Future research directed toward identifying best screening methods and HBV risk tools will be necessary to reduce the risk of reactivation of HBV infection after chemotherapy.
Abstract
Purpose:
Patients with hepatitis B virus (HBV) infection are at risk for reactivation after chemotherapy. Effective prophylaxis is available but depends on detection of prior infection. Previous studies have shown low screening rates, but no large-scale US studies have been conducted. We sought to determine predictors of screening and positive HBV test results in patients receiving chemotherapy.
Methods:
We conducted a retrospective cohort study of patients with newly diagnosed cancer who received chemotherapy between January 2004 and September 2007 at a comprehensive cancer center. We determined rates and predictors of screening for HBV infection with HB surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc) tests as well as the prevalence and predictors of positive results. We explored rates of acutely elevated liver function tests and liver decompensation after chemotherapy.
Results:
Of 10,729 new patients who received chemotherapy, 1,787 (16.7%) underwent HBsAg or anti-HBc screening. Less than 20% of patients with HBV risk factors were screened, even though their odds of HBV infection were increased four-fold compared with those without risk factors. The prevalence of chronic HBV infection was 1.5%. whereas 7.4% had positive anti-HBc only. The strongest predictors of HBV screening were having a history of HBV infection, hematologic malignancy, and rituximab treatment (P < .001). Asian ethnicity was not a significant predictor of screening, despite being a strong and highly significant predictor of positive test results (P < .001).
Conclusion:
HBV screening among patients with cancer is low, especially among those known to be at high risk for HBV infection. Future research directed toward identifying best screening methods and HBV risk tools will be necessary to reduce the risk of reactivation of HBV infection after chemotherapy.
Introduction
Patients with chronic hepatitis B virus (HBV) infection are at risk for reactivation after chemotherapy.1,2 Patients who have recovered from previous HBV infection and patients with occult chronic HBV infection are also at risk for reactivation.3 Reactivation may cause interruptions in chemotherapy and, in severe cases, lead to liver failure and death.4–6 Administration of oral anti-HBV medications before chemotherapy can reduce the risk of reactivation by more than 79% in patients with chronic HBV infection7; however, prophylaxis can only be initiated after HBV infection has been identified.
In the United States, the prevalence of chronic HBV infection as manifested by positive results on both hepatitis B surface antigen (HBsAg) and immunoglobulin G antibody to hepatitis B core antigen (anti-HBc) testing is less than 1% overall8 but may be as high as 3% to 9% among high-risk groups.8,9 The US prevalence of convalescent or occult chronic HBV infection as manifested by a negative HBsAg test result but a positive anti-HBc test result has been reported to be 5% to 8% overall10–12 and up to 15% to 46% in some high-risk groups.13,14
There is general agreement about the importance of HBV screening among patients with cancer; however, there are differing opinions about the best screening approach. The Centers for Disease Control and Prevention (CDC) has recommended that all patients be screened for HBV infection before administration of any immunosuppression,8 a recommendation endorsed by the Institute of Medicine.15 The National Comprehensive Cancer Network has recommended that patients undergoing intensive immunosuppressive therapies be screened for prior HBV infection.16 The American Association for the Study of Liver Diseases has recommended that all persons at high risk for HBV be screened for prior HBV infection before chemotherapy.17 And the American Society of Clinical Oncology (ASCO) has recommended that only certain patients—those at high risk for HBV infection or those who will be receiving highly immunosuppressive therapies such as stem-cell transplantation or rituximab—be screened for HBV infection before chemotherapy.18 Despite differences about which patients should be screened, all guidelines indicate that some form of systematic screening is needed to identify patients at risk for reactivation so that prophylaxis may be initiated. We hypothesized that patients with cancer with risk factors for HBV infection are not being systematically screened for HBV at the onset of chemotherapy. We tested our hypothesis by retrospectively studying determinants of HBV screening and test results in a cohort of patients with newly diagnosed cancer who received chemotherapy at The University of Texas MD Anderson Cancer Center (Houston, TX).
Methods
Patient Identification
In this retrospective cohort study, we used the MD Anderson Tumor Registry, Pharmacy, Laboratory Results, and Patient Account databases. This study was approved by our institutional review board, which waived informed consent requirement. We reviewed the tumor registry to identify a cohort of patients with newly diagnosed cancer registered between January 1, 2004, and September 30, 2007. We included patients age ≥ 18 years who had a new diagnosis of cancer and were anticipating first administration of chemotherapy at MD Anderson.
Demographics
Through the tumor registry, we obtained information on age, sex, race/ethnicity, and date of birth. We reported age as a continuous variable and ethnicity as white, black, Hispanic, Asian, or other. For patients with ethnicity classified as other, we used the patient birthplace to assist in classification of Asian race, a practice that has been used in other studies, especially in situations where multiple Asian groups have been studied.19,20 Second, we used Asian surnames to find Asian patients who might have been incorrectly classified as other. This method has been successfully used in previous studies on cancer control issues to identify Asian Americans in large administrative databases.19–21
Types of Cancer and Chemotherapy
Using the tumor registry, we classified malignancies as solid tumors or hematologic malignancies (acute leukemia, chronic leukemia, Hodgkin's lymphoma, non-Hodgkin's lymphoma, other hematologic system cancer, multiple myeloma, and leukemia–not otherwise specified). Liver and bile duct cancers were grouped together as primary liver cancers. In subgroup analyses, we excluded these patients because of the etiologic relationship between HBV infection and primary liver cancers. We classified solid tumors into three stage categories: local or locally advanced disease, distant metastases, and unstaged disease. Because stem-cell transplantation is not routinely captured in our patient account database, we did not include it in this analysis.
We classified chemotherapy agents according to the American Cancer Society classification22 (Appendix Table A1, online only). Using the pharmacy database, we included chemotherapy delivered by intravenous, intramuscular, subcutaneous, intraperitoneal, or intra-arterial routes but not by enteral tube or unknown routes. We excluded oral chemotherapy because we were not able to monitor adherence. We excluded patients who received investigational chemotherapy as part of a clinical trial because screening might be required by protocol and not represent usual physician practices.
We identified the first time that a patient received chemotherapy at our institution (first administration) and then searched for another administration at least 4 but fewer than 8 weeks after the first administration (second administration). Any chemotherapy drug administered within the first 7 days after first or second chemotherapy administration was included.
HBV Risk Factors and History
Through the medical informatics database, patients with at least one International Classification of Diseases, version 9 (ICD-9), diagnosis code related to nonspecific hepatitis, other liver conditions, hepatitis C virus (HCV), or human immunodeficiency virus infection anytime before HBV screening test or second chemotherapy were considered to have a risk factor for HBV infection. Patients who had an ICD-9 code for HBV entered before the first HBV screening test were considered to have a history of HBV infection. The ICD-9 codes are detailed in Table 1.
Table 1.
Characteristics of the Study Population by HBV Screening Status
| Characteristic | Total (N = 10,729) |
HBV Screening |
||||
|---|---|---|---|---|---|---|
| Yes (n = 1,787) |
No (n = 8,942) |
|||||
| No. | % | No. | % | No. | % | |
| Age, years | ||||||
| Mean | 54.9 | 51.5 | 55.5 | |||
| SD | 13.8 | |||||
| Sex | ||||||
| Male | 4,866 | 45.4 | 1,060 | 21.8 | 3,806 | 78.2 |
| Female | 5,863 | 55.6 | 727 | 12.4 | 5,136 | 87.6 |
| Ethnicity | ||||||
| White | 7,810 | 72.8 | 1,310 | 16.8 | 6,500 | 83.2 |
| Hispanic | 1,279 | 11.9 | 230 | 18.0 | 1,049 | 82.0 |
| Black | 1,138 | 10.6 | 139 | 12.2 | 999 | 87.8 |
| Asian | 266 | 2.5 | 45 | 16.9 | 221 | 83.1 |
| Other | 236 | 2.2 | 63 | 26.7 | 173 | 73.3 |
| US residence | 10,428 | 97.2 | 1,716 | 16.5 | 8,712 | 83.5 |
| History of HBV infection* | 95 | 0.9 | 65 | 68.4 | 30 | 31.6 |
| HBV risk factors† | 2,612 | 24.3 | 513 | 19.6 | 2,099 | 80.4 |
| Cancer type | ||||||
| Solid tumor | 9,009 | 84.0 | 555 | 6.2 | 8,454 | 93.8 |
| Hematologic malignancy | 1,720 | 16.0 | 1,232 | 71.6 | 488 | 28.4 |
| Chemotherapy type | ||||||
| Chemotherapy/nonimmunotherapy | 8,315 | 77.5 | 887 | 10.7 | 7,428 | 89.3 |
| Immunotherapy, excluding rituximab | 1,293 | 12.1 | 100 | 7.7 | 1,193 | 92.3 |
| Rituximab | 1,121 | 10.4 | 800 | 71.4 | 321 | 28.6 |
NOTE. HBV screening refers to either HBsAg or anti-HBc screening test ordered; all comparisons between screened and unscreened patients are statistically significant using χ2 test (P < .001).
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; ICD-9, International Classification of Diseases, version 9; SD, standard deviation.
Patients who had an ICD-9 code for hepatitis B (070.2, 070.3, 070.20, 070.21, 070.22, 070.23, 070.310, 070.31, 070.32, 070.33, or v02.61) entered before HBV screening were considered to have a history of HBV infection.
Patients with at least one of the following ICD-9 diagnoses codes entered into the database anytime before HBV screening were considered to have a risk factor for HBV infection: (A) hepatitis, not specific (codes 070, 070.4, 070.49, 070.5, 070.59, 070.6, 070.9, 571.4, 571.40, 571.41, 571.42, 571.49, 573.1, 573.2, 573.3, v02.6, v02.60, and v02.69); (B) other liver conditions (codes 571, 571.0, 571.0, 571.2, 571.3, 571.5, 571.6, 571.8, 571.9, 572, 572.0, 572.8, 573, 573.8, 573.9, 782.4, 789.1, and 794.8); (C) hepatitis C (codes 070.41, 070.44, 070.51, 070.54, 070.7, 070.70, 070.71, and v02.62); and (D) human immunodeficiency disease (codes 042, 042.0, 042.1, 042.2, 043, 043.0, 043.1, 043.2, 043.3, 044.0, 044.9, 079.53, 795.71, 795.8, v08, and v65.44).
Outcome Measures
We searched the laboratory results database for evidence of HBsAg or anti-HBc testing (Ortho-Clinical Diagnostics, Rochester, NY). To comprehensively capture screening efforts, we defined screening as an HBsAg or anti-HBc test ordered in the period from 2 months before the first chemotherapy administration to receipt of the second administration. We defined a positive HBV test result as a positive result on HBsAg or anti-HBc testing or both tests. We did not include antibody to hepatitis B surface antigen (anti-HBs) because it was not routinely tested during the study period. We considered patients with positive HBsAg and anti-HBc to have chronic HBV infection. At the physician's discretion, HBV DNA testing was performed in patients who tested positive for HBsAg or anti-HBc. At our institution, although there is no official policy on HBV screening, several clinics order screening tests in the routine care of patients with hematologic malignancies; however, physicians must order these tests themselves.
Not all patients with HBV infection had HBV DNA level at baseline or during chemotherapy available, and thus we were not able to fully characterize reactivation. Instead, we described clinical outcomes of acute abnormalities of liver tests and liver decompensation, although these outcomes do not substitute for reactivation. Patients with ALT ≥ 100 IU/L and total bilirubin ≥ 2.5 mg/dL anytime after chemotherapy (until death or end of data period [April 2011]) were categorized as having abnormalities of liver tests. Patients with ALT ≥ 100 IU/L plus international normalized ratio ≥ 1.5, ascites, or encephalopathy were categorized as having liver decompensation.
Statistical Analyses
Primary outcomes were prevalence of HBV screening and positive HBV test results during the screening period. We used χ2 tests to examine characteristics of patients who were screened compared with those who were not. We used SAS software, version 9.13 (SAS Institute, Cary, NC), for statistical analyses. An exploratory two-stage method was used to identify factors predictive of outcomes. First, through univariate analysis, we assessed the associations of these outcomes with age, sex, ethnicity, US residence, risk factors for HBV infection, history of HBV infection, cancer type, and chemotherapy type to identify clinically relevant predictors. Second, potential predictors with P ≤ .20 were entered into multivariable logistic regression models to ascertain their independent predictive ability. We created two different multivariable logistic regression models to determine factors related to HBV screening and HBV test results (positive results for both HBsAg and anti-HBc testing v negative results on both tests). Because of the small number of patients without a history of HBV infection who had positive HBsAg and anti-HBc test results, we ran the last model without the variable HBV history. Final models were identified using a stepwise method, which ensured the independent predictability of included model variables. The Hosmer and Lemeshow goodness-of-fit tests were used to evaluate the fit of the logistic regression models to our data. We examined rate of abnormalities of liver tests and liver decompensation among patients with HBV infection.
Results
Patient Characteristics
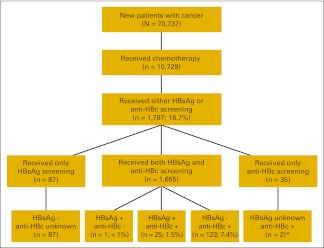
Of 70,737 patients with newly diagnosed cancer seen at MD Anderson during the study period, 10,729 (15.2%) received at least two administrations of chemotherapy according to our criteria (Table 1). Most patients had solid tumors, and of these, 56.3% had local or locally advanced disease, 34.1% had distant metastases, and 9.6% had unstaged disease; 65 patients had primary liver cancers. Among the patients with hematologic malignancies, most had lymphoma (62.4%), leukemia (25.4%), or multiple myeloma (6.4%). Over 52% of the patients with hematologic malignancies received rituximab. Most patients were from the United States, and approximately half (n = 4,637; 44.5%) were from Houston, Texas. In our cohort, 21.5% of the patients (n = 2,308) were tested for anti-HCV, and 4.5% (n = 105) had a positive result. Of these, none had a positive HBsAg test, but 26 patients (24.8%) had an isolated positive anti-HBc test. In the group who tested negative for anti-HCV (n = 2,203), 24 patients (1%) had a positive HBsAg test, and 92 patients (4.2%) had an isolated positive anti-HBc test.
Predictors of HBV Screening
Among 10,729 new patients who received chemotherapy, 1,787 (16.7%) were screened for HBV infection before chemotherapy. We compared characteristics of these patients with those of patients who were not screened (n = 8,942).
We found a similar ethnic distribution in the screened and unscreened populations. The proportion of patients with an HBV risk factor was higher in the screened population (513 of 1,787; 28.7%) than in the unscreened population (2,099 of 8,942; 23.5%; P < .001). The screened population also had significantly higher proportions of patients with hematologic malignancies (1,232 of 1,787; 68.9% v 288 of 8,942; 5.5%) and patients treated with rituximab-containing regimens (800 of 1,787; 44.8% v 321 of 8,942; 3.6%).
Among Asian patients, 16.9% underwent HBV screening, and among patients with risk factors, 19.6% were screened (Table 1). The prevalence of screening was 5.9% (530 of 8,944) among patients with solid tumors excluding primary liver cancers, 38.5% (25 of 65) among patients with primary liver cancers, and 71.6% (1,232 of 1,720) among patients with hematologic malignancies.
On univariate analysis, the following factors were associated with screening: age, sex, ethnicity, US residence, history of HBV infection, HBV risk factors, cancer type, and chemotherapy type. In the multivariable logistic regression model of screening (Table 2), younger age, male sex, history of HBV infection, and HBV risk factors predicted HBV testing. The odds of undergoing HBV screening were 30% less for blacks than whites. The odds of screening were 22 times greater for patients with hematologic malignancies than for patients with solid tumors. The odds of screening were four times greater for patients who were anticipated to initiate rituximab therapy than for patients who did not receive immunotherapy. We also modeled HBsAg and anti-HBc testing separately and found similar results.
Table 2.
Predictors of HBV Screening* in Multivariable Logistic Regression Analysis
| Predictor | Screened for HBV† |
|
|---|---|---|
| OR | 95% CI | |
| Age | 0.98 | 0.98 to 0.99‡ |
| Sex | ||
| Male | 1.5 | 1.3 to 1.7‡ |
| Female | Reference | |
| Ethnicity | ||
| Hispanic | 0.9 | 0.7 to 1.1 |
| Black | 0.7 | 0.6 to 0.9§ |
| Asian | 1.3 | 0.8 to 2.0 |
| Other | 1.4 | 0.9 to 2.2 |
| White | Reference | |
| History of HBV infection | ||
| Yes | 10.2 | 5.9 to 17.6‡ |
| No | Reference | |
| HBV risk factors | ||
| Yes | 1.6 | 1.3 to 1.9‡ |
| No | Reference | |
| Cancer type | ||
| Hematologic malignancy | 21.5 | 18.3 to 25.2‡ |
| Primary liver cancer | 7.0 | 3.9 to 11.8‡ |
| Solid tumor, excluding primary liver cancer | Reference | |
| Chemotherapy type | ||
| Rituximab | 4.2 | 3.4 to 5.1‡ |
| Immunotherapy, excluding rituximab | 1.0 | 0.8 to 1.2 |
| Chemotherapy/nonimmunotherapy | Reference | |
Abbreviations: anti-HBc, antibody to hepatitis B core antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; OR, odds ratio.
Either HBsAg or anti-HBc test.
Total of 1,752 patients had HBsAg screening test; 1,700 had anti-HBc screening test; 1,665 had both HBsAg and anti-HBc screening tests.
P < .001.
P < .01.
Results of HBV Tests
Among 1,787 patients who underwent screening, the prevalence of either a positive HBsAg or anti-HBc result was 8.5% (151 of 1,787; Appendix Fig A1, online only). Among 1,665 patients screened using both tests, the prevalence of chronic HBV infection was 1.5%. The prevalence of having a positive anti-HBc result but negative HBsAg test was 7.4%. Among 1,541 patients who had either both tests positive or both tests negative, the prevalence of chronic HBV infection was nearly 40% among Asian patients and 4% among patients with HBV risk factors (Table 3). Among 151 patients with positive HBsAg and/or anti-HBc screening tests, 25 patients (17%) developed abnormalities of liver tests after chemotherapy, and nearly 20% of patients had liver decompensation.
Table 3.
Odds of Positive Results on Both HBsAg and Anti-HBc Tests Versus Both Tests Negative in Univariate and Multivariable Logistic Regression Analyses
| Predictor | No. | % | Both HBsAg and Anti-HBc Positive (n = 25) |
|||
|---|---|---|---|---|---|---|
| Univariate Analysis* |
Multivariable Analysis |
|||||
| OR | 95% CI | OR | 95% CI | |||
| Age, years | 1.0 | 0.97 to 1.01 | ||||
| Mean | 48.8 | |||||
| SD | 11.0 | |||||
| Sex | ||||||
| Male | 20 of 914 | 2.2 | 2.8 | 1.0 to 7.5† | 7.4 | 2.0 to 27.0‡ |
| Female | 5 of 627 | 0.8 | Reference | Reference | ||
| Ethnicity | ||||||
| Hispanic | 3 of 204 | 1.5 | 4.3 | 1.0 to 19.4 | 4.5 | 1.0 to 20.7 |
| Black | 3 of 98 | 3.1 | 9.1 | 2.0 to 41.3‡ | 9.7 | 2.1 to 45.6‡ |
| Asian | 12 of 31 | 38.7 | 182.3 | 53.9 to 616.9§ | 270.8 | 65.4 to 1,109.0§ |
| Other | 3 of 49 | 6.1 | 18.8 | 4.1 to 86.6‡ | 13.0 | 2.6 to 64.6‡ |
| White | 4 of 1,159 | 0.4 | Reference | Reference | ||
| Residence | ||||||
| Outside United States | 2 of 59 | 3.4 | 2.2 | 0.5 to 9.7 | ||
| United States | 23 of 1,482 | 1.6 | Reference | |||
| HBV risk factors | ||||||
| Yes | 17 of 414 | 4.1 | 6.0 | 2.6 to 14.0§ | 3.9 | 1.4 to 10.5‡ |
| No | 8 of 1,127 | 0.7 | Ref. | Reference | ||
| Cancer type | ||||||
| Hematologic malignancy | 11 of 1,097 | 1.0 | 0.4 | 0.2 to 0.9† | 0.7 | 0.3 to 2.1 |
| Primary liver cancer | 3 of 21 | 14.3 | 6.2 | 1.6 to 24.3‡ | 8.3 | 1.5 to 46.3† |
| Solid tumor, excluding primary liver cancer | 11 of 423 | 2.6 | Reference | Reference | ||
| Chemotherapy type | — | |||||
| Rituximab | 6 of 709 | 0.9 | 0.5 | 0.2 to 1.8 | — | |
| Immunotherapy, excluding rituximab | 5 of 80 | 6.3 | 3.5 | 1.2 to 10.0† | — | |
| Chemotherapy/nonimmunotherapy | 14 of 752 | 1.9 | Reference | — | ||
NOTE. Model includes 1,541 patients who had either both tests positive (n = 25) versus both tests negative (n = 1,516). Age and residence variables were not entered in the multivariable model because their P > .20. Empty cells with dashes refer to variables not retained in the final step of the multivariable model because their P > .05.
Abbreviations: HBsAg, hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen; OR, odds ratio; SD, standard deviation.
P ≤ .20 for the following predictors in the univariate logistic regression model, which were entered into the multivariable model: sex, ethnicity, HBV risk factors, cancer type, and chemotherapy type. History of HBV infection was not entered into the model because of small numbers of patients without a history HBV infection but with positive HBsAg and anti-HBc test results.
P < .05.
P < .01.
P < .001.
Predictors of Positive Results
We examined factors related to having chronic HBV infection versus negative results on both tests (Table 3). We did not include history of HBV in this model because of the small numbers of patients (n = 5) with positive HBsAg and anti-HBc test results without a history of HBV. On univariate analysis, sex, ethnicity, HBV risk factors, cancer type, and chemotherapy type were associated with positive results on both tests. On multivariable analysis, male sex, Asian ethnicity, black ethnicity, and HBV risk factors predicted positive results on both tests. Not surprisingly, primary liver cancer also predicted positive results on both tests. We also explored factors related to having a positive anti-HBc but negative HBsAg test, and we found similar results.
Discussion
Our study is the first to our knowledge to examine determinants of HBV screening at the onset of chemotherapy in a large population of patients with cancer undergoing chemotherapy in the United States. Our study was conducted before the CDC recommendation, and our results could serve as a baseline against which results of future studies of HBV screening can be compared. We found low rates of HBV screening among patients at high risk for HBV infection and potentially at risk for reactivation after chemotherapy.
Although patients with HBV risk factors had four-fold increased odds of positive HBV screening test results, less than 20% were screened for HBV infection. Previously, Tran et al23 found that 38% of American Medical Association oncologists reported screening patients with risk factors for HBV infection, and Khokhar et al24 found that 86% of oncologists reported screening patients with HBV risk factors. These studies show that oncologists may be screening based on HBV risk factors; however, they represent self-reported screening behavior, which may overestimate screening rates.25,26
Although certain ethnic groups have higher risks of HBV infection, we found that many of these patients were not screened. Although Asian and black patients had a high likelihood of infection on screening, only 17% Asians and 12% of blacks in our study were screened. The prevalence of past or present HBV infection in the United States has been reported to be 12.2% among blacks27 and 8.9% to 13.4% among Asian Americans.9
Given our finding that the rate of chronic HBV infection before chemotherapy was less than 2% overall but higher among Asian patients and patients with other HBV risk factors, at minimum, patients with risk factors should be screened for HBV infection. However, to optimize selective screening, providers need tools to predict HBV infection and reactivation in different patient subgroups—tools that do not yet exist. At present, many patients with HBV may not know their own risk or that they are infected.9,28 They might not even have identifiable HBV risk factors. Previous studies of antenatal HBV screening showed that HBV in nearly 45% to 65% of patients with infection would have been missed if only patients with known risk factors were screened.29,30 Thus, until risk models are developed and clinically applicable risk tools are incorporated into medical practice, universal screening for HBV before chemotherapy could be considered, paralleling universal antenatal screening.30–33 Rigorous future studies should examine whether risk-based or universal screening for HBV infection before chemotherapy should be implemented.
ASCO has recommended screening patients known to have HBV risk factors or who are anticipating highly immunosuppressive therapies, such as stem-cell transplantation and rituximab.18 Our finding of low screening rates among patients with risk factors for HBV infection indicates that physicians were not systematically screening for HBV among select high-risk groups during our study period.
An interesting finding of this study was that substantial numbers of patients had positive anti-HBc but negative HBsAg test results. These patients may be convalescent from previous infection (if anti-HBs positive) or have occult HBV infection (if anti-HBs negative). Covalently closed circular DNA may persist indefinitely in hepatocytes of patients after acute infection who have positive anti-HBc and negative HBsAg.34,35 Further study about the natural history and role of prophylaxis among these patients is needed because previous studies have shown that patients with positive anti-HBc and negative HBsAg may be at risk of reactivation after chemotherapy.3,36–41 Screening strategies using anti-HBc as the first test (with reflex testing of HBsAg, anti-HBs, and HBV DNA) should be explored because anti-HBc is a reliable serologic marker of HBV infection, whether chronic, convalescent, or occult.42,43
The main limitations of our study result from its retrospective nature. Because we did not have HBV DNA data at baseline or during chemotherapy, we described outcomes of abnormalities of liver tests and liver decompensation in patients with HBV after chemotherapy; however, these outcomes cannot substitute for reactivation because they could alternatively be explained by drug toxicity, infiltrative malignancy, or other causes of liver injury. We did not explore these outcomes in patients who were without HBV infection, and this is a weakness of our study design because these patients could also have abnormalities of liver tests and liver decompensation after chemotherapy. We were not able to ascertain patients' complete HBV risk factors,17 and this may have decreased the accuracy of screening rates. Furthermore, screening rates may have reflected providers' investigating unspecified liver disease rather than screening to prevent reactivation of HBV infection. Our single-institution study may have inherent biases; however, our findings are based on a substantial sample of insured and indigent patients whose physicians drive screening. Unfortunately, patients' race was not self-identified, which limits the accuracy of this variable, and the proportion of nonwhite patients is lower at MD Anderson (25%; Tumor Registry Department New Patient Profile, 2010)44 than in the overall US population (36%),45 limiting the generalizability of findings.
In conclusion, the findings of our study, reflecting a period before the 2008 release of the CDC recommendation for widespread HBV screening, indicate that the overall rate of HBV screening was low. Additionally, we found that screening was low among patients with selected risk factors for HBV infection. Data from population-based studies are seriously lacking, and recent studies of screening practices have been limited because of recall bias46 or have not incorporated risk factors for reactivation.47 To ascertain appropriate screening strategies for patients with cancer with HBV infection, oncologists need sound evidence to make effective clinical decisions. Future large and prospective studies to identify best screening methods as well as risk models for HBV infection and reactivation will be important to elucidate solutions for this serious complication of chemotherapy.
Acknowledgment
We thank the following individuals for their assistance with institutional databases: Sarah Taylor (tumor registry), Chun Feng and Frank Hung (pharmacy informatics), and Weiming Shi (patient account). We would also like to acknowledge Susan Lackey, MPH, for administrative support; Stephanie Deming, BA, Department of Scientific Publications, MD Anderson, for editing the manuscript; and Anna Lok, MD, Holly Holmes, MD, and Rohit Loomba, MD, for manuscript review. Supported by the American Cancer Society, Bristol-Myers Squibb, and the National Institutes of Health through MD Anderson Cancer Center Support Grant No. CA016672; by National Cancer Institute Career Development Award No. K07 CA132955 (J.P.H.); and by Midcareer Investigator Award No. K24 AR053593 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (M.E.S.-A.). Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, June 4-8, 2010; the 47th Annual Meeting of ASCO, Chicago, IL, June 3-7, 2011; Multinational Association of Supportive Care in Cancer (MASCC) International Symposium, Vancouver, British Columbia, Canada, June 24-26, 2010; ASCO Gastrointestinal Cancers Symposium, San Francisco, CA, January 20-22, 2011; MASCC International Symposium, Athens, Greece, June 23-25, 2011; and 62nd Annual Meeting of the American Association for the Study of Liver Diseases, San Francisco, CA, November 4-8, 2011.
Appendix
Table A1.
Classification of Chemotherapy Drugs Received by Study Population
| Classification | Drugs |
|---|---|
| Alkylating agents | Busulfan, carboplatin, carmustine, chlorambucil, cisplatin, cyclophosphamide, dacarbazine, ifosfamide, lomustine, mechlorethamine, melphalan, oxaliplatin, procarbazine, streptozocin, temozolomide |
| Antimetabolites | Azacitidine, capecitabine, cladribine, clofarabine, cytarabine, cytarabine liposome, decitabine, fludarabine, fluorouracil, gemcitabine, hydroxyurea, mercaptopurine, methotrexate, pemetrexed, pentostatin, thioguanine |
| Mitotic inhibitors | Docetaxel, ixabepilone, vinblastine, vincristine, vinorelbine |
| Antitumor antibiotics | Bleomycin, dactinomycin, daunorubicin, doxorubicin, epirubicin HCl, idarubicin, mitomycin, mitoxantrone |
| Immunotherapy | Aldesleukin, alemtuzumab, bevacizumab, cetuximab, gemtuzumab ozogamicin, interferon alfa-2b, rituximab |
| Hormone therapy | Anastrozole, bicalutamide, estramustine phosphate, exemestane, flutamide, fulvestrant, goserelin, letrozole, leuprolide, megestrol, mitotane, nilutamide, tamoxifen |
| Targeted therapy | Bortezomib, dasatinib, erlotinib, everolimus, imatinib, lapatinib, sorafenib, sunitinib, temsirolimus, vorinostat |
| Topoisomerase inhibitors | Etoposide, irinotecan, topotecan |
| Miscellaneous chemotherapy drugs | Asparaginase, denileukin diftitox, pegaspargase, porfimer |
| Differentiating agents | Arsenic trioxide, bexarotene, tretinoin |
Figure A1.
Hepatitis B virus screening and results among new patients with cancer who received chemotherapy at MD Anderson Cancer Center, 2004-2007. Abbreviations: HBsAg, hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Jessica P. Hwang, Gilead (C); John M. Vierling, Gilead (C), Bristol-Myers Squibb (C), Roche (C) Stock Ownership: None Honoraria: None Research Funding: Jessica P. Hwang, Bristol-Meyers Squibb; John M. Vierling, Gilead, Bristol-Myers Squibb, Roche Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Jessica P. Hwang, Michael J. Fisch, John M. Vierling, Maria E. Suarez-Almazor
Financial support: Jessica P. Hwang
Administrative Support: Jessica P. Hwang, Michael J. Fisch, Mark J. Routbort, Lincy S. Lal
Provision of study materials or patients: Mark J. Routbort, Lincy S. Lal
Collection and assembly of data: Jessica P. Hwang, Hong Zhang, Mark J. Routbort, Lincy S. Lal
Data analysis and interpretation: Jessica P. Hwang, Hong Zhang, Michael A. Kallen, John M. Vierling, Maria E. Suarez-Almazor
Manuscript writing: Jessica P. Hwang, Michael J. Fisch, Hong Zhang, John M. Vierling, Maria E. Suarez-Almazor
Final approval of manuscript: All authors
References
- 1.Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(suppl):S156–S165. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- 2.Sorrell MF, Belongia EA, Costa J, et al. National Institutes of Health Consensus Development Conference statement: Management of hepatitis B. Ann Intern Med. 2009;150:104–110. doi: 10.7326/0003-4819-150-2-200901200-00100. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, Liang RH, Chiu EK, et al. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy: Report of a prospective study. Gastroenterology. 1991;100:182–188. doi: 10.1016/0016-5085(91)90599-g. [DOI] [PubMed] [Google Scholar]
- 4.Yeo W, Chan PK, Hui P, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: A prospective study. J Med Virol. 2003;70:553–561. doi: 10.1002/jmv.10430. [DOI] [PubMed] [Google Scholar]
- 5.Barclay S, Pol S, Mutimer D, et al. The management of chronic hepatitis B in the immunocompromised patient: Recommendations from a single topic meeting. J Clin Virol. 2008;41:243–254. doi: 10.1016/j.jcv.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Kwak LW, Halpern J, Olshen RA, et al. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: Results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]
- 7.Loomba R, Rowley A, Wesley R, et al. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1–20. [PubMed] [Google Scholar]
- 9.Lin SY, Chang ET, So SK. Why we should routinely screen Asian American adults for hepatitis B: A cross-sectional study of Asians in California. Hepatology. 2007;46:1034–1040. doi: 10.1002/hep.21784. [DOI] [PubMed] [Google Scholar]
- 10.McQuillan GM, Coleman PJ, Kruszon-Moran D, et al. Prevalence of hepatitis B virus infection in the United States: The National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kottiri BJ, Friedman SR, Euler GL, et al. A community-based study of hepatitis B infection and immunization among young adults in a high-drug-use neighborhood in New York City. J Urban Health. 2005;82:479–487. doi: 10.1093/jurban/jti095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ioannou GN. Hepatitis B virus in the United States: Infection, exposure, and immunity rates in a nationally representative survey. Ann Intern Med. 2011;154:319–328. doi: 10.7326/0003-4819-154-5-201103010-00006. [DOI] [PubMed] [Google Scholar]
- 13.French AL, Operskalski E, Peters M, et al. Isolated hepatitis B core antibody is associated with HIV and ongoing but not resolved hepatitis C virus infection in a cohort of US women. J Infect Dis. 2007;195:1437–1442. doi: 10.1086/515578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shuler CM, Fiore AE, Neeman R, et al. Reduction in hepatitis B virus seroprevalence among U.S.-born children of foreign-born Asian parents: Benefit of universal infant hepatitis B vaccination. Vaccine. 2009;27:5942–5947. doi: 10.1016/j.vaccine.2009.07.087. [DOI] [PubMed] [Google Scholar]
- 15.Colvin HM, Mitchell AE, editors. Washington, DC: Institute of Medicine; 2010. Hepatitis and Liver Cancer: A National Strategy for Prevention and Control of Hepatitis B and C. [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Practice guidelines in oncology: Prevention and treatment of cancer-related infections, 2009. www.nccn.org/professionals/physician_gls/pdf/infections.pdf.
- 17.Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 18.Artz AS, Somerfield MR, Feld JJ, et al. American Society of Clinical Oncology provisional clinical opinion: Chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. J Clin Oncol. 2010;28:3199–3202. doi: 10.1200/JCO.2010.30.0673. [DOI] [PubMed] [Google Scholar]
- 19.Keegan TH, Gomez SL, Clarke CA, et al. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California. Int J Cancer. 2007;120:1324–1329. doi: 10.1002/ijc.22432. [DOI] [PubMed] [Google Scholar]
- 20.Lauderdale DS, Huo D. Cancer death rates for older Asian-Americans: Classification by race versus ethnicity. Cancer Causes Control. 2008;19:135–146. doi: 10.1007/s10552-007-9079-4. [DOI] [PubMed] [Google Scholar]
- 21.Lauderdale DS, Kestenbaum B. Asian American ethnic identification by surname. Popul Res Policy Rev. 2000;19:283–300. [Google Scholar]
- 22.American Cancer Society. Chemotherapy principles: An in-depth discussion of the techniques and its role in cancer treatment, 2010. www.cancer.org/acs/groups/cid/documents/webcontent/002995-pdf.pdf.
- 23.Tran TT, Rakoski MO, Martin P, et al. Screening for hepatitis B in chemotherapy patients: Survey of current oncology practices. Aliment Pharmacol Ther. 2010;31:240–246. doi: 10.1111/j.1365-2036.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- 24.Khokhar OS, Farhadi A, McGrail L, et al. Oncologists and hepatitis B: A survey to determine current level of awareness and practice of antiviral prophylaxis to prevent reactivation. Chemotherapy. 2008;55:69–75. doi: 10.1159/000183731. [DOI] [PubMed] [Google Scholar]
- 25.McPhee SJ, Richard RJ, Solkowitz SN. Performance of cancer screening in a university general internal medicine practice: Comparison with the 1980 American Cancer Society Guidelines. J Gen Intern Med. 1986;1:275–281. doi: 10.1007/BF02596202. [DOI] [PubMed] [Google Scholar]
- 26.Montano DE, Phillips WR. Cancer screening by primary care physicians: A comparison of rates obtained from physician self-report, patient survey, and chart audit. Am J Public Health. 1995;85:795–800. doi: 10.2105/ajph.85.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasley A, Kruszon-Moran D, Kuhnert W, et al. The prevalence of hepatitis B virus infection in the United States in the era of vaccination. J Infect Dis. 2010;202:192–201. doi: 10.1086/653622. [DOI] [PubMed] [Google Scholar]
- 28.Hwang JP, Mohseni M, Gor BJ, et al. Hepatitis B and hepatitis C prevalence and treatment referral among Asian Americans undergoing community-based hepatitis screening. Am J Public Health. 2010;100(suppl 1):S118–S124. doi: 10.2105/AJPH.2009.162776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brook MG, Lever AM, Kelly D, et al. Antenatal screening for hepatitis B is medically and economically effective in the prevention of vertical transmission: Three years experience in a London hospital. Q J Med. 1989;71:313–317. [PubMed] [Google Scholar]
- 30.Centers for Disease Control. Prevention of perinatal transmission of hepatitis B virus: Prenatal screening of all pregnant women for hepatitis B surface antigen. MMWR Morb Mortal Wkly Rep. 1988;37:341–346. [PubMed] [Google Scholar]
- 31.Grosheide PM, Wladimiroff JW, Heijtink RA, et al. Proposal for routine antenatal screening at 14 weeks for hepatitis B surface antigen: Dutch Study Group on Prevention of Neonatal Hepatitis. BMJ. 1995;311:1197–1199. doi: 10.1136/bmj.311.7014.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dwyer MJ, McIntyre PG. Ante-natal screening for hepatitis B surface antigen: An appraisal of its value in a low prevalence area. Epidemiol Infect. 1996;117:121–131. doi: 10.1017/s0950268800001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boxall E. Screening of pregnant women for hepatitis B. Vaccine. 1998;16:S30–S33. doi: 10.1016/s0264-410x(98)00289-8. [DOI] [PubMed] [Google Scholar]
- 34.Wursthorn K, Wedemeyer H, Manns MP. Managing HBV in patients with impaired immunity. Gut. 2010;59:1430–1445. doi: 10.1136/gut.2009.195834. [DOI] [PubMed] [Google Scholar]
- 35.Vierling JM. The immunology of hepatitis B. Clin Liver Dis. 2007;11:727–759. doi: 10.1016/j.cld.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Matsue K, Kimura S, Takanashi Y, et al. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer. 2010;116:4769–4776. doi: 10.1002/cncr.25253. [DOI] [PubMed] [Google Scholar]
- 37.Yeo W, Chan TC, Leung NW, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605–611. doi: 10.1200/JCO.2008.18.0182. [DOI] [PubMed] [Google Scholar]
- 38.Palmore TN, Shah NL, Loomba R, et al. Reactivation of hepatitis B with reappearance of hepatitis B surface antigen after chemotherapy and immunosuppression. Clin Gastroenterol Hepatol. 2009;7:1130–1137. doi: 10.1016/j.cgh.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koo YX, Tay M, Teh YE, et al. Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol. 2011;90:1219–1223. doi: 10.1007/s00277-011-1241-0. [DOI] [PubMed] [Google Scholar]
- 40.Ferraro D, Pizzillo P, Di Marco V, et al. Evaluating the risk of hepatitis B reactivation in patients with haematological malignancies: Is the serum hepatitis B virus profile reliable? Liver Int. 2009;29:1171–1177. doi: 10.1111/j.1478-3231.2009.02071.x. [DOI] [PubMed] [Google Scholar]
- 41.Viganò M, Vener C, Lampertico P, et al. Risk of hepatitis B surface antigen seroreversion after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46:125–131. doi: 10.1038/bmt.2010.70. [DOI] [PubMed] [Google Scholar]
- 42.Laperche S, Guitton C, Smilovici W, et al. Blood donors infected with the hepatitis B virus but persistently lacking antibodies to the hepatitis B core antigen. Vox Sang. 2001;80:90–94. doi: 10.1046/j.1423-0410.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- 43.Pondé RA, Cardoso DD, Ferro MO. The underlying mechanisms for the ‘anti-HBc alone’ serological profile. Arch Virol. 2010;155:149–158. doi: 10.1007/s00705-009-0559-6. [DOI] [PubMed] [Google Scholar]
- 44. Reference deleted.
- 45.The US Census Bureau. State and County QuickFacts: USA. http://quickfacts.census.gov/qfd/states/00000.html.
- 46.Day F, Link E, Thursky K, et al. Current hepatitis B screening practices and clinical experience of reactivation in patients undergoing chemotherapy for solid tumors: A nationwide survey of medical oncologists. J Oncol Pract. 2011;7:141–147. doi: 10.1200/JOP.2010.000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol. 2011;29:3270–3277. doi: 10.1200/JCO.2011.35.1635. [DOI] [PubMed] [Google Scholar]