Abstract
Mitochondria are essential for ensuring numerous fundamental physiological processes such as cellular energy, redox balance, modulation of Ca2+ signaling and important biosynthetic pathways. They also govern the cell fate by participating in the apoptosis pathway.
The mitochondrial shape, volume, number and distribution within the cells are strictly controlled. The regulation of these parameters has an impact on mitochondrial function, especially in the central nervous system, where trafficking of mitochondria is critical to their strategic intracellular distribution, presumably according to local energy demands. Thus, the maintenance of a healthy mitochondrial population is essential to avoid the impairment of the processes they regulate: for this purpose, cells have developed mechanisms involving a complex system of quality control to remove damaged mitochondria, or to renew them. Defects of these processes impair mitochondrial function and lead to disordered cell function, i.e., to a disease condition. Given the standard role of mitochondria in all cells, it might be expected that their dysfunction would give rise to similar defects in all tissues. However, damaged mitochondrial function has pleiotropic effects in multicellular organisms, resulting in diverse pathological conditions, ranging from cardiac and brain ischemia, to skeletal muscle myopathies to neurodegenerative diseases. In this review, we will focus on the relationship between mitochondrial (and cellular) derangements and Ca2+ dysregulation in neurodegenerative diseases, emphasizing the evidence obtained in genetic models. Common patterns, that recognize the derangement of Ca2+ and energy control as a causative factor, have been identified: advances in the understanding of the molecular regulation of Ca2+ homeostasis, and on the ways in which it could become perturbed in neurological disorders, may lead to the development of therapeutic strategies that modulate neuronal Ca2+ signaling.
Keywords: Mitochondria, Calcium signaling, Neurodegenerative diseases, Mitochondrial quality control, Neurons
1. Mitochondria in neuronal Ca2+ homeostasis
Ca2+ is the main second messenger that helps the biochemical machinery of neurons to connect their depolarization status with synaptic activity, which is their most crucial activity. Thus, neurons have developed extensive and intricate Ca2+ signaling pathways to cope with the requirement. Neuronal Ca2+ signals are mainly generated by the influx through the voltage dependent or ligand activated Ca2+ channels, such as ionotropic glutamate receptors (VOCs and ROCs) [1]. The release of Ca2+ from intracellular stores (i.e. the endo/sarcoplasmic reticulum, ER/SR) by the opening of the inositol 1,4,5 trisphosphate (InsP3R) and the ryanodine (RyR) receptors contributes to the transmission of Ca2+ signals [2]. The strict control of intracellular Ca2+ concentration is operated by Ca2+ binding proteins and by energy demanding Ca2+ transport proteins. The plasma membrane Ca2+ ATPase (PMCA) and the plasma membrane Na+/Ca2+ exchanger (NCX) extrude Ca2+ into the extracellular space (however, the NCX can also operate in the reverse mode). The Ca2+ pumps of the intracellular organelles, i.e., the ER/SR Ca2+ATPase (SERCA) and the secretory pathway Golgi Ca2+ ATPase (SPCA) pumps, accumulate Ca2+ in the intracellular stores [3]. Mitochondria also contribute to the spatiotemporal tuning of the cytosolic Ca2+ concentration thanks to Ca2+ uptake and release systems. The transport of Ca2+ by these systems controls how much Ca2+ enters the cell, the Ca2+ concentration in cytoplasmic microdomains, the frequency of oscillatory cytosolic Ca2+ signals and the rate of propagation of a Ca2+ signal. In turn, mitochondria use their Ca2+ transporting activity to modulate the rate of ATP synthesis in a number of ways, i.e., by activating Krebs cycle (TCA) dehydrogenases, by promoting the supply of oxidizable substrates and by regulating the activity of the ATP synthase [4,5].
The electron transport chain (ETC), i.e., the molecular machinery for energy production, is organized in five protein complexes located in the inner mitochondrial membrane. Three of these complexes (I, III, IV) pump protons (H+) across the inner membrane, thus establishing the electrochemical gradient which is then used by complex V, the ATP synthase, to produce ATP. During their activity, electrons are transported from the reduced substrates, that accepted reducing equivalents from the TCA cycle (NADH and FADH2), to the oxygen which is converted to H2O. The electron transport, especially in complex I and III, also generates the free radical superoxide (O2−•), most of which is converted to hydrogen peroxide (H2O2) by the manganese superoxide dismutase (MnSOD). H2O2 is in turn converted to water by glutathione peroxidase and catalase. However, it can also generate the highly reactive hydroxyl radical (OH•) by reacting with Fe2+ or Cu2+. Another highly reactive species, the peroxynitrite (ONOO−), is produced by the reaction of O2−• with nitric oxide, and free radicals may be generated by the activity of the outer mitochondrial membrane (OMM) monoamine oxidases (MAOA and MAOB), enzymes involved in the metabolism of amines like serotonin, norepinephrine and dopamine. All these oxygen reactive species can induce membrane lipid peroxidation (damaging protein and DNA) or nitration of mitochondrial proteins at tyrosine residues, with consequent loss of function. However, it must be underlined that O2−• and H2O2 also serve important signaling functions in physiological processes (for a comprehensive review see Schon and Przedborski [6]).
The ETC, by generating the electrochemical gradient and, in turn, a difference of membrane potential across the inner mitochondrial membrane, also sustains Ca2+ transfer into the mitochondrial matrix which occurs downhill the gradient through a low affinity uniporter. Two antiporters, the Na+/Ca2+ exchanger and the H+/Ca2+ exchanger, move Ca2+ out of the matrix, allowing the return of mitochondrial Ca2+ to basal values after cell stimulation [7]. Thus, impaired abilities of neurons to maintain appropriate cellular energy levels, and increased generation of mitochondrial reactive oxygen species (ROS) may affect Ca2+ signaling.
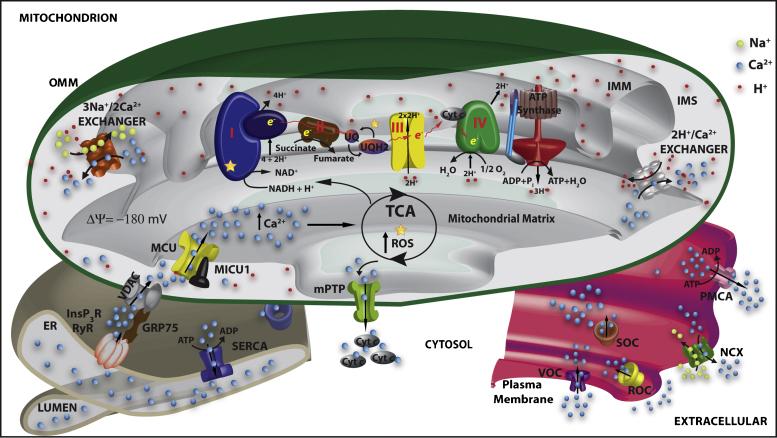
The functional properties of mitochondrial Ca2+ transporters have been extensively characterized, and very recently some of them, i.e. the mitochondrial Ca2+ uniporter (MCU), as well as one of its regulators (MICU1), and the Na+/Ca2+ antiporter (NCLX) have also been molecularly identified [8–11]. Fig. 1 summarizes the main players in mitochondrial Ca2+ transport.
Fig. 1.
The main players in mitochondrial Ca2+ transport. Ca2+ flows into the cytoplasm upon cell stimulation and the opening of the inositol trisphosphate receptors (InsP3R), the ryanodine receptors (RyR) at the ER/SR membranes and/or of the plasma membrane associated voltage (VOC), receptor (ROC) and store (SOC) operated calcium channels. The generation of localized high Ca2+ concentration microdomains drives Ca2+ into the mitochondrial matrix via the mitochondria Ca2+ uniporter (MCU), this action being potentiated by the voltage-dependent anion channel (VDAC) that, through GRP75, enhanced the ER Ca2+ transfer. MICU1 is a MCU associated protein that acts as MCU regulator. The efflux mechanism depends on the activity of the H+/Ca2+ and the Na+/Ca2+ exchangers. Increased mitochondrial Ca2+ concentration stimulates TCA cycle enzymes generating NADH and increasing ATP synthesis and ROS production. Sustained increases in mitochondrial Ca2+ concentration sensitize mitochondria to permeability transition pore (mPTP) opening with consequent release of cytochrome c (cyt c) and induction of apoptosis. Cytosolic Ca2+ clearance depends on the activity of the plasma membrane Ca2+ ATPase (PMCA), of the plasma membrane Na+/Ca2+ exchanger (NCX) and of the ER/SR Ca2+ATPase (SERCA). ER, Endoplasmic reticulum; OMM, outer mitochondrial membrane; IMS, intermembrane mitochondrial space; IMM, inner mitochondrial membrane.
Mitochondrial Ca2+ accumulation depends on the proton electrochemical gradient that drives its rapid accumulation through MCU that, upon cell stimulation, becomes exposed to microdomains of high Ca2+ concentration generated by the opening of the Ca2+ channels in the membrane of the ER. The Ca2+ microdomains are sensed by the MCU thanks to the close contact of mitochondria with the ER Ca2+ channels and match its low Ca2+ affinity [12]. The tethers linking the ER and mitochondria have been visualized [13] and, more recently, mitofusin 2 has been demonstrated to be an essential component of these structures [14]. The concept of mitochondria tethering has been extended also to the plasma membrane and the Golgi compartment [15,16].
In addition to the Ca2+ transporting systems on the inner mitochondrial membrane (IMM), recent data have revealed an important role of the OMM permeability. Attention has been drawn to the potential role of a large-conductance channel, commonly referred to as the mitochondrial permeability transition pore (mPTP), in modulating mitochondrial Ca2+. As to the pathophysiological role, mPTP is most likely involved in the swelling and fragmentation of the mitochondrial network that underlie the release of caspase cofactors from mitochondria. Its molecular identity is still unknown, but it appears to be a multiprotein complex activated by various pathophysiological conditions (e.g. Ca2+ increases in the mitochondrial matrix and oxidation of critical cysteins). The putatively essential components/regulators are the voltage dependent anion channel (VDAC) of the OMM, the adenine nucleotide transporter (ANT) of the IMM and cyclophilin D [17]. Interestingly, it has been shown that VDAC channels clustered at the ER/mitochondrial contact sites to play a key role in the rapid transfer of the high Ca2+ microdomain from the surface of mitochondria to the intermembrane space to which the MCU is exposed [18]. VDAC shuttles between open and closed states (with reference to metabolite transport) and in the closed state shows a higher permeability to Ca2+. Remarkably, Ca2+ itself appears to control the conductance of VDAC, thus suggesting that, during the Ca2+ signal, the OMM barrier is dynamically reduced and mitochondrial Ca2+ uptake facilitated.
2. Mitochondrial Ca2+ dysregulation and affected processes
Moderate increases in mitochondrial Ca2+ concentration are necessary and sufficient to adjust ATP production to cell demand, but mitochondrial Ca2+ overload (MCO) unequivocally leads to disruption of mitochondrial membrane integrity, permeability transition, irreversible oxidative damage and loss of ATP production, finally emerging in cell death in different pathological conditions. MCO can result essentially from three mechanisms: (i) increased mitochondrial Ca2+ uptake, following release from the ER and Ca2+ influx from the extracellular space; (ii) reduced Ca2+ extrusion through the mitochondrial Na+/Ca2+ exchanger; and (iii) changes of mitochondrial Ca2+ buffering. However, before MCO culminates in cell death, mitochondrial Ca2+ dysregulation originates in a plethora of disturbances that cells try to control by modulating mitochondrial Ca2+ related activities. In the following sections we will discuss them with respect to their regulation by Ca2+, bearing in mind that they represent the mitochondrial quality control system. Their impairment is a hallmark of the initial phases of neurodegeneration.
2.1. Mitochondrial trafficking and fusion/fission
The dynamic properties of mitochondria are critical to all cells but may be particularly important in neurons, due to their unique morphology. Synaptic mitochondria clear Ca2+ from the cytosol either by directly taking it up or by providing ATP for the Ca2+ extrusion (PMCA and NCX) and uptake (SERCA) systems. Thus, mitochondrial movements and fusion/fission processes are required, not only during cell division to properly distribute mitochondria to daughter cells, but also to transport mitochondria to their potential sites of action. At the molecular level, mitochondrial dynamics are mediated by three GTPases, namely mitofusins (Mfn1/2), optic atrophy1 (OPA1) and dynamin related protein 1 (Drp1), which are responsible for the mitochondrial fusion/fission process, and by the kinesin and dynein motors that, through the action of the adaptor protein Milton and the atypical GTPase Miro, mediate the traveling of mitochondria along cytoskeletal tracks. These two pathways are strictly connected since the balance between mitochondrial fission and fusion governs the shape and the number of mitochondria, but also their function and distribution. Intriguingly, Ca2+ acts as the common molecular switch for both processes [19,20].
Mfns are integral OMM proteins and mediate mitochondrial fusion, together with OPA1. OPA1 is located in the intermembrane space, and, by interacting with the IMM, promotes its fusion and cristae remodeling. The process requires a proton gradient, thus mitochondria that are metabolically compromised are prevented from fusing. Mitochondrial fission is governed by the action of Drp1, which is recruited to mitochondria by several post-translational modifications such as phosphorylation and sumoylation. Ca2+ controls Drp1-dependent fission through calcineurin and CaM-kinase activities, but also by a Miro-dependent Ca2+ induced mechanism that enhances fusion at resting Ca2+ concentration, and promotes fragmentation at high Ca2+ levels [21], thus linking the motility process to fusion/fission. As to Mfns, no direct link with Ca2+ regulation has been proposed. However, it must be taken into account that Mfn2 has been shown to favor ER-mitochondria tethering, implying that it could modulate the ER-mitochondria Ca2+ transfer through this physical coupling [14]. As to the motility process, Miro proteins are anchored to the OMM, interact with Milton and kinesin motors and, thanks to the presence of two Ca2+ binding EF-motifs in the cytosolic domain, they confer Ca2+ sensitivity to mitochondrial trafficking.
2.2. Mitochondrial Ca2+ buffering
Recent work by different groups has clarified that the role of mitochondrial Ca2+ uptake is not limited to the control of organelle function, but also has a direct impact on the Ca2+ signals evoked by agonist stimulation in the cytosol. Seminal observation in studies by Friel and Tsien [22] in bullfrog sympathetic neurons showed that a FCCP-sensitive store may influence the degree of activation of intracellular Ca2+-dependent processes. Two mechanisms are responsible for this effect. The first operates in the microdomains where mitochondria and Ca2+ channels come in close contact. Here, the efficiency of mitochondrial Ca2+ accumulation accounts for the rapid clearing of the high Ca2+ concentration at the mouth of the release channels, and thus reduces the (positive or negative) feedback effect of the cation on the channel itself [15,23]. Thus, this close co-positioning on one hand allows the MCU to rapidly import large amounts of Ca2+ into the lumen of the organelle (and thus to provide cellular ATP to fuel energy-requiring processes) and, on the other hand, to modulate Ca2+ channels activity. The strategic distribution of mitochondria in the different cell compartments is critical in the regulation and may differ among cells types. In HeLa and GH3 cells, the activation of capacitative Ca2+ entry mechanism (CCE) results in no differences in mitochondrial Ca2+ uptake between the organelles population close to the plasma membrane and in that more deeply located, suggesting that mitochondria are excluded from the regions where the store operated Ca2+ channels (SOCs) are located and/or activated [24]. Indeed, electron microscopy analysis in RBL-1 mast cells has revealed that very few mitochondria were found beneath the plasma membrane, and that SOCs activation failed to change their pattern of distribution [25]. In T cells, mitochondria migrate instead to cell periphery following Ca2+ entry, thus acting as Ca2+ buffers and preventing SOCs inactivation [26].
The second mechanism by which mitochondrial Ca2+ uptake affects cytosolic Ca2+ signals has been initially demonstrated in pancreatic acinar cells, but has been also shown in neurons [27]. Pancreatic acinar cells have a defined polarized morphology, and the occurrence of cellular Ca2+ signals in different cellular locations has different physiological consequences. The spreading of the Ca2+ signal is prevented by clustered mitochondria that accumulate Ca2+, thus acting as a “firewall”. When mitochondrial “buffering” is overwhelmed (e.g. upon intense cell stimulation, or when the Ca2+ uptake ability of mitochondria is impaired), Ca2+ can freely diffuse to the rest of the cell, with physiological and pathological consequences. In addition to prevent the propagation of a Ca2+signal, mitochondria can also delay its propagation to specific cellular compartments, i.e. to the nucleus, thus having a role in the control of gene expression [28]. For example, Ca2+ uptake and release from mitochondria generate prolonged cytosolic Ca2+ elevations that trigger the nuclear import of the transcription factor NFAT (nuclear factor of activated T-cells), which has a role in synaptic plasticity, axonal growth, and neuronal survival [29]. Furthermore, the localization of mitochondria in cell districts where other Ca2+ removal systems are scarce or absent, for example in dendrites or axons, represents a key element in the Ca2+ handling.
2.3. Mitophagy: organelle-selective autophagic process
Autophagy degrades cellular components by encapsulating them in a double-membrane structure, the autophagosome, which fuses with lysosomes [30]. In this way, autophagy recycles intracellular components to compensate for nutrient deprivation, but also selectively eliminates organelles or protein aggregates to maintain quality control. Recently, selective mitochondrial autophagy, known as mitophagy, has been proposed as a principal mechanism for damaged mitochondria removal. The selective mitochondria elimination prevents the cytotoxic release of pro-apoptotic molecules and the extension of mitochondrial damage to the entire mitochondrial population. Mitophagy is generally preceded by mitochondrial fission [31], which implies that the regulation of mitochondria shape is an essential aspect of mitochondrial quality control.
Different Ca2+-signaling related proteins have been identified in the molecular toolkit of autophagy. Ca2+ has a dual role, being both pro-autophagic and anti-autophagic in different situations. Autophagy appears directly dependent on the levels of ER Ca2+ and on the activity of InsP3R. However, there are opposite views on its action. The antiapoptotic proteins Bcl-2 and Bcl-XL have been reported to mediate the decrease of the ER Ca2+ levels, and of the Ca2+ release, thus being protective against apoptotic cell death [32]. These proteins have also been shown to inhibit autophagy by lowering ER Ca2+ levels [33] and by interacting with both the InsP3R and the autophagic protein beclin 1. Thus, Bcl-2 proteins may have an inhibitory role on both apoptotic and autophagic pathways. However, the mechanisms which trigger these events are yet to be elucidated. On one hand, it has been shown that the constitutive Ca2+ transfer from ER to mitochondria through the InsP3R essential to sustain mitochondrial function and bioenergetics [34]: when this signal was abolished, the increased AMP/ATP ratio promoted AMPK activation and stimulation of autophagy [34]. On the other hand, however, it has been reported that elevation of cytosolic Ca2+ by agents which deplete the ER Ca2+ store resulted in increased autophagy, and that the addition of the Ca2+ buffer BAPTA-AM prevented its induction [35]. An interesting model has been proposed to reconcile the inhibitory and the stimulatory role of Ca2+ signal in autophagy: in healthy cells, spontaneous InsP3-mediated Ca2+ release from the ER is essential to sustain mitochondria bioenergetics. When this signal is missing, ATP depletion accounts for activation of autophagy. Instead, under stress situation, Ca2+ signaling is enhanced and elevated cytosolic Ca2+ stimulates autophagic flux (for a detailed discussion of these aspects see the comprehensive review by Parys and co-workers [36]).
As to mitochondria, several pathways indicate their possible link with the autophagic machinery. General consensus has been reached that two proteins, the mitochondrial serine/threonine kinase PINK1 and the E3 ubiquitin ligase parkin, the mutations of which are the major cause of autosomal recessive familial Parkinson's disease, are key to directing mitochondria to the mitophagy process. They are proposed to function in the same pathway, with PINK1 acting upstream of parkin, and being responsible for parkin recruitment to mitochondria by a kinase-dependent mechanism [37]. Once parkin has reached the OMM, it ubiquinates a series of targets among which mitofusins, [38–40], Drp1 [41], VDAC1 [40,42], and Bcl-2 [43], suggesting that these changes could be early events in the process of mitophagy. Very recently, it has also been shown that PINK1 phosphorylates Miro, thus promoting its proteasomal degradation by triggering parkin [44]. This finding is particularly intriguing since the Miro yeast homolog has been shown to regulate the ER-mitochondria contact sites [45].
Thus, PINK1 phosphorylation of substrates may represent a necessary early event that triggers the subsequent action of parkin and of the proteasome. Whether mitochondrial Ca2+ dysregulation could be relevant in these actions remains an unexplored possibility that has been suggested by the studies on the roles of PINK1/parkin (see below, Section 3.2).
2.4. Mitochondrial pathway for apoptotic cell death
Apoptosis (programmed cell death) occurs in all multicellular organisms during normal tissue development, and its dysregulation is responsible for the origin of many pathological conditions neurodegeneration and cancer.
The Ca2+ link with the apoptotic pathways has been established clearly by the finding that the anti-apoptotic Bcl-2 protein lowers the Ca2+ ER content and that Ca2+ can sensitize cells to apoptotic challenges, acting on the mitochondrial checkpoint [32]. The finding that Bcl-2, in addition to being localized in the cytoplasm and at the nuclear envelope, is also associated to the ER and mitochondrial membranes further reinforced the link. Bcl-2 and Bcl-XL, the other anti-apoptotic member of Bcl-2 family, directly interact with the InsP3R on the ER membrane, and sensitize it to low agonist doses, promoting the leakage of Ca2+ from ER. These effects are reverted by overexpression of the pro-apoptotic protein Bax [46]. The knock down of Bax and of Bak, another pro-apoptotic Bcl-2 family member protein, reduces the Ca2+ content of the ER; instead, Bax overexpression increases it [47,48]. Accordingly, double knockout Bax/Bak mouse embryonic fibroblasts (MEF) are resistant to apoptotic stimuli [49].
The amount of Ca2+ released from the ER is critical to the transduction of the signal in mitochondria and the modulation of the InsP3R opening by pro or antiapoptotic proteins is a key element that determines mitochondrial changes in physio/pathological responses. Mitochondria are the depositories of proapoptotic proteins like Smac/DIABLO, Omi/HtrA2, AIF and EndoG which are in equilibrium with the antiapoptotic proteins XIAP, cIAP-1 and cIAP-2, their relationship finely regulating cell death and life balance. Thus, the role of mitochondria and Ca2+ is an essential determinant: Ca2+ loads in the matrix sensitize the mPTP to apoptotic stimuli, inducing its opening, mitochondrial changes in morphology, and the release of cytochrome c [50] and caspase activation [51,52].
Numerous studies have also established a link between mitochondrial dynamics and the apoptotic pathway. Loss of mitofusins and OPA1 leads to mitochondrial fragmentation and enhances sensitivity to cell death stimuli, while their overexpression leads to increased survival in neurons [53,54]. The molecular basis of this action could be traced back to the cytochrome c release and caspase activation induced by mitochondrial fragmentation. Interestingly, however, Bax and Bak proteins have been found to associate with components of the fission/fusion machinery suggesting their mutual cross-talk [55].
3. Mitochondrial Ca2+ dysregulation and neurodegenerative diseases: the cause or the consequence?
Neurodegenerative diseases are a large group of heterogeneous disorders characterized by the selective death of neuronal subtypes. A number of studies suggest that the alteration of Ca2+ homeostasis is a hallmark of these pathologies; in particular, Ca2+-dependent mitochondrial dysfunction, defects in morphology and trafficking may be critical to the degeneration of neurons in Alzheimer's (AD), Parkinson's (PD) and Huntington's (HD) diseases, in amyotrophic lateral sclerosis (ALS) and in demyelinating diseases [56–61]. The molecular etiology of AD is causally related to the altered synaptic bioenergetics and function. Cognitive defects in AD patients correlate with the loss of dendritic spines and synapses [62], and alterations in mitochondrial function [61], morphology and dynamics [63–65] are particularly important. As to PD, our understanding of the molecular mechanisms has dramatically improved after the discovery of rare familial forms linked to mutations in LRRK2, α-synuclein (α-syn), parkin, DJ-1 and PINK1. Functional studies suggest that these proteins have important roles in regulating the balance between mitochondrial fission/fusion processes [66,67] and in controlling mitochondria motility along microtubules [44]. In HD mitochondrial abnormalities and oxidative damage, defects in the activity of complex II (and III) of the respiratory chain have been observed, and it has been proposed that mutant huntingtin may sensitize mPTP opening in model cells, thus disrupting mitochondrial Ca2+ homeostasis and increasing the susceptibility to apoptotic stimuli [68,69].
As to ALS, the familial cases are related to mutations in the mitochondrial Cu/Zn superoxide dismutase (SOD1) gene, which result in the alteration of complex II and IV of the respiratory chain, and possibly in the abnormal structure and number of mitochondria in motor neurons and in skeletal muscles [70].
The evidence that mitochondrial abnormalities and oxidative damage play a central role in the pathogenesis of several neurodegenerative diseases is thus well supported. The molecular determinants of the defects, however, are still debated. The possibility that dysregulation of the mitochondrial Ca2+ could be a common feature of the molecular etiology of these diseases is now gaining momentum. The different neurodegenerative pathologies will be discussed separately in the next sections.
3.1. Alzheimer's disease
AD is the first cause of dementia in aged people worldwide. It begins with mild, slowly progressing loss of memory and then continued with debilitating symptoms such as complete loss of cognitive abilities and bodily functions, ultimately leading to death. Although AD cases are mostly sporadic, with the symptoms first appearing after age 60, a small fraction of cases (1–2%) is genetically inherited and characterized by an early age of onset (<60). Three genes, namely the amyloid-precursor protein (APP) and the presenilin-1 and 2 genes (PSEN-1 and 2), have been identified as responsible for the Familial forms of Alzheimer's disease (FAD). The study of their gene products has allowed to gain new insights on the pathogenic mechanisms of the condition [71]. Sporadic and familial AD are both characterized by the presence of extracellular amyloid plaques. They are composed of aggregates of amyloid β (Aβ) peptides derived from the amyloid precursor protein (APP) cleavage by the β- and γ-secretase, and intracellular neurofibrillary tangles, formed by filaments of hyperphosphorylated tau protein [72,73]. Based on the toxicity evoked by the deposition of Aβ aggregates, the amyloidogenic pathway has been proposed as the main pathological event at the basis of the AD pathogenesis [74]. However, the finding that the oligomeric soluble fraction of the Aβ protein [75], rather than the fibrillar and insoluble form, is crucial to the impairment of the cognitive function [76] has led to the possibility of an alternative hypothesis. Disturbances in Ca2+ signaling have been found in both sporadic [77,78] and familial cases of AD [79]. The development of a Ca2+ dysregulation hypothesis was supported by the finding that Aβ oligomers can insert in the plasma membrane and form ion conducting channels, thus possibly mediating excitotoxicity by enhancing Ca2+ influx [80] and eventual neurodegeneration [81]. Fig. 2 summarizes the main findings on this issue. Aβ oligomers have been found to induce massive Ca2+ transfer between ER and mitochondria [82] and mitochondrial Ca2+ overload [83]. Altered mitochondrial morphology and/or distribution have been found in neurons from brains of AD patients [84,85] and in model cells upon Aβ [86], APP [87] and FAD-Presenilin-1 [88] expression. Excessive Ca2+ taken up into mitochondria increases ROS production, inhibits ATP synthesis, induces the opening of the mPTP, and the release of cytochrome c, triggering the initiation of apoptosis [89,90]. Aβ can also accumulate in mitochondria and interact with specific intra-mitochondrial targets, directly leading to the dysfunction of this organelle [91]. A study on brain mitochondria from an AD animal model has shown that Aβ interacts with Cyclophilin D (CypD) and promotes the opening of the mPTP, thereby causing neuronal injury and a decline in cognitive functions. Accordingly, genetic ablation of CypD renders brain mitochondria more resistant to mPTP opening [92], suggesting it could represent a potential therapeutic target.
Fig. 2.
Mitochondrial dysfunctions and Ca2+ homeostasis in AD. Peptide β-amyloid (Aβ) oligomers affect mitochondrial functionality either by increasing cytosolic Ca2+ concentration through a pore-forming mechanism at the plasma membrane and/or by enhancing ER Ca2+ release. Possible mitochondrial Aβ accumulation impairs mitochondrial energy metabolism, leading to mitochondrial oxidative damage. Aβ may sensitize mPTP opening by interacting with cyclophilin D (CypD). FAD-linked mutant presenilins (PSs) may alter the expression/sensitivity of ER Ca2+ release channels (RyR and InsP3R) leading to an exaggerated ER Ca2+ release and abnormal mitochondrial Ca2+ uptake. A reduction in SERCA activity has also been described. Wild-type PSs, but not the FAD mutants, were reported to form Ca2+ permeable leak channels in the ER. PSs have also been found in mitochondria.
The “Ca2+ hypothesis” is also strongly supported by studies on FAD. It has been shown that mutated presenilins (PSs) may contribute to the dysregulation of Ca2+ homeostasis that, in turn, may be responsible for the mitochondrial impairment observed in AD [86]. PS1 and PS2 are located in different intracellular compartments such as the ER, the Golgi apparatus [93], and mitochondria, where they participate to the formation of active γ-secretase complexes [94,95]. They have been proposed to function as low conductance Ca2+-leak channels in the ER membrane, thus contributing to maintain physiological Ca2+ concentration within the ER. Mutations causing AD have been shown to abrogate this channel activity, and result in enhanced ER Ca2+ levels and, following cell stimulation, increased cytosolic Ca2+ transients [96,97]. Nevertheless, a number of studies have instead observed either no alteration or reduced ER Ca2+ levels in FAD PS-expressing cells [79,98–101]. These studies have proposed that, rather to function as Ca2+ leak channels, PS are able to modulate Ca2+ leak by modulating InsP3Rs and/or RyRs permeability [102] and, possibly, by reducing SERCA activity [103]. FAD mutant PS1 and PS2 have been shown to interact with the InsP3R and to enhance Ca2+ release from the ER at low physiological concentrations of agonist [104,105], thus reducing ER Ca2+ content rather than augmenting it. Interestingly, PSs have been found to be enriched in the ER-mitochondrial-associated membranes (MAM) [106] and, it is worth mentioning that the T122R PS2 mutation has been shown to enhance the ER-mitochondria tethering [107]. Thus, two opposite scenarios can be envisaged: the increased ER-mitochondria interaction could potentially result in toxic mitochondrial Ca2+ overload or, alternatively, it could result in a compensatory phenomenon to ensure proper Ca2+ signaling to mitochondria when ER Ca2+ levels are reduced. On the other hand, it must also be taken into account that the enhanced recruitment of mitochondria close to ER could be simply the consequence of dysfunctional Ca2+ handling by the store, since mitochondria dynamics is tightly regulated by Ca2+.
3.2. Parkinson's disease
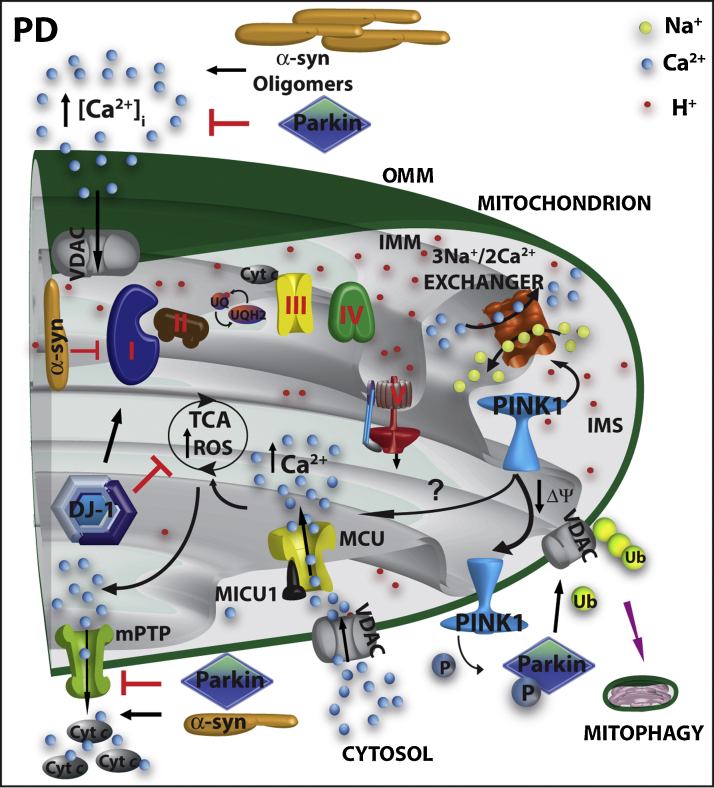
PD is the second most common progressing neurodegenerative disorder. It is characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta and by the accumulation of intracellular inclusions called Lewy bodies. Like the AD, most of the cases of PD are sporadic and probably caused by environmental toxins, but about 5–10% of the cases are of genetic origin. Evidence linking mitochondrial dysfunctions and oxidative stress with PD originated from a seminal study on chemically induced Parkinsonism in 1983, in which drug addicts were shown to develop rapid onset PD-like symptoms after injection of heroin contaminated with 1-methyl-4-phenyl-1,2,3,6-tetra-hydropyridine (MPTP) [108]. The study demonstrated that the active metabolite of MPTP was a potent inhibitor of complex I of the respiratory chain and a substrate of the dopamine transporter. It accumulated in dopaminergic neurons to which it conferred toxicity and eventually produced cell death. Further studies on humans and on animal models exposed to the complex I inhibitors rotenone, paraquat and 6-hydroxydopamine (6-OHDA) have linked mitochondrial exposure to toxins to the degeneration of dopaminergic neurons, dopamine depletion, and increased oxidative stress [109]. The subsequent discovery of hereditary forms of PD caused by dominant and recessive mutations in nuclear genes encoded proteins functionally related to mitochondria has added additional importance to the study of mitochondrial dysfunction in PD [110]. The products of two dominantly inherited genes, α-synuclein (α-syn) and LRRK2 (dardarin), and of several autosomal recessive inherited genes, DJ-1, parkin, PINK1, and Omi/HTRA2, have been found to be localized in, and/or to interfere with, mitochondria. The number of reports on this relationship is increasing exponentially: general consensus has now been reached on the role of PINK1/parkin in the regulation of mitophagy (see above), and emerging evidence indicates that also α-syn and DJ-1 may participate in the pathways of mitochondrial quality control by regulating mitochondrial shape.
Very few papers have considered the possibility that these proteins may also interfere with mitochondrial Ca2+ signaling. The proposed mechanisms by which this could occur are schematized in Fig. 3. α-syn has been described to modulate Ca2+ influx, suggesting that its oligomers could enhance the plasma membrane ion permeability [111] either by becoming directly inserted into the plasma membrane to form a pore [112], or by modulating the open probability of the plasma membrane Ca2+ channels [113]. However, the link with the PD pathogenesis is still missing, and controversial effects have been reported on the intracellular Ca2+ increase by PD-related α-syn mutants [111,113]. Recent studies have shown that α-syn can associate to mitochondria and that its accumulation increases mitochondrial Ca2+ levels and, as a consequence, nitric oxide levels, oxidative damage and cytochrome c release [114]. However, these observations are controversial [115] and we have obtained evidence that α-syn plays a dual opposite role on the modulation of mitochondrial Ca2+ accumulation depending on its level of expression and its intracellular distribution [116]. We have recently reported that moderate level of α-syn overexpression enhances mitochondrial Ca2+ homeostasis by augmenting ER-mitochondria contact sites thus, possibly, sustaining cell bioenergetics. Accordingly, α-syn “loss of function” impaired mitochondrial Ca2+ transients and enhanced autophagic process [116]. Interestingly, it has been recently shown that glial cells obtained from the mesencephalon of mice overexpressing mutated human α-syn, displayed severe mitochondrial damage, including morphological changes and reduced Ca2+-storage capacity [117]: the reduced mitochondrial Ca2+ buffering capacity could, in turn, lead to an increase in cytosolic Ca2+ with consequent calpain activation [118].
Fig. 3.
Mitochondrial dysfunctions and Ca2+ homeostasis in PD. α-syn monomers impair complex I and complex III activity, while oligomeric α-syn has been shown to potentiate intracellular Ca2+ influx through the VOC. DJ-1 scavenges mitochondrial ROS and sustain complex I activity. PINK1, possibly, modulates the activity of the mitochondrial Na+/Ca2+ exchanger and/or of the MCU. Additionally, together with parkin, it acts as a sensor to direct damaged mitochondria to the mitophagy process. Parkin attenuates protein misfolding and may protect against apoptotic stimuli, by preventing the opening of mPTP.
DJ-1 is a multifunctional protein and, despite its predominant role as an antioxidant [119], it also appears to have a role in maintaining the cytosolic basal Ca2+ concentration and to permit the depolarization-induced Ca2+ release from the SR in muscle cells [120]. An interesting link between DJ-1, Ca2+ handling and mitochondria has been reported by studies of the properties of L-type voltage dependent Ca2+ channels of dopaminergic neurons of the substantia nigra. These channels are characterized by a specific pore forming subunit (Cav1.3) which confers to the channel the property to be opened at relatively hyperpolarized potentials, allowing them to sustain the pacemaking activity which characterises these neurons [121]. The sustained engagement of these channels during pacemaking activity has an obvious high metabolic cost, and induces a very large Ca2+ penetration into the neurons. Surmeier and co-workers have elegantly demonstrated that, in dopaminergic neurons, the enhanced Ca2+ entry created an oxidant stress in mitochondria that thus became specifically vulnerable. Interestingly, the situation was further exacerbated by DJ-1 knockout, implying an essential role of DJ-1 in protecting dopaminergic neurons by Ca2+-induced mitochondrial uncoupling and ROS production during physiological pace-making [122].
More compelling evidence for a possible role of mitochondrial Ca2+ dysfunction in the pathogenesis of PD comes from studies on the mitochondrial kinase PINK1. The first suggestions arose from the finding that the expression of mutant, but not of wt, PINK1 exacerbated the mitochondrial defects observed in a cellular model of PD expressing mutated A53T α-syn. The defects, i.e., loss of ΔΨm, increased mitochondrial size with loss of cristae and reduced ATP levels, were partially recovered by cyclosporine A and, fully rescued by the inhibitor of MCU ruthenium red, thus leading to the suggestion that mitochondrial Ca2+ uptake was involved [123]. Other studies have expanded the investigation of the role of PINK1 in mitochondrial Ca2+ metabolism, but the results are controversial. In one case the absence of PINK1 induced mitochondrial Ca2+ accumulation, possibly as a consequence of the impairment of mitochondrial Ca2+ efflux through the mitochondrial Na+/Ca2+ exchanger [124]. In another study, PINK1 depletion impaired mitochondrial Ca2+ uptake (with no effect on Ca2+ extrusion) and consequently ATP production [125]. Further studies are evidently necessary to clarify the molecular targets of PINK1 action. However, it is interesting to note that an increased sensitivity of mitochondria to Ca2+-induced permeability has been shown to precede dopaminergic defects in PINK1-deficient mice, suggesting that mitochondrial Ca2+ degeneration could be an early event in the pathogenesis of PD [126].
3.3. Huntington's disease
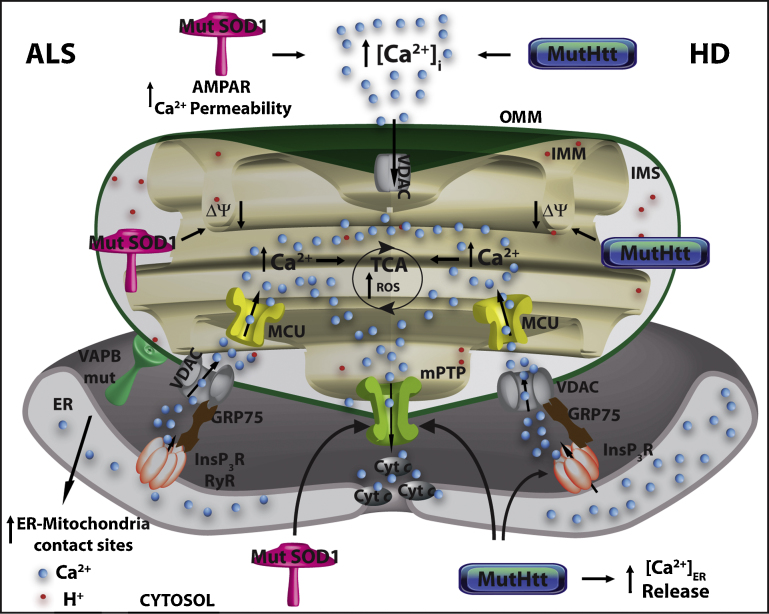
HD is an autosomal-dominant disease characterized by the loss of striatal GABAergic neurons. Major symptoms are motility impairment (chorea), dementia, and other neuropsychiatric defects. The genetic cause is the abnormal expansion of CAG repeats (corresponding to a poly-glutamine stretch (poly-Q)) in exon 1 of the gene coding for Huntingtin protein (Htt). The mutant forms of Htt contain more than 35 repeats and there is a direct correlation between the number of glutamines over 35 and the severity of the disease. Htt is a large protein (348 kDa) mainly localized in the cytosol, but it is also found in the ER, mitochondria, nucleus and Golgi compartment. Its function is presently unclear, although its involvement has been suggested in cellular pathways like transcription regulation, mitochondrial function, ROS production, apoptosis and organelles trafficking [127,128]. It is, nevertheless, essential to life: its homozygous deletion causes death at the embryonic age in mice, and its heterozygous deletion causes neurodegeneration [129]. The role of the poly-Q expansion at the N-terminal is not known, but it has been reported that its ablation in mice ameliorates motor/behavioral deficits, and extends lifespan [130]. The poly-Q expansion is cleaved off proteolitically, caspase 6 being most likely responsible for the cleavage [131]. The cleaved fragments increase probability of protein misfolding events and show propensity to aggregate and to form fibrils and oligomers [132,133]. However, whether the Htt fragment aggregates play a role in HD pathogenesis, and whether the oligomers (and/or aggregates) are toxic or protective, is still debated [133,134]. The link between Ca2+ homeostasis and HD has been established in a number of studies, most of them focused on mitochondrial Ca2+ handling dysfunction. The results have been controversial and no unequivocal conclusion can be drawn. Fig. 4 summarizes the main pathways that have been proposed, and a succinct overview of the data present in the literature will be presented here. As it was found in other neurodegenerative diseases, mitochondria isolated from lymphoblast of HD patients and from the brain of HD mice models have impaired ability to take up Ca2+. They are more susceptible to Ca2+ overload, with the vulnerability proportional to the length of the mutant Htt poly-Q expansion [135,136]. The matrix Ca2+ overload favors mPTP opening, which has been shown to be prevented by CsA in studies on mitochondria isolated from striatal neurons expressing mutant Htt [137] and from muscle of HD model R6/2 mice [138]. These findings have been confirmed also in cultured cells, e.g., the expression of mutant Htt in mouse immortalized striatal cells made mitochondria unable to handle large Ca2+ loads, very likely because of induced mPTP opening. The observed decrease of mitochondrial Ca2+, appears to be a compensatory attempt to prevent the mitochondrial Ca2+ stress that would irreversibly damage the organelles and eventually lead to cell death [69]. However, the ablation of cyclophilin D in HD mouse models failed to induce any improvement in the disease progression, despite the increased Ca2+-buffering capacity of their mitochondria [139–141]. These controversial findings are possibly related to the different experimental conditions, but also to the loss of the contribution of the ER compartment in experiments performed in isolated mitochondria. In fact, a number of reports have described the involvement of ER Ca2+ handling in HD cellular models. Htt was shown to influence intracellular Ca2+ signaling by acting on the InsP3R activity and expression [69,142–144], and striatal precursors of HD neurons display increased Ca2+ content in the ER [145] that could compromise mitochondria Ca2+ handling. Another aspect of mitochondria in other neurodegenerative diseases is shared by HD: the mitochondrial fission/fusion balance is affected by the presence of mutant Htt in different HD models, possibly as a consequence of the enhanced calcineurin activity, and thus of the calcineurin-dependent Drp1 recruitment to mitochondria. The enhanced mitochondria fragmentation led to enhanced apoptotic sensitivity, thus accounting for an early role of Ca2+ dysregulation in the death of striatal neurons [145].
Fig. 4.
Mitochondrial dysfunctions and Ca2+ homeostasis in ALS and HD. Mutant SOD1 in ALS and mutant Htt in HD enhance intracellular Ca2+ permeability, impair mitochondrial membrane potential and increase the susceptibility to mitochondrial Ca2+ overload, thus inducing mPTP opening, the release of cytochrome c (cyt c) and apoptosis. Mutant Htt increases ER Ca2+ release by acting on the InsP3R. Mutant VAPD (a protein found associated with mutant forms of ALS) enhances ER-mitochondria tethering, leading to an augmented mitochondrial Ca2+ uptake. Increased mitochondrial Ca2+ concentration, in turn, stimulates TCA cycle enzymes generating NADH and increasing ATP synthesis and ROS production.
3.4. Amyotrophic lateral sclerosis
ALS is a neurodegenerative disease caused by the progressive loss of motor neurons in motor cortex, brain stem and spinal cord [146]. Like other neurodegenerative disease, ALS presents alterations of mitochondrial morphology and function, accumulation of phosphorylated neurofilaments in the axons and somatic accumulation of protein inclusions formed by TDP-43 (TAR DNA binding protein-43) and ubiquitin [147–149]. ALS is a sporadic disease and despite several hypotheses implicating oxidative stress, proteasome dysfunction, glutamatergic excitotoxicity and mitochondria dysfunction, its etiology remains elusive [150]. However, in the last years, several mutations in some proteins were identified to be causally related to familial forms of ALS: among them, those in superoxide dismutase 1 (SOD1) are the most frequent, accounting for the 20% of the familial cases [151]. As previously mentioned, SOD1 is key enzyme in the defense against oxidative stress, as it converts the superoxide anion in hydrogen peroxide. It has been proposed that its mutations could cause a gain of toxic function [147]. At the moment, the best model for the study of the disease is represented by transgenic mice expressing mutant SOD1 [150].
To understand the role of Ca2+ dysfunction in ALS, it is important to underline that ALS-vulnerable motor neurons, like spinal and brain stem neurons, have very low Ca2+ buffering capacity as compared to not-vulnerable neurons. This specific characteristic accounts for the rapid and low cost recovery of Ca2+ transients that, in physiological condition, is necessary for the rapid relaxation times during high-frequency rhythmic activity. However, under pathological conditions it may generate the appearance of high Ca2+ concentration microdomains that would account for excitotoxic cell damage [152]. Another interesting characteristic of these neurons is their enrichment in Ca2+-permeable glutamate AMPA-receptor channels. Under overstimulation, they mediate glutamate-excitotoxicity, which results in selective motor neuron degeneration and death [153–155]. The Ca2+ permeability of AMPA receptor is regulated by the GluR2 subunit, and motor neurons express low amount of GluR2 mRNA in comparison with other neurons [156–158]. The GluR2 expression level is regulated by the astrocytes surrounding the motor neurons, and its Ca2+ permeability is controlled by a mechanism of RNA editing that replaces a positively charged arginine with a neutral glutamine, thus making the channel impermeable to Ca2+. Reduced editing results in acute neurodegeneration in mice and, accordingly, defective editing was detected in motor neurons of individuals affected by sporadic ALS [159]. The absence of the GluR2 subunit accelerated motor neurons degeneration in mutant SOD1 mice, whereas the induction of GluR2 expression increased life span of these mice [160,161]. It has also been shown that mutant SOD1 augmented AMPA receptor permeability to Ca2+ [162].
Experimental evidence shows enhancement of glutamate in motor neurons of patients affected by sporadic and genetic ALS [163,164] possibly due to the loss of the astroglial glutamate transporter GLT1 and thus to a decrease of glutamate uptake by the surrounding astrocytes. However, contrasting findings have shown that the chronic elevation of glutamate by the impairment of its transport has no effect on spinal motor neurons in vivo [165].
In the last years an increasing number of papers pointed out the importance of mitochondrial dysfunctions, in particular of defects in the electron transport chain and in the morphology of the organelles, both in sporadic and familial ALS [166]. Fig. 4 summarizes the main findings. As previously discussed, different mitochondrial populations may be strategically located close to the ER or to the plasma membrane Ca2+ channels to promptly buffer high Ca2+ microdomains generated by cell stimulation. This aspect is of particular relevance for neuronal cells that undergo continuous stimulation [167], and especially for motor neurons that have reduced levels of Ca2+ buffering proteins, like paravalbumin, calbindin, etc. [168,169]. Although not numerous, the studies on mitochondria support a causative role for Ca2+ overload in neurons from individuals affected by sporadic and genetic ALS. Mitochondria at the synapse of motoneurons of mutant SOD1 mice display a greater membrane potential depolarization after Ca2+ uptake, in line with the proposal that the Ca2+-buffering capacity of these organelles could be compromised [170]. Accordingly, the overexpression of mutant SOD1 in neuroblastoma cells increases the cytosolic Ca2+ level [171]. Emerging evidence thus supports the MCO hypothesis, in which mPTP opening, cytochrome c release, and activation of the apoptotic cascade are direct consequences. Interestingly, the ablation of cyclophilin D in SOD1 mutant mice delays disease onset, and CsA treatment is beneficial [172,173].
Finally, it is appropriate to mention another interesting possibility that may link mitochondrial Ca2+ dysfunction to ALS pathogenesis: the vesicle-associated membrane protein (VAMP)-associated protein B (VAPB) has been found to be mutated in familial ALS [174]. VAPB is an ER membrane anchored-protein which exposed its C-terminal domain on the cytosolic side [175] and has been reported to induce ER-stress and consequent Ca2+-mediated death in motor neurons [176]. It has then been found that VAPB is enriched in the MAMs, where it interacts with PTPIP51 (a mitochondrial outer membrane protein) and, where it is necessary to support ER-mitochondria Ca2+ transfer, since its siRNA-mediated silencing disrupts Ca2+ signaling between these two organelles [175]. Interestingly, a mutant form of VAPB (P56S), but not the wild-type protein, induces mitochondria clustering, impaired mitochondria Ca2+ uptake and increased cytosolic Ca2+ levels. In turn, these alterations result in an impairment of the anterograde axonal transport of mitochondria towards the cell periphery [177].
4. Conclusions
The picture emerging from the study of the pathogenesis of neurodegenerative conditions appears extremely complex and multifaceted. Intriguingly, common elements are shared by most of them making crucial to investigate on these aspects. Defects in the respiratory chain and oxidative stress have been recognized in almost all neurodegenerative disorders. However, reviewing the most recent literature, they do not appear as the primary cause, but rather the result of impaired mitochondria function. Compromised mitochondrial quality control and Ca2+ handling are instead suggested to be primary events. Therefore, any approach aimed at the modulation of mitochondrial dynamics and/or stimulation of mitochondrial biogenesis may be beneficial for the treatment of the diseases. Targeting mitochondrial Ca2+ dysregulation may represent a great challenge, at least to delay the insurgence of neurodegenerative symptoms and alleviate their progression. Thus, both in basic and applied research there is growing interest on this topic, further accentuated by the recent identification of key molecules (the NCX, MICU1, a uniporter regulator and MCU, the uniporter itself).
Conflict of interest statement
No conflict of interest statement.
Acknowledgements
The authors wish to thank Ernesto Carafoli (Venetian Institute of Molecular Medicine, Padova, Italy) for critical reading the manuscript.
Work performed by the authors on the topic was supported over the years by grants to M.B. from the Italian National Research Council (CNR), the Italian Ministry of University and Research (PRIN 2003, 2005 and 2008), the Telethon Foundation (Project GGP04169) and the local founding of the University of Padova (Progetto di Ateneo 2008).
References
- 1.Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Reviews Molecular Cell Biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 2.Berridge M.J. Inositol trisphosphate and calcium signalling mechanisms. Biochimica et Biophysica Acta. 2009;1793:933–940. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Brini M., Carafoli E. Calcium pumps in health and disease. Physiological Reviews. 2009;89:1341–1378. doi: 10.1152/physrev.00032.2008. [DOI] [PubMed] [Google Scholar]
- 4.Mammucari C., Patron M., Granatiero V., Rizzuto R. Molecules and roles of mitochondrial calcium signaling. Biofactors. 2011;37:219–227. doi: 10.1002/biof.160. [DOI] [PubMed] [Google Scholar]
- 5.Calì T., Ottolini D., Brini M. Mitochondrial Ca2+ as a key regulator of mitochondrial activities. Advances in mitochondrial medicine. Advances in Experimental Medicine and Biology Series. 2012;942:53–73. doi: 10.1007/978-94-007-2869-1_3. [DOI] [PubMed] [Google Scholar]
- 6.Schon E.A., Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drago I., Pizzo P., Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO Journal. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 13.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 14.de Brito O.M., Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 15.Hoth M., Fanger C.M., Lewis R.S. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. Journal of Cell Biology. 1997;137:633–648. doi: 10.1083/jcb.137.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzo P., Lissandron V., Capitanio P., Pozzan T. Ca(2+) signalling in the Golgi apparatus. Cell Calcium. 2011;50:184–192. doi: 10.1016/j.ceca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Ricchelli F., Sileikyte J., Bernardi P. Shedding light on the mitochondrial permeability transition. Biochimica et Biophysica Acta. 2011;1807:482–490. doi: 10.1016/j.bbabio.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Szabadkai G., Bianchi K., Varnai P., De Stefani D., Wieckowski M.R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. Journal of Cell Biology. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Hajnoczky G. Ca2+-dependent regulation of mitochondrial dynamics by the Miro–Milton complex. International Journal of Biochemistry and Cell Biology. 2009;41:1972–1976. doi: 10.1016/j.biocel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacAskill A.F., Kittler J.T. Control of mitochondrial transport and localization in neurons. Trends in Cell Biology. 2010;20:102–112. doi: 10.1016/j.tcb.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Saotome M., Safiulina D., Szabadkai G., Das S., Fransson A. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20728–20733. doi: 10.1073/pnas.0808953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friel D.D., Tsien R.W. An FCCP-sensitive Ca2+ store in bullfrog sympathetic neurons and its participation in stimulus-evoked changes in [Ca2+]i. Journal of Neuroscience. 1994;14:4007–4024. doi: 10.1523/JNEUROSCI.14-07-04007.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 24.Giacomello M., Drago I., Bortolozzi M., Scorzeto M., Gianelle A. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Molecular Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Glitsch M.D., Bakowski D., Parekh A.B. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO Journal. 2002;21:6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintana A., Schwarz E.C., Schwindling C., Lipp P., Kaestner L. Sustained activity of calcium release-activated calcium channels requires translocation of mitochondria to the plasma membrane. Journal of Biological Chemistry. 2006;281:40302–40309. doi: 10.1074/jbc.M607896200. [DOI] [PubMed] [Google Scholar]
- 27.Walsh C., Barrow S., Voronina S., Chvanov M., Petersen O.H. Modulation of calcium signalling by mitochondria. Biochimica et Biophysica Acta. 2009;1787:1374–1382. doi: 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Deisseroth K., Mermelstein P.G., Xia H., Tsien R.W. Signaling from synapse to nucleus: the logic behind the mechanisms. Current Opinion in Neurobiology. 2003;13:354–365. doi: 10.1016/s0959-4388(03)00076-x. [DOI] [PubMed] [Google Scholar]
- 29.Kim M.S., Usachev Y.M. Mitochondrial Ca2+ cycling facilitates activation of the transcription factor NFAT in sensory neurons. Journal of Neuroscience. 2009;29:12101–12114. doi: 10.1523/JNEUROSCI.3384-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z., Klionsky D.J. Mammalian autophagy: core molecular machinery and signaling regulation. Current Opinion in Cell Biology. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO Journal. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinton P., Rizzuto R. Bcl-2 and Ca2+ homeostasis in the endoplasmic reticulum. Cell Death and Differentiation. 2006;13:1409–1418. doi: 10.1038/sj.cdd.4401960. [DOI] [PubMed] [Google Scholar]
- 33.Vicencio J.M., Ortiz C., Criollo A., Jones A.W., Kepp O. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death and Differentiation. 2009;16:1006–1017. doi: 10.1038/cdd.2009.34. [DOI] [PubMed] [Google Scholar]
- 34.Cardenas C., Miller R.A., Smith I., Bui T., Molgo J. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakaki K., Wu J., Kaufman R.J. Protein kinase Ctheta is required for autophagy in response to stress in the endoplasmic reticulum. The Journal of Biological Chemistry. 2008;283:15370–15380. doi: 10.1074/jbc.M710209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decuypere J.P., Bultynck G., Parys J.B. A dual role for Ca(2+) in autophagy regulation. Cell Calcium. 2011;50:242–250. doi: 10.1016/j.ceca.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Imai Y., Lu B. Mitochondrial dynamics and mitophagy in Parkinson's disease: disordered cellular power plant becomes a big deal in a major movement disorder. Current Opinion in Neurobiology. 2011;21:935–941. doi: 10.1016/j.conb.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka A., Cleland M.M., Xu S., Narendra D.P., Suen D.F. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. Journal of Cell Biology. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gegg M.E., Cooper J.M., Chau K.Y., Rojo M., Schapira A.H. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Human Molecular Genetics. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan N.C., Salazar A.M., Pham A.H., Sweredoski M.J., Kolawa N.J. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Human Molecular Genetics. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., Song P., Du L., Tian W., Yue W. Parkin ubiquitinates Drp1 for proteasome-dependent degradation: implication of dysregulated mitochondrial dynamics in Parkinson disease. Journal of Biological Chemistry. 2011;286:11649–11658. doi: 10.1074/jbc.M110.144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geisler S., Holmstrom K.M., Skujat D., Fiesel F.C., Rothfuss O.C. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biology. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 43.Chen D., Gao F., Li B., Wang H., Xu Y. Parkin mono-ubiquitinates Bcl-2 and regulates autophagy. Journal of Biological Chemistry. 2010;285:38214–38223. doi: 10.1074/jbc.M110.101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y.L. PINK1 and Parkin Target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kornmann B., Osman C., Walter P. The conserved GTPase Gem1 regulates endoplasmic reticulum-mitochondria connections. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:14151–14156. doi: 10.1073/pnas.1111314108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White C., Li C., Yang J., Petrenko N.B., Madesh M. The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nature Cell Biology. 2005;7:1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danial N.N., Korsmeyer S.J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 48.Chami M., Prandini A., Campanella M., Pinton P., Szabadkai G. Bcl-2 and Bax exert opposing effects on Ca2+ signaling, which do not depend on their putative pore-forming region. Journal of Biological Chemistry. 2004;279:54581–54589. doi: 10.1074/jbc.M409663200. [DOI] [PubMed] [Google Scholar]
- 49.Scorrano L., Oakes S.A., Opferman J.T., Cheng E.H., Sorcinelli M.D. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 50.Pacher P., Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. EMBO Journal. 2001;20:4107–4121. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kroemer G., Reed J.C. Mitochondrial control of cell death. Nature Medicine. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 52.Pinton P., Giorgi C., Siviero R., Zecchini E., Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jahani-Asl A., Cheung E.C., Neuspiel M., MacLaurin J.G., Fortin A. Mitofusin 2 protects cerebellar granule neurons against injury-induced cell death. The Journal of Biological Chemistry. 2007;282:23788–23798. doi: 10.1074/jbc.M703812200. [DOI] [PubMed] [Google Scholar]
- 54.Barsoum M.J., Yuan H., Gerencser A.A., Liot G., Kushnareva Y. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. The EMBO Journal. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jahani-Asl A., Germain M., Slack R.S. Mitochondria: joining forces to thwart cell death. Biochimica et Biophysica Acta. 2010;1802:162–166. doi: 10.1016/j.bbadis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Lee H.C., Wei Y.H. Mitochondrial role in life and death of the cell. Journal of Biomedical Science. 2000;7:2–15. doi: 10.1007/BF02255913. [DOI] [PubMed] [Google Scholar]
- 57.Chang D.T., Rintoul G.L., Pandipati S., Reynolds IJ Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiology of Disease. 2006;22:388–400. doi: 10.1016/j.nbd.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Forte M., Gold B.G., Marracci G., Chaudhary P., Basso E. Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7558–7563. doi: 10.1073/pnas.0702228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weihofen A., Thomas K.J., Ostaszewski B.L., Cookson M.R., Selkoe D.J. Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry. 2009;48:2045–2052. doi: 10.1021/bi8019178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mattson M.P., Gleichmann M., Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rintoul G.L., Reynolds I.J. Mitochondrial trafficking and morphology in neuronal injury. Biochimica et Biophysica Acta. 2010;1802:143–150. doi: 10.1016/j.bbadis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Shankar G.M., Walsh D.M. Alzheimer's disease: synaptic dysfunction and Abeta. Molecular Neurodegeneration. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iijima-Ando K., Hearn S.A., Shenton C., Gatt A., Zhao L. Mitochondrial mislocalization underlies Abeta42-induced neuronal dysfunction in a Drosophila model of Alzheimer's disease. PLoS One. 2009;4:e8310. doi: 10.1371/journal.pone.0008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao X.L., Wang W.A., Tan J.X., Huang J.K., Zhang X. Expression of beta-amyloid induced age-dependent presynaptic and axonal changes in Drosophila. Journal of Neuroscience. 2010;30:1512–1522. doi: 10.1523/JNEUROSCI.3699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang X., Su B., Lee H.G., Li X., Perry G. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. Journal of Neuroscience. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nature Reviews Molecular Cell Biology. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whitworth A.J., Pallanck L.J. The PINK1/Parkin pathway: a mitochondrial quality control system? Journal of Bioenergetics and Biomembranes. 2009;41:499–503. doi: 10.1007/s10863-009-9253-3. [DOI] [PubMed] [Google Scholar]
- 68.Panov A.V., Gutekunst C.A., Leavitt B.R., Hayden M.R., Burke J.R. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nature Neuroscience. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 69.Lim D., Fedrizzi L., Tartari M., Zuccato C., Cattaneo E. Calcium homeostasis and mitochondrial dysfunction in striatal neurons of Huntington disease. Journal of Biological Chemistry. 2008;283:5780–5789. doi: 10.1074/jbc.M704704200. [DOI] [PubMed] [Google Scholar]
- 70.Faes L., Callewaert G. Mitochondrial dysfunction in familial amyotrophic lateral sclerosis. Journal of Bioenergetics and Biomembranes. 2011;43:587–592. doi: 10.1007/s10863-011-9393-0. [DOI] [PubMed] [Google Scholar]
- 71.Goedert M., Spillantini M.G. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- 72.LaFerla F.M., Green K.N., Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nature Reviews Neuroscience. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 73.Mattson M.P. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 75.Demuro A., Parker I., Stutzmann G.E. Calcium signaling and amyloid toxicity in Alzheimer disease. The Journal of Biological Chemistry. 2010;285:12463–12468. doi: 10.1074/jbc.R109.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kayed R., Head E., Thompson J.L., McIntire T.M., Milton S.C. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 77.Tolar M., Keller J.N., Chan S., Mattson M.P., Marques M.A. Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1999;19:7100–7110. doi: 10.1523/JNEUROSCI.19-16-07100.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boada M., Antunez C., Lopez-Arrieta J., Galan J.J., Moron F.J. CALHM1 P86L polymorphism is associated with late-onset Alzheimer's disease in a recessive model. Journal of Alzheimer's Disease. 2010;20:247–251. doi: 10.3233/JAD-2010-1357. [DOI] [PubMed] [Google Scholar]
- 79.Zatti G., Ghidoni R., Barbiero L., Binetti G., Pozzan T. The presenilin 2 M239I mutation associated with familial Alzheimer's disease reduces Ca2+ release from intracellular stores. Neurobiology of Disease. 2004;15:269–278. doi: 10.1016/j.nbd.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 80.Demuro A., Mina E., Kayed R., Milton S.C., Parker I. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. The Journal of Biological Chemistry. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 81.Khachaturian Z.S. Calcium hypothesis of Alzheimer's disease and brain aging. Annals of the New York Academy of Sciences. 1994;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- 82.Ferreiro E., Oliveira C.R., Pereira C.M. The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway. Neurobiology of Disease. 2008;30:331–342. doi: 10.1016/j.nbd.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Sanz-Blasco S., Valero R.A., Rodriguez-Crespo I., Villalobos C., Nunez L. Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs. PLoS One. 2008;3:e2718. doi: 10.1371/journal.pone.0002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirai K., Aliev G., Nunomura A., Fujioka H., Russell R.L. Mitochondrial abnormalities in Alzheimer's disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang X., Su B., Lee H.G., Li X., Perry G. Impaired balance of mitochondrial fission and fusion in Alzheimer's disease. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:9090–9103. doi: 10.1523/JNEUROSCI.1357-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 87.Wang X., Su B., Siedlak S.L., Moreira P.I., Fujioka H. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pigino G., Morfini G., Pelsman A., Mattson M.P., Brady S.T. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:4499–4508. doi: 10.1523/JNEUROSCI.23-11-04499.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brustovetsky N., Dubinsky J.M., Antonsson B., Jemmerson R. Two pathways for tBID-induced cytochrome c release from rat brain mitochondria: BAK- versus BAX-dependence. Journal of Neurochemistry. 2003;84:196–207. doi: 10.1046/j.1471-4159.2003.01545.x. [DOI] [PubMed] [Google Scholar]
- 90.Jiang D., Sullivan P.G., Sensi S.L., Steward O., Weiss J.H. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. The Journal of Biological Chemistry. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 91.Caspersen C., Wang N., Yao J., Sosunov A., Chen X. Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer's disease. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 92.Du H., Guo L., Fang F., Chen D., Sosunov A.A. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nature Medicine. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Annaert W.G., Levesque L., Craessaerts K., Dierinck I., Snellings G. Presenilin 1 controls gamma-secretase processing of amyloid precursor protein in pre-golgi compartments of hippocampal neurons. Journal of Cell Biology. 1999;147:277–294. doi: 10.1083/jcb.147.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ankarcrona M., Hultenby K. Presenilin-1 is located in rat mitochondria. Biochemical and Biophysical Research Communications. 2002;295:766–770. doi: 10.1016/s0006-291x(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 95.Hansson C.A., Frykman S., Farmery M.R., Tjernberg L.O., Nilsberth C. Nicastrin, presenilin, APH-1, and PEN-2 form active gamma-secretase complexes in mitochondria. The Journal of Biological Chemistry. 2004;279:51654–51660. doi: 10.1074/jbc.M404500200. [DOI] [PubMed] [Google Scholar]
- 96.Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S.F. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer's disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nelson O., Tu H., Lei T., Bentahir M., de Strooper B. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. Journal of Clinical Investigation. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giacomello M., Barbiero L., Zatti G., Squitti R., Binetti G. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer's disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiology of Disease. 2005;18:638–648. doi: 10.1016/j.nbd.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 99.Zatti G., Burgo A., Giacomello M., Barbiero L., Ghidoni R. Presenilin mutations linked to familial Alzheimer's disease reduce endoplasmic reticulum and Golgi apparatus calcium levels. Cell Calcium. 2006;39:539–550. doi: 10.1016/j.ceca.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 100.Lessard C.B., Lussier M.P., Cayouette S., Bourque G., Boulay G. The overexpression of presenilin2 and Alzheimer's-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cellular Signalling. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 101.Kasri N.N., Kocks S.L., Verbert L., Hebert S.S., Callewaert G. Up-regulation of inositol 1,4,5-trisphosphate receptor type 1 is responsible for a decreased endoplasmic-reticulum Ca2+ content in presenilin double knock-out cells. Cell Calcium. 2006;40:41–51. doi: 10.1016/j.ceca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 102.Shilling D., Mak D.O., Kang D.E., Foskett J.K. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. Journal of Biological Chemistry. 2012;287:10933–10934. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brunello L., Zampese E., Florean C., Pozzan T., Pizzo P. Presenilin-2 dampens intracellular Ca2+ stores by increasing Ca2+ leakage and reducing Ca2+ uptake. Journal of Cellular and Molecular Medicine. 2009;13:3358–3369. doi: 10.1111/j.1582-4934.2009.00755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheung K.H., Shineman D., Muller M., Cardenas C., Mei L. Mechanism of Ca2+ disruption in Alzheimer's disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheung K.H., Mei L., Mak D.O., Hayashi I., Iwatsubo T. Gain-of-function enhancement of IP3 receptor modal gating by familial Alzheimer's disease-linked presenilin mutants in human cells and mouse neurons. Science Signaling. 2010;3:ra22. doi: 10.1126/scisignal.2000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Area-Gomez E., de Groof A.J., Boldogh I., Bird T.D., Gibson G.E. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. American Journal of Pathology. 2009;175:1810–1816. doi: 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zampese E., Fasolato C., Kipanyula M.J., Bortolozzi M., Pozzan T. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2777–2782. doi: 10.1073/pnas.1100735108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Langston J.W., Ballard P.A., Jr. Parkinson's disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. New England Journal of Medicine. 1983;309:310. doi: 10.1056/nejm198308043090511. [DOI] [PubMed] [Google Scholar]
- 109.Cicchetti F., Lapointe N., Roberge-Tremblay A., Saint-Pierre M., Jimenez L. Systemic exposure to paraquat and maneb models early Parkinson's disease in young adult rats. Neurobiology of Disease. 2005;20:360–371. doi: 10.1016/j.nbd.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 110.DiMauro S., Schon E.A. Mitochondrial disorders in the nervous system. Annual Review of Neuroscience. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 111.Danzer K.M., Haasen D., Karow A.R., Moussaud S., Habeck M. Different species of alpha-synuclein oligomers induce calcium influx and seeding. Journal of Neuroscience. 2007;27:9220–9232. doi: 10.1523/JNEUROSCI.2617-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lashuel H.A., Petre B.M., Wall J., Simon M., Nowak R.J. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. Journal of Molecular Biology. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 113.Furukawa K., Matsuzaki-Kobayashi M., Hasegawa T., Kikuchi A., Sugeno N. Plasma membrane ion permeability induced by mutant alpha-synuclein contributes to the degeneration of neural cells. Journal of Neurochemistry. 2006;97:1071–1077. doi: 10.1111/j.1471-4159.2006.03803.x. [DOI] [PubMed] [Google Scholar]
- 114.Parihar M.S., Parihar A., Fujita M., Hashimoto M., Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. International Journal of Biochemistry and Cell Biology. 2009;41:2015–2024. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 115.Hettiarachchi N.T., Parker A., Dallas M.L., Pennington K., Hung C.C. alpha-Synuclein modulation of Ca2+ signaling in human neuroblastoma (SH-SY5Y) cells. Journal of Neurochemistry. 2009;111:1192–1201. doi: 10.1111/j.1471-4159.2009.06411.x. [DOI] [PubMed] [Google Scholar]
- 116.Cali T., Ottolini D., Negro A., Brini M. Alpha-synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. Journal of Biological Chemistry. 2012;287:17914–17929. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmidt S., Linnartz B., Mendritzki S., Sczepan T., Lubbert M. Genetic mouse models for Parkinson's disease display severe pathology in glial cell mitochondria. Human Molecular Genetics. 2011;20:1197–1211. doi: 10.1093/hmg/ddq564. [DOI] [PubMed] [Google Scholar]
- 118.Esteves A.R., Arduino D.M., Swerdlow R.H., Oliveira C.R., Cardoso S.M. Dysfunctional mitochondria uphold calpain activation: contribution to Parkinson's disease pathology. Neurobiology of Disease. 2010;37:723–730. doi: 10.1016/j.nbd.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 119.Taira T., Saito Y., Niki T., Iguchi-Ariga S.M., Takahashi K. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Reports. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shtifman A., Zhong N., Lopez J.R., Shen J., Xu J. Altered Ca2+ homeostasis in the skeletal muscle of DJ-1 null mice. Neurobiology of Aging. 2011;32:125–132. doi: 10.1016/j.neurobiolaging.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guzman J.N., Sanchez-Padilla J., Chan C.S., Surmeier D.J. Robust pacemaking in substantia nigra dopaminergic neurons. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:11011–11019. doi: 10.1523/JNEUROSCI.2519-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guzman J.N., Sanchez-Padilla J., Wokosin D., Kondapalli J., Ilijic E. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marongiu R., Spencer B., Crews L., Adame A., Patrick C. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson's disease by disturbing calcium flux. Journal of Neurochemistry. 2009;108:1561–1574. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E. PINK1-associated Parkinson's disease is caused by neuronal vulnerability to calcium-induced cell death. Molecular Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Heeman B., Van den Haute C., Aelvoet S.A., Valsecchi F., Rodenburg R.J. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. Journal of Cell Science. 2011;124:1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- 126.Akundi R.S., Huang Z., Eason J., Pandya J.D., Zhi L. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in pink1-deficient mice. PLoS One. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.The Huntington's Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 128.Giacomello M., Hudec R., Lopreiato R. Huntington's disease, calcium, and mitochondria. Biofactors. 2011;37:206–218. doi: 10.1002/biof.162. [DOI] [PubMed] [Google Scholar]
- 129.Thayer S.A., Miller R.J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. Journal of Physiology. 1990;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zheng S., Clabough E.B., Sarkar S., Futter M., Rubinsztein D.C. Deletion of the huntingtin polyglutamine stretch enhances neuronal autophagy and longevity in mice. PLoS Genetics. 2010;6:e1000838. doi: 10.1371/journal.pgen.1000838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Graham R.K., Deng Y., Slow E.J., Haigh B., Bissada N. Cleavage at the caspase-6 site is required for neuronal dysfunction and degeneration due to mutant huntingtin. Cell. 2006;125:1179–1191. doi: 10.1016/j.cell.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 132.Williams A.J., Paulson H.L. Polyglutamine neurodegeneration: protein misfolding revisited. Trends in Neurosciences. 2008;31:521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.DiFiglia M., Sapp E., Chase K.O., Davies S.W., Bates G.P. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 134.Martindale D., Hackam A., Wieczorek A., Ellerby L., Wellington C. Length of huntingtin and its polyglutamine tract influences localization and frequency of intracellular aggregates. Nature Genetics. 1998;18:150–154. doi: 10.1038/ng0298-150. [DOI] [PubMed] [Google Scholar]
- 135.Choo Y.S., Johnson G.V., MacDonald M., Detloff P.J., Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Human Molecular Genetics. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 136.Gellerich F.N., Gizatullina Z., Nguyen H.P., Trumbeckaite S., Vielhaber S. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. Journal of Biological Chemistry. 2008;283:30715–30724. doi: 10.1074/jbc.M709555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Milakovic T., Quintanilla R.A., Johnson G.V. Mutant huntingtin expression induces mitochondrial calcium handling defects in clonal striatal cells: functional consequences. Journal of Biological Chemistry. 2006;281:34785–34795. doi: 10.1074/jbc.M603845200. [DOI] [PubMed] [Google Scholar]
- 138.Gizatullina Z.Z., Lindenberg K.S., Harjes P., Chen Y., Kosinski C.M. Low stability of Huntington muscle mitochondria against Ca2+ in R6/2 mice. Annals of Neurology. 2006;59:407–411. doi: 10.1002/ana.20754. [DOI] [PubMed] [Google Scholar]
- 139.Brustovetsky N., LaFrance R., Purl K.J., Brustovetsky T., Keene C.D. Age-dependent changes in the calcium sensitivity of striatal mitochondria in mouse models of Huntington's disease. Journal of Neurochemistry. 2005;93:1361–1370. doi: 10.1111/j.1471-4159.2005.03036.x. [DOI] [PubMed] [Google Scholar]
- 140.Oliveira J.M., Jekabsons M.B., Chen S., Lin A., Rego A.C. Mitochondrial dysfunction in Huntington's disease: the bioenergetics of isolated and in situ mitochondria from transgenic mice. Journal of Neurochemistry. 2007;101:241–249. doi: 10.1111/j.1471-4159.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- 141.Perry G.M., Tallaksen-Greene S., Kumar A., Heng M.Y., Kneynsberg A. Mitochondrial calcium uptake capacity as a therapeutic target in the R6/2 mouse model of Huntington's disease. Human Molecular Genetics. 2010;19:3354–3371. doi: 10.1093/hmg/ddq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kaltenbach L.S., Romero E., Becklin R.R., Chettier R., Bell R. Huntingtin interacting proteins are genetic modifiers of neurodegeneration. PLoS Genetics. 2007;3:e82. doi: 10.1371/journal.pgen.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tang T.S., Tu H., Chan E.Y., Maximov A., Wang Z. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang H., Li Q., Graham R.K., Slow E., Hayden M.R. Full length mutant huntingtin is required for altered Ca2+ signaling and apoptosis of striatal neurons in the YAC mouse model of Huntington's disease. Neurobiology of Disease. 2008;31:80–88. doi: 10.1016/j.nbd.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Costa V., Giacomello M., Hudec R., Lopreiato R., Ermak G. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington's disease to apoptotic stimuli. EMBO Molecular Medicine. 2010;2:490–503. doi: 10.1002/emmm.201000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duffy L.M., Chapman A.L., Shaw P.J., Grierson A.J. Review: the role of mitochondria in the pathogenesis of amyotrophic lateral sclerosis. Neuropathology and Applied Neurobiology. 2011;37:336–352. doi: 10.1111/j.1365-2990.2011.01166.x. [DOI] [PubMed] [Google Scholar]
- 147.Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature Reviews Neuroscience. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 148.Wong P.C., Pardo C.A., Borchelt D.R., Lee M.K., Copeland N.G. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 149.Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochemical and Biophysical Research Communications. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 150.Dion P.A., Daoud H., Rouleau G.A. Genetics of motor neuron disorders: new insights into pathogenic mechanisms. Nature Reviews Genetics. 2009;10:769–782. doi: 10.1038/nrg2680. [DOI] [PubMed] [Google Scholar]
- 151.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 152.von Lewinski F., Keller B.U. Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends in Neurosciences. 2005;28:494–500. doi: 10.1016/j.tins.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 153.Ikonomidou C., Qin Qin Y., Labruyere J., Olney J.W. Motor neuron degeneration induced by excitotoxin agonists has features in common with those seen in the SOD-1 transgenic mouse model of amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology. 1996;55:211–224. doi: 10.1097/00005072-199602000-00010. [DOI] [PubMed] [Google Scholar]
- 154.Rothstein J.D., Jin L., Dykes-Hoberg M., Kuncl R.W. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carriedo S.G., Sensi S.L., Yin H.Z., Weiss J.H. AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro. Journal of Neuroscience. 2000;20:240–250. doi: 10.1523/JNEUROSCI.20-01-00240.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Van Damme P., Van Den Bosch L., Van Houtte E., Callewaert G., Robberecht W. GluR2-dependent properties of AMPA receptors determine the selective vulnerability of motor neurons to excitotoxicity. Journal of Neurophysiology. 2002;88:1279–1287. doi: 10.1152/jn.2002.88.3.1279. [DOI] [PubMed] [Google Scholar]
- 157.Heath P.R., Tomkins J., Ince P.G., Shaw P.J. Quantitative assessment of AMPA receptor mRNA in human spinal motor neurons isolated by laser capture microdissection. Neuroreport. 2002;13:1753–1757. doi: 10.1097/00001756-200210070-00012. [DOI] [PubMed] [Google Scholar]
- 158.Kawahara Y., Kwak S., Sun H., Ito K., Hashida H. Human spinal motoneurons express low relative abundance of GluR2 mRNA: an implication for excitotoxicity in ALS. Journal of Neurochemistry. 2003;85:680–689. doi: 10.1046/j.1471-4159.2003.01703.x. [DOI] [PubMed] [Google Scholar]
- 159.Kawahara Y., Ito K., Sun H., Aizawa H., Kanazawa I. Glutamate receptors: RNA editing and death of motor neurons. Nature. 2004;427:801. doi: 10.1038/427801a. [DOI] [PubMed] [Google Scholar]
- 160.Van Damme P., Braeken D., Callewaert G., Robberecht W., Van Den Bosch L. GluR2 deficiency accelerates motor neuron degeneration in a mouse model of amyotrophic lateral sclerosis. Journal of Neuropathology and Experimental Neurology. 2005;64:605–612. doi: 10.1097/01.jnen.0000171647.09589.07. [DOI] [PubMed] [Google Scholar]
- 161.Tateno M., Sadakata H., Tanaka M., Itohara S., Shin R.M. Calcium-permeable AMPA receptors promote misfolding of mutant SOD1 protein and development of amyotrophic lateral sclerosis in a transgenic mouse model. Human Molecular Genetics. 2004;13:2183–2196. doi: 10.1093/hmg/ddh246. [DOI] [PubMed] [Google Scholar]
- 162.Spalloni A., Albo F., Ferrari F., Mercuri N., Bernardi G. Cu/Zn-superoxide dismutase (GLY93→ALA) mutation alters AMPA receptor subunit expression and function and potentiates kainate-mediated toxicity in motor neurons in culture. Neurobiology of Disease. 2004;15:340–350. doi: 10.1016/j.nbd.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 163.Rothstein J.D., Van Kammen M., Levey A.I., Martin L.J., Kuncl R.W. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Annals of Neurology. 1995;38:73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 164.Rothstein J.D., Martin L.J., Kuncl R.W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. New England Journal of Medicine. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 165.Tovar Y.R.L.B., Santa-Cruz L.D., Zepeda A., Tapia R. Chronic elevation of extracellular glutamate due to transport blockade is innocuous for spinal motoneurons in vivo. Neurochemistry International. 2009;54:186–191. doi: 10.1016/j.neuint.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 166.Grosskreutz J., Van Den Bosch L., Keller B.U. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 167.Bianchi K., Rimessi A., Prandini A., Szabadkai G., Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochimica et Biophysica Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 168.Appel B., Givan L.A., Eisen J.S. Delta-Notch signaling and lateral inhibition in zebrafish spinal cord development. BMC Developmental Biology. 2001;1:13. doi: 10.1186/1471-213X-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fahandejsaadi A., Leung E., Rahaii R., Bu J., Geula C. Calbindin-D28K, parvalbumin and calretinin in primate lower motor neurons. Neuroreport. 2004;15:443–448. doi: 10.1097/00001756-200403010-00012. [DOI] [PubMed] [Google Scholar]
- 170.David G. Mitochondrial clearance of cytosolic Ca(2+) in stimulated lizard motor nerve terminals proceeds without progressive elevation of mitochondrial matrix [Ca(2+)] Journal of Neuroscience. 1999;19:7495–7506. doi: 10.1523/JNEUROSCI.19-17-07495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Carri M.T., Ferri A., Battistoni A., Famhy L., Gabbianelli R. Expression of a Cu,Zn superoxide dismutase typical of familial amyotrophic lateral sclerosis induces mitochondrial alteration and increase of cytosolic Ca2+ concentration in transfected neuroblastoma SH-SY5Y cells. FEBS Letters. 1997;414:365–368. doi: 10.1016/s0014-5793(97)01051-x. [DOI] [PubMed] [Google Scholar]
- 172.Martin L.J., Gertz B., Pan Y., Price A.C., Molkentin J.D. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Experimental Neurology. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kirkinezos I.G., Hernandez D., Bradley W.G., Moraes C.T. An ALS mouse model with a permeable blood–brain barrier benefits from systemic cyclosporine A treatment. Journal of Neurochemistry. 2004;88:821–826. doi: 10.1046/j.1471-4159.2003.02181.x. [DOI] [PubMed] [Google Scholar]
- 174.Nishimura A.L., Mitne-Neto M., Silva H.C., Richieri-Costa A., Middleton S. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. American Journal of Human Genetics. 2004;75:822–831. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.De Vos K.J., Morotz G.M., Stoica R., Tudor E.L., Lau K.F. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Human Molecular Genetics. 2012;21:1299–12311. doi: 10.1093/hmg/ddr559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Langou K., Moumen A., Pellegrino C., Aebischer J., Medina I. AAV-mediated expression of wild-type and ALS-linked mutant VAPB selectively triggers death of motoneurons through a Ca2+-dependent ER-associated pathway. Journal of Neurochemistry. 2010;114:795–809. doi: 10.1111/j.1471-4159.2010.06806.x. [DOI] [PubMed] [Google Scholar]
- 177.Morotz G.M., De Vos K.J., Vagnoni A., Ackerley S., Shaw C.E. Amyotrophic lateral sclerosis-associated mutant VAPBP56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Human Molecular Genetics. 2012;21:1979–1988. doi: 10.1093/hmg/dds011. [DOI] [PMC free article] [PubMed] [Google Scholar]