med. 2000;132:705–710. doi: 10.7326/0003-4819-132-9-200005020-00004. [DOI] [PubMed] [Google Scholar]
- 20.Urquhart R, Rayson D, Porter GA, et al. Quantifying limitations in chemotherapy data in administrative health databases: Implications for measuring the quality of colorectal cancer care. Health Pol. 2011;7:32–40. [PMC free article] [PubMed] [Google Scholar]
- 21.Jessup JM, Stewart A, Greene FL, et al. Adjuvant chemotherapy for stage III colon cancer: Implications of race/ethnicity, age, and differentiation. JAMA. 2005;294:2703–2711. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 22.Mitry E, Bouvier AM, Esteve J, et al. Improvements in colorectal cancer survival: A population-based study. Eur J Cancer. 2005;41:2297–2303. doi: 10.1016/j.ejca.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Cronin DP, Harlan LC, Potosky AL, et al. Patterns of care for adjuvant therapy in a random population-based sample of patients diagnosed with colorectal cancer. Am J Gastroenterol. 2006;101:2308–2318. doi: 10.1111/j.1572-0241.2006.00775.x. [DOI] [PubMed] [Google Scholar]
- 24.Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 25.Lima IS, Yasui Y, Scarfe A, et al. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta, Canada. Cancer. 2011;117:3833–3840. doi: 10.1002/cncr.25954. [DOI] [PubMed] [Google Scholar]
- 26.Renouf D, Kennecke H, Gill S. Trends in chemotherapy utilization for colorectal cancer. Clin Colorectal Cancer. 2008;7:386–389. doi: 10.3816/CCC.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 27.Cree M, Tonita J, Turner D, et al. Comparison of treatment received versus long-standing guidelines for stage III colon and stage II/III rectal cancer patients diagnosed in Alberta, Saskatchewan, and Manitoba in 2004. Clin Colorectal Cancer. 2009;8:141–145. doi: 10.3816/CCC.2009.n.023. [DOI] [PubMed] [Google Scholar]
- 28.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman IH, Rapp T, Onukwugha E, et al. Effect of age on survival benefit of adjuvant chemotherapy in elderly patients with stage III colon cancer. J Am Geriatr Soc. 2009;57:1403–1410. doi: 10.1111/j.1532-5415.2009.02355.x. [DOI] [PubMed] [Google Scholar]
- 31.Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA. 2010;303:1037–1045. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rayson D, Saint-Jacques N, Younis T, et al. Comparison of elapsed times from breast cancer detection to first adjuvant therapy in Nova Scotia in 1999/2000 and 2003/2004. CMAJ. 2007;176:327–332. doi: 10.1503/cmaj.060825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saint-Jacques N, Rayson D, Al-Fayea T, et al. Waiting times in early-stage non-small cell lung cancer (NSCLC) J Thorac Oncol. 2008;3:865–870. doi: 10.1097/JTO.0b013e318180210c. [DOI] [PubMed] [Google Scholar]
- 34.Hershman D, Hall MJ, Wang X, et al. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581–2588. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed S, Ahmad I, Zhu T, et al. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: A population-based study. Dis Colon Rectum. 2010;53:1432–1438. doi: 10.1007/DCR.0b013e3181e78815. [DOI] [PubMed] [Google Scholar]
- 36.Bayraktar UD, Chen E, Bayraktar S, et al. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer. 2011;117:2364–2370. doi: 10.1002/cncr.25720. [DOI] [PubMed] [Google Scholar]
- 37.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon cancer. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 38.Andr é T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 39.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 40.British Columbia Cancer Agency. Gastrointestinal. www.bccancer.bc.ca/HPI/CancerManagementGuidelines/Gastrointestinal/default.htm.
- 41.Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010;46:1049–1055. doi: 10.1016/j.ejca.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 43.Davidoff AJ, Rapp T, Onukwugha E, et al. Trends in disparities in receipt of adjuvant therapy for elderly stage III colon cancer patients, the role of the medical oncologist evaluation. Med Care. 2009;47:1229–1236. doi: 10.1097/MLR.0b013e3181b58a85. [DOI] [PubMed] [Google Scholar]
- 44.Luo R, Giordano SH, Freeman JL, et al. Referral to medical oncology: A crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006;11:1025–1033. doi: 10.1634/theoncologist.11-9-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers SO, Jr, Ayanian JZ, Ko CY, et al. Surgeons' volume of colorectal cancer procedures and collaborative decision-making about adjuvant therapies. Ann Surg. 2009;250:895–900. doi: 10.1097/SLA.0b013e3181afe0c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkin EB, Kim SH, Casper ES, et al. Desire for information and involvement in treatment decisions: Elderly cancer patients' preferences and their physicians' perceptions. J Clin Oncol. 2007;25:5275–5280. doi: 10.1200/JCO.2007.11.1922. [DOI] [PubMed] [Google Scholar]
- 47.Abrams TA, Brightly R, Mao J, et al. Patterns of adjuvant chemotherapy use in a population-based cohort of patients with resected stage II or III colon cancer. J Clin Oncol. 2011;29:3255–3262. doi: 10.1200/JCO.2011.35.0058. [DOI] [PubMed] [Google Scholar]