Abstract
Stimulation of mitochondrial oxidative metabolism by Ca2+ is now generally recognised as important for the control of cellular ATP homeostasis. Here, we review the mechanisms through which Ca2+ regulates mitochondrial ATP synthesis. We focus on cardiac myocytes and pancreatic β-cells, where tight control of this process is likely to play an important role in the response to rapid changes in workload and to nutrient stimulation, respectively. We also describe a novel approach for imaging the Ca2+-dependent regulation of ATP levels dynamically in single cells.
Abbreviations: [ATP/ADP]c, cytosolic ATP/ADP ratio; [Ca2+]c, [Ca2+]m, cytosolic and mitochondrial free concentrations of calcium; EC, excitation-contraction; ICEU, intracellular energy unit; KATP, ATP-sensitive K+ channel; MCU, mitochondrial uniporter; mNCX/NCLX, mitochondrial sodium-calcium exchanger; Pi, PPi, inorganic phosphate and pyrophosphate, respectively; RuR, ruthenium red
Keywords: ATP, Mitochondria, Calcium, Heart, Beta cell, Islet, Insulin, Secretion, Diabetes
1. Introduction
Mitochondria are the major site of oxidative metabolism, and hence ATP synthesis, in eukaryotic cells. Thus, whereas the glycolytic metabolism of glucose generates 2 ATP molecules, 36–38 are generated by the reactions of the citrate cycle and the oxidation of the resulting NADH and FADH2 by the respiratory chain [1]. The latter reactions are tightly controlled by intramitochondrial Ca2+ in a mechanism designed to ensure that ATP synthesis is closely coupled to the cell's energetic needs. We discuss this form of regulation in detail here, taking as examples two cell types with contrasting energetic needs, the cardiac myocyte and the pancreatic β cell.
2. Ca2+ regulation of intramitochondrial oxidative metabolism: a brief historical perspective
Mitochondrial oxidative metabolism has long been recognised as subject to complex regulation by several factors, notably the concentrations of ADP and substrate(s) [2]. However, regulation by Ca2+ has emerged in recent years as a further important means of controlling this vital aspect of cell function. Mammalian mitochondria have a huge capacity for Ca2+ uptake and for many years it was thought that these organelles served as mobilisable intracellular reservoirs of Ca2+ ions [3]. Studies in the 1970s and 80s by Denton and McCormack [4] as well as Hansford and Castro [5] and Crompton [6] indicated instead that under basal conditions total mitochondrial Ca2+ content was low and that increases in cytosolic free Ca2+ ([Ca2+]c) in response to extrinsic agents (nutrients, hormones, neurotransmitters, etc.), were likely to provoke increases in intramitochondrial free Ca2+ ([Ca2+]m) concentrations. Thus, based on the properties of a group of key intramitochondrial dehydrogenases (later characterised in some detail – see the following sections) [7,8], it was proposed that the uptake of Ca2+ ions from the cytosol increased [Ca2+]m from ∼0.1 to 10 μM or more. The consequent activation of oxidative metabolism would then provide an increased supply of reducing equivalents to drive respiratory chain activity and ATP synthesis. Export of mitochondrial ATP in exchange for ADP was anticipated then to meet an increased ATP demand to fuel energy-requiring processes in the cytosol such as ion pumping, contraction, exocytosis etc. The biochemical analyses (e.g. [9]) upon which the above model was based were subsequently complemented and reinforced in the 1990s by highly selective measurements of intramitochondrial free Ca2+ in living cells by Rizzuto, Pozzan, Rutter and others [10,11]. These experiments used recombinant expression of the Ca2+-sensitive photoprotein aequorin, targeted to the mitochondrial matrix by in-frame fusion with the signal peptide of cytochrome c subunit VIII. Later extended by Rutter et al. to measurements at the level of single cells [12], this approach provided evidence that basal intramitochondrial Ca2+ levels were indeed low (similar to or lower than those in the cytosol, i.e. ≤100 nM) and that the free concentrations of the ion increased within mitochondria in response to Ca2+-mobilising agonists. Subsequent refinements of these approaches, including the use of GFP-based probes, highlighted the importance of close contacts between the endoplasmic reticulum and mitochondria in the uptake of Ca2+ by the latter [13] and demonstrated that mitochondrial Ca2+ increases stably elevate mitochondrial [ATP] [14].
3. Mitochondrial Ca2+ transport: roles of the mitochondrial uniporter MCU and the Na+/Ca2+ exchanger, mNCX/NCLX
An impediment to progress in this field has been that the mitochondrial Ca2+ transport proteins were not purified or cloned, and that use of the inhibitors of these pathways is problematic in living cells. However, recent work has very likely identified the proteins responsible for both the Ca2+ influx and Ca2+ efflux pathways in mitochondria (see below).
Increases in [Ca2+]c are relayed to the mitochondria by the mitochondrial Ca2+ uniporter (MCU), resulting in activation of mitochondrial dehydrogenases and stimulation of ATP synthesis. Average [Ca2+]m is controlled by the activities of the MCU and the efflux pathway, the mitochondrial Na+/Ca2+ exchanger (mNCX). Ca2+ uptake is respiration-dependent, thus high rates of uptake can effectively lower the mitochondrial membrane potential (ΔΨm) (reviewed in [15]). This phenomenon can be observed clearly in isolated mitochondria at supraphysiological concentrations of extramitochondrial Ca2+; however, under physiological conditions of Ca2+ uptake, a change in ΔΨm has not been detected in isolated cardiomyocytes using fluorescent indicators. Thus, Ca2+ uptake would not normally be expected to lower the membrane potential, at least for a sustained period, or to such an extent that it would inhibit ATP synthesis. Nonetheless, transient and small decreases in mitochondrial membrane potential may occur during cytosolic Ca2+ peaks in other systems, and can be observed in pancreatic β cells by following rhodamine-123 accumulation [16] or sensitive fluorescent ATP probes (Tarasov and Rutter, unpublished and see below).
The Ca2+ transport pathways of mitochondria are covered in more detail by other reviews in this issue. However, a brief discussion of their properties specifically with regard to energy production will be given here.
Early studies on isolated mitochondria characterised the MCU as a low affinity, high capacity transporter of Ca2+, whereas the mNCX had a much lower Vmax, saturating at [Ca2+]m below 1 μM (reviewed in [17,15]). Thus, it was predicted that net Ca2+ influx would occur only when external [Ca2+] rose above about 500 nM, much higher than normal resting [Ca2+]c of 100–200 nM [18]. Studies in living adult rat myocytes [Ca2+]m confirmed this prediction, since [Ca2+]m remained less than [Ca2+]c until the latter rose above 500 nM [18]. However, the seminal paper by Rizzuto, Pozzan and colleagues in 1992, using aequorin targeted to mitochondria, revealed that these organelles were capable of taking up Ca2+ on a fast timescale, due to their proximity to intracellular Ca2+ stores [10] so they would effectively “see” much higher [Ca2+]c than that of the bulk cytosol. In some cells and species [Ca2+]m has been reported to change in response to physiological, submicromolar, changes in [Ca2+]c; but, in cardiomyocytes for example, the reflected changes in [Ca2+]m were small, 10–20 nM [19].
Research into the mitochondrial Ca2+ transporters is now likely to undergo another step change, with the identification of proteins corresponding to both MCU and mNCX. Two papers published simultaneously in 2011 identified a Ca2+ uptake pathway in mitochondria, inhibited by ruthenium red: Baughman et al. [20] identified the MCU as a novel predicted transmembrane protein (CCD109A) that formed oligomers in the inner membrane, and interacted with their previously identified regulator protein, MICU1. Silencing MCU in cultured cells or in vivo in mouse liver abrogated Ca2+ uptake, but membrane potential and respiration remain intact. A point mutation removed the sensitivity to ruthenium red (RuR). The other paper [21] identified MCU as a 40 kD protein with 2 transmembrane regions. RNA silencing of MCU in HeLa cells reduced mitochondrial Ca2+ uptake, and purified MCU reconstituted in bilayers exhibited Ca2+ channel activity that was sensitive to RuR.
In 2010, Palty et al. [22] identified NCLX in mitochondria – this protein is a novel NCX, lying in an ancestral branch of the NCX superfamily. The protein occurred as 50, 70 and 100 kD forms in mitochondrial fractions of mouse heart and brain; the 100 kD form likely representing dimers. The authors demonstrated that mitochondrial Ca2+ efflux was enhanced upon over-expression of NCLX in HEK 293 cells and inhibited by CGP 371457, and that Ca2+ efflux was greatly reduced using an anti-NCLX siRNA.
4. Ca2+ sensitive mitochondrial enzymes
4.1. FAD-glycerol phosphate dehydrogenase (FAD-GPDH)
Physiological levels of [Ca2+]c regulate FAD-GPDH (K0.5 ≃ 0.1 μM in rat liver mitochondrial extracts [23]), via a cytosol-facing EF-hand binding site for Ca2+ [24]. Increases in extramitochondrial Ca2+ lowered the KM for DL-glycerolphosphate (K0.5 = 30–100 nM) in permeabilized INS1 β cells [25].
4.2. Pyruvate dehydrogenase phosphatase (PDHP)
The 50 MDa pyruvate dehydrogenase (PDH) multi-enzyme complex catalyses the irreversible reaction: pyruvate + CoA + NAD → acetyl-CoA + NADH2 + CO2, with the product, acetyl-CoA, then entering the citrate cycle or fatty acid synthesis. The complex comprises multiple copies of three enzymes: E1 (that decarboxylates pyruvate); E2 (forms acetyl-CoA) and E3 (reduces NAD+ to NADH).
The activity of the PDH complex is a rate-limiting step for glucose oxidation and is therefore reflected in the rate of respiration and ATP synthesis in mammalian tissues [26]. The regulation of the PDH complex activity is achieved via end-product inhibition and reversible phosphorylation by a PDH kinase, which inhibits the activity of E1 subunit via phosphorylation at three sites around S293 [27,28]. A Mg2+-dependent PDH phosphatase, in turn, dephosphorylates the E1 subunit.
The activity of the Ca2+-regulated phosphatase depends on the association of the catalytic subunit of the predominating isoform 1 of PHD phosphatase (PDHP1c) and the L2 domain of the E2 subunit of the PDH complex, which is potentiated by Ca2+ [29]. PDHP1c and the L2 domain of the E2 subunit lack Ca2+ binding motifs so the binding site for the cation is likely to be formed jointly by residues of both interacting proteins [30]. Ca2+ activates the phosphatase in heart mitochondrial extracts with K0.5 of ∼1 μM [31] and extramitochondrial Ca2+ stimulates phosphatase activity over the physiological range (0.1–1.0 μM), in rat heart [32] liver [33] and adipose tissue [34] mitochondria.
4.3. NAD+-isocitrate dehydrogenase (NAD-ICDH)
NAD-ICDH is a hetero-octamer consisting (in eukaryotes) of 2 × (2α,β,γ) subunits [35]. The enzyme binds ∼2Ca2+/octamer [7] but the binding site is as yet unknown. In the presence of ADP, Ca2+ decreased the KM for isocitrate ∼8-fold [36]) with a K0.5 for Ca2+ of 1.2 μM in mitochondrial extracts [37] and 5–43 μM (increasing with ATP/ADP ratio), in toluene-permeabilised mitochondria [38]. ADP (or ATP) and Mg2+-isocitrate are required for Ca2+ binding [7]. The lower sensitivity of this enzyme to Ca2+ compared to other intramitochondrial dehydrogenases may extend the range over which Ca2+ is able to modulate oxidative metabolism by these organelles [8].
4.4. 2-Oxoglutarate dehydrogenase (OGDH)
The OGDH complex, similarly to the PDH complex, consists of multiple copies of three subunits: E1 (α-ketoacid decarboxylase), E2 (dihydrolipoyl transacetylase) and E3 (dihydrolipoamide dehydrogenase) [39]. The activity of the complex is directly regulated by Ca2+, most likely via the E1 subunit [40] (K0.5 = 1–7 μM in mitochondrial extracts) [7]. Each enzyme complex binds ∼3.5Ca2+ ions, suggestive of a Ca2+ binding site at E1 dimer interfaces [7]. Ca2+ decreases the KM for 2-oxoglutarate with little effect on Vmax [41]. The K0.5 for extramitochondrial Ca2+ is ∼0.15 μM in intact heart mitochondria [37,42].
4.5. F1–FO ATP synthase
The possibility that the rate of ATP production can be regulated independently from the respiration rate or mitochondrial membrane potential (Δψm) has been demonstrated in intact mitochondria [43] and confirmed using control theory analysis [44]. Early in vitro [45] and in vivo [46] studies reported F1–FO synthase as a potential target of this regulation. Although shown to bind Ca2+ directly [47], the F1–FO ATP synthase is likely to be regulated via post-translational modifications and phosphorylation of the γ-subunit was sensitive to mitochondrial Ca2+ [48] with a K0.5 in the μM range. Recent work has identified a protein that binds to the F1–FO synthase in Ca2+-dependent manner, resulting in an increased capacity for ATP production [49].
4.6. Other potential mitochondrial targets for Ca2+
Cytochrome c oxidase (COX) is allosterically inhibited by ATP/ADP (binding to the matrix domain of subunit IV). This inhibition is switched on by the cAMP-dependent phosphorylation of subunits II (and/or) III and Vb by protein kinase A (PKA), and results in an enhanced H+/e-stoichiometry and therefore more efficient energy transduction. Ca2+-dependent phosphatase reverses the cAMP-mediated effect (in vitro or in isolated mitochondria) [50]. cAMP is likely to stimulate the phosphorylation at Tyr304 of subunit I, which decreases the Vmax and increases the KM. COX binds Ca2+ with a Kd of around 1 μM, competitively with Na+ [51].
Malate–aspartate shuttle activity. Ca2+ activates this shuttle in brain mitochondria (K0.5 = 0.3 μM) [52]. The aspartate–glutamate transporter has a cytosolic EF domain and is also regulated by [Ca2+]c, albeit in the supraphysiological range [53].
Pyrophosphatase activity in liver and heart mitochondria is inhibited by Ca2+, due to competition of CaPPi with MgPPi (K0.5 = 12 μM) [54]. The accumulation of PPi is expected to result in an increase in mitochondrial volume and activation of the respiratory chain.
The ATP-Mg2+/Pi transporter (SCaMC1). This transporter is stimulated by extramitochondrial Ca2+ and may regulated the total content of adenine nucleotides inside mitochondria, and hence alter Ca2+ buffering. SCaMC1 is overexpressed in some cancer cell lines, and this has been proposed to contribute to the ability of the cells to withstand Ca2+ loads that would otherwise trigger opening of the mitochondrial permeability transition pore [56].
Ca2+ reportedly potentiates fatty acid oxidation to β-hydroxybutyrate [55] though the underlying mechanisms are not understood.
5. Role of mitochondrial Ca2+ transport in specific cell types
5.1. Cardiac myocyte
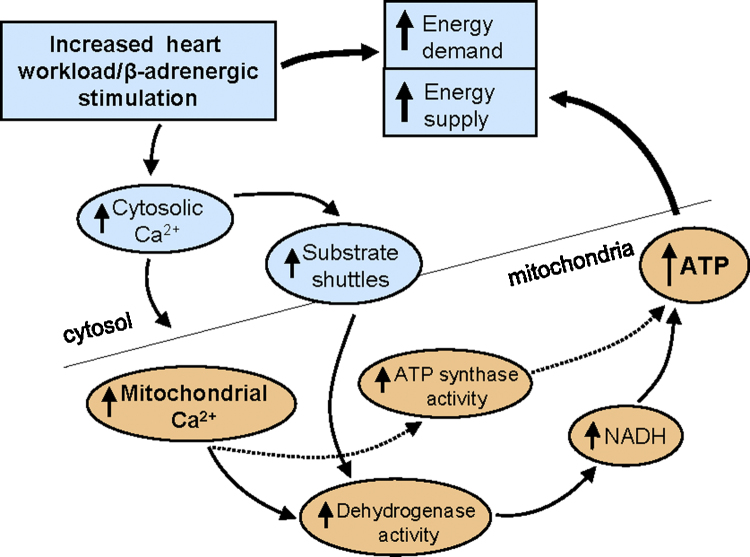
Fig. 1 presents a summary of the model proposed for mitochondrial Ca2+ uptake in the control of ATP synthesis in the heart.
Fig. 1.
Role of Ca2+ uptake by mitochondria in the heart.
Oxidative phosphorylation in mitochondria provides over 90% of ATP production in the heart [45]. The overall equation is given below, although estimates of the number of ATP molecules synthesised per molecule of NADH vary:
| NADH + H+ + O2 + 2.5ADP + 2.5Pi → NAD+ + H2O + 2.5 ATP |
ATP production can potentially be regulated by several different mechanisms, such as increases in NADH/NAD+ and ADP/ATP. It was originally proposed that changes in the ADP/ATP ratio were the main regulator of ATP synthesis [2], and this was easily demonstrated in isolated mitochondria. However, later experiments using isolated beating rat hearts found that high energy phosphate levels did not change under conditions that imposed a large increase in energy demand, e.g. increases in workload of the heart [57,58]. In fact utilisation of ATP under these conditions was exactly matched by an increase in ATP production. Changes in ATP are initially buffered by the phosphocreatine system but this only lasts for a few seconds, implying the existence of alternative mechanisms for rapidly increasing ATP synthesis.
This role was later found to be fulfilled by intramitochondrial Ca2+, following the discovery of the activation by Ca2+ of mitochondrial enzymes, as discussed above, namely the matrix dehydrogenases PDH [31], NAD-ICDH [37], OGDH [9], and possibly the ATP synthase [59]. The observations led Denton, McCormack and colleagues to propose that an increase in the supply of reducing equivalents in the form of NADH and FADH2 resulting from Ca2+ activation of the dehydrogenases would increase ATP production; thus suggesting a parallel activation model of stimulation of contraction and ATP synthesis by Ca2+ [60,61] (and see Fig. 1). The mitochondrial F1F0ATP synthase (ATPase) may also be stimulated by Ca2+ in this setting [43,47,62,63].
Whether Ca2+ transport into mitochondria occurs in the heart, and whether or not [Ca2+]m changes on a beat-to-beat basis in this organ, has remained controversial for some time. Based on work in isolated mitochondria, the MCU and mNCX pathways could certainly not respond quickly enough to the very rapid (ms) changes in [Ca2+]c that occur during excitation-contraction (EC) coupling. However, more recent work using isolated myocytes has suggested that mitochondrial Ca2+ transients do occur during EC coupling in neonatal and adult cardiac myocytes [64–66]. There is conflicting data on this in the literature since other studies reported that mitochondrial Ca2+ accumulation occurred over tens of seconds in cardiac myocytes [18,67,68]. Part of the conflict may be due to different species or stage of development (neonate versus adult), although why and how mitochondrial Ca2+ spiking occurs in some species but not others remains unknown. However, there is agreement that the main role of MCU and mNCX under physiological conditions is to translate or decode cytosolic Ca2+ signals in the mitochondria, so that Ca2+ activation of mitochondrial metabolism occurs.
In addition to the known proximity of mitochondria to the ER in a variety of cell types, there is now evidence for a direct physical coupling that persists during isolation of mitochondria (reviewed in [69]). This evidence was provided in heart by isolation of mitochondrial fractions with associated SR components that were highly resistant to purification [70]. These “SR appendices” were capable of transferring Ca2+ directly to mitochondria (measured with rhod-2 fluorescence) upon stimulation with caffeine (which activated the ryanodine receptor, thus triggering release of Ca2+ from the SR), and of increasing the NADH autofluorescence signal, suggesting activation of the dehydrogenases. This was the first direct evidence that Ca2+ transfer directly from the SR to mitochondria is capable of activating oxidative metabolism [70].
Activation of ATP synthesis in the heart by mitochondrial Ca2+. Parallel measurements of [Ca2+]m and [Ca2+]c in rat myocytes revealed that resting [Ca2+]m was about 80 nM compared with resting [Ca2+]c of 150 nM [18]. Whereas in mitochondria isolated from beating hearts the [Ca2+]m was estimated to be slightly higher, about 170 nM [71]. Upon rapid stimulation in presence of an adrenergic agonist (myocytes), or increased workload (isolated hearts), the value of [Ca2+]m increased to 500–1000 nM [18,71]. These values of [Ca2+]m under physiological conditions are within the range for activation of the dehydrogenases (see above).
There is also evidence to suggest that extramitochondrial Ca2+ regulates substrate supply into mitochondria via the aspartate–glutamate carrier (aralar) and the malate–aspartate shuttle (see above). This newer mechanism has been proposed to act as a “gas pedal, supplying…substrate on demand” [72].
A problem in elucidating the relationship between [Ca2+]m and [ATP] was that although ATP has been measured in whole hearts, and now in animals and humans using non-invasive 31P-NMR (reviewed in [73]), only relatively slow responses were measured, and so it could not be determined whether ATP was varying beat-to-beat, or during the time taken for mitochondria to accumulate sufficient Ca2+ to activate the dehydrogenases.
We directly measured ATP in mitochondria and cytosol, [ATP]m and [ATP]c, respectively, using targeted luciferase [64], and [Ca2+]m and [Ca2+]c using targeted aequorin: in adult cardiac myocytes stimulated to contract at 2 Hz, there was no change in [ATP] in either compartment on addition of isoproterenol, despite an increase in contractile force and increases in both [Ca2+]m and [Ca2+]c. This indicated that [ATP]c was extremely well-buffered in myocytes, i.e. that the rate of ATP supply and/or synthesis can keep pace with that of ATP breakdown, even under conditions that imposed a large energy demand on the cell. This lack of change in [ATP] is in agreement of studies using whole hearts [74].
However, when we allowed cells to rest, then suddenly stimulated to contract in the presence of isoproterenol (“standing-start” experiments), [ATP]m showed a significant transient drop (up to 22% in some cells) followed by recovery to higher than initial levels [64]. These changes likely reflect an initial activation of ATP-requiring processes in the cytosol, such as ion pumps and contractile proteins, which would cause a drop in [ATP]m before a time-dependent activation of mitochondrial oxidative metabolism by Ca2+ stimulated ATP synthesis [75]. The changes in [ATP]c were much smaller than those in the mitochondrial matrix, again suggesting that even when [ATP]m does change, the cytosolic energy supply is very rapidly matched to the increased demand. The lag phase where [ATP]m falls before recovering, exactly matches the time course for [Ca2+]m to increase [64,76].
Similarly it has been generally observed that under conditions of adequate oxygen consumption, NAD(P)H levels remain constant in beating hearts and myocytes, again highlighting that ATP supply and demand are extremely well-coupled. The only time NAD(P)H was observed to decrease was in similar experiments to the “standing start” experiments described above, where there was an initial drop in NAD(P)H levels before recovery, again being slightly preceded by an increase in [Ca2+]m [77].
Do ADP and Ca2+ have joint role in regulating ATP production in myocytes? Balaban [73] has argued that, given the importance of ensuring a rapid response system of ATP synthesis in the myocardium, more than one mechanism is likely to operate to coordinate ATP supply and demand. Whether [Ca2+]m is absolutely required to maintain ATP levels during continuous or large increases in energy demand is not yet known and the idea of [Ca2+]m being the only factor that is important physiologically in regulating ATP supply and demand is not universally accepted. Our own studies show that in rat cardiac myocytes when stimulation rate is increased from 0.2 Hz to 4 Hz, which presumably causes a large increase in ATP demand, there is very little change in [Ca2+]m unless an adrenergic agonist is also present [67]; presumably this may increase the systolic [Ca2+]c sufficiently that MCU is activated to increase [Ca2+]m.
One possibility is again that of the subcellular arrangement of mitochondria and proximity to other cell compartments: Saks and colleagues have put forward the idea that the subcellular architecture changes during contraction, causing a decrease in the apparent KM of ADP for stimulating respiration, and removing diffusion limits of ADP that occur in resting cells (reviewed in [78]). This requires the mitochondria to be organised in structural units with ATP consuming processes in the cell, such as myofibrils, SR and sarcolemmal ATPases, so-called “intracellular energy units (ICEUs)” – where channelling of ADP occurs.
This group also argues that [Ca2+]m may not be the sole factor, or possibly not a major factor, in regulating ATP supply and demand in the heart, since modelling studies have predicted that the increase in [Ca2+]m in the heart would only be enough to stimulate respiration 2–3 fold, whereas increases of 10–20-fold are seen in vivo [43,79]. Therefore localised regulation of [ADP] in the ICEUs would present a method of stimulating ATP synthesis by mitochondria without changes in bulk [ADP] or [ATP] [80]. However, it is not clear how far these studies can be extrapolated to the physiological situation of rapidly beating cardiac myocytes, either cells or whole hearts that are continually shortening and re-lengthening. If the authors are correct, the theory also implies that control of respiration by ADP is operating on a millisecond timescale (in rat cardiac myocytes, for example). Alternatively, respiration could be sensitive to the time-averaged local [ADP], in a similar manner as we suggested above for rapid changes in [Ca2+]m.
Implications for cardiac disease. Pathologically, an increase in [Ca2+]m has been associated with the transition from reversible to irreversible cell injury in ischaemia/reperfusion injury of the heart, and a major contributing factor to this injury is Ca2+-induced opening of the mitochondrial permeability transition pore [81,82]. A role for [Ca2+]m in heart failure, and for the mitochondrial Ca2+ efflux pathway in the treatment of this condition has also been suggested [83].
Both MCU and mNCX have been proposed as possible targets for cardioprotective drugs (reviewed in [76]). However, protective effects of RuR have been found in ischaemia reperfusion injury ([84,85]), although it is not certain that the drug was acting on MCU since it is not specific and also affects SR Ca2+ flux [86]. Indeed we found, using a myocyte model of hypoxia/reoxygenation injury, that RuR was not protective [87], but that inhibiting the mNCX with clonazepam was. We concluded that the route of Ca2+ entry into mitochondria under hypoxic conditions is likely to be the mNCX rather than MCU [87,88].
The mNCX has also been considered a target for cardioprotection in heart failure. Recent work from O’Rourke's group has shown that dysregulation of Na+ homeostasis in heart failure may be a primary cause of mitochondrial dysfunction: in a guinea-pig model of heart failure (induced by aortic constriction), intracellular [Na+] was 16 mM compared with 5 mM in control cells [89]. Rapid pacing of the cells induced a decrease in NADH fluorescence, an indirect indicator of respiratory chain activity, whereas this was maintained in controls. CGP 37157, an inhibitor of mNCX, was able to prevent the decrease in NADH in the failing myocytes. It is thus likely to restore ATP levels although this has yet to be shown directly: earlier work showed that the mNCX is capable of regulating [Ca2+]m and dehydrogenase activity since adding Na+ to isolated mitochondria shifts the activation curves for PDH and OGDH by Ca2+ to the right [32].
5.2. The pancreatic islet β-cell
Insulin secretion from pancreatic β-cells is prompted by increases in blood glucose over the physiological range (4–8 mM), thanks to the expression of high KM glucose transporters (Glut2 in rodents) and glucokinase [90]. Defective β-cell glucose sensitivity [91,92] as well as a decrease in numbers of these fuel-sensing cells [93,94] result in hyperglycaemia and eventually type 2 diabetes.
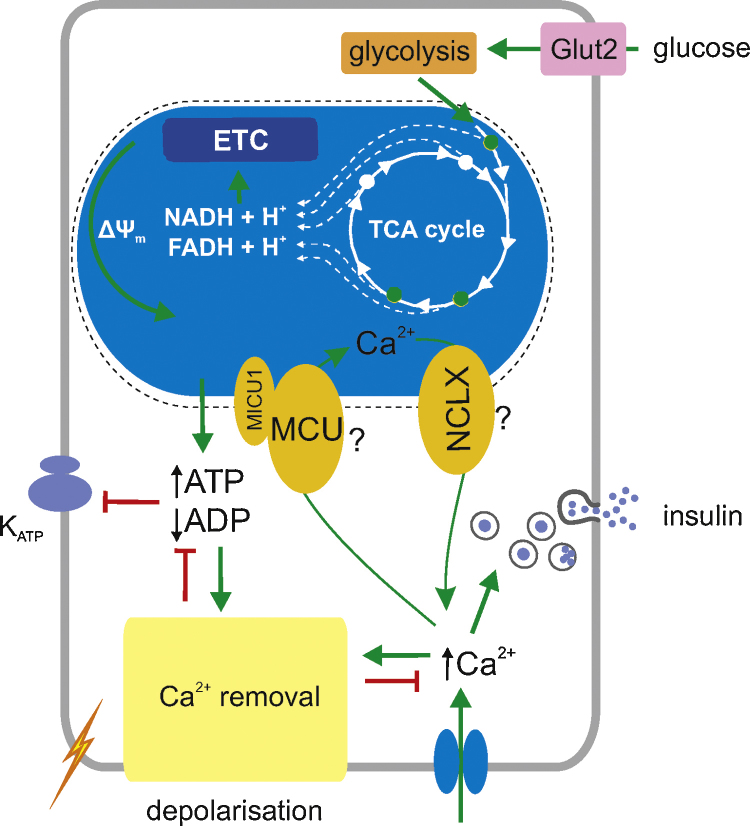
The metabolic configuration of β-cells is adapted to favour the complete oxidation of glucose by mitochondria [95] through the suppression of genes involved in the production of lactate (LDHA and the plasma membrane monocarboxylate transporter, SLC16A1/MCT1) [96–99], and the expression at high levels of FAD-GPDH [24,25,100]. Consequently, increases in extracellular glucose are obligatorily linked to increased flux through the citrate cycle [100] and lead to clear elevations in cytosolic ATP/ADP ratio [101], which block ATP-sensitive K+ (KATP) channels on the plasma membrane [102]. This triggers plasma membrane depolarisation, electrical activity and Ca2+ influx into the cytosol via voltage-gated Ca2+ channels [103]. The elevated [Ca2+]c then triggers exocytosis of insulin granules [90,104] (Fig. 2). Other, more poorly defined “KATP-independent” effects of glucose, possibly involving the inhibition of AMP-activated protein kinase [105,106], also contribute to “amplifying” effects of the sugar on secretion [107]. Changes in ATP/ADP ratio also regulate exocytosis directly [108], modulating the effects of cAMP [109,110].
Fig. 2.
Potential role of Ca2+ uptake by mitochondria in the pancreatic β-cell. ETC, electron transport chain. See the text for further details of the Ca2+ sensitive intramitochondrial dehydrogenases.
In contrast to most tissues including the heart (see above), cytosolic ATP changes over a relatively wide range in β-cells [111] and this is likely to play a key-signalling role. Our early measurements of glucose-induced ATP dynamics in MIN6 and primary islet β cells [75,112,113], using the recombinant bioluminescent reporter firely luciferase (an approach whose sensitivity is relatively poor), suggested a monophasic elevation of ATP occurs in response to high glucose, although evidence for oscillations was also obtained [113,114]. Significant differences between the glucose-induced changes in the bulk cytosol and a sub-plasma membrane “microdomain”, as well as the mitochondrial matrix, were also demonstrated [75]. Importantly, blockade of Ca2+ influx with EGTA or through the inhibition of voltage-gated Ca2+ channels substantially inhibited glucose-induced ATP/ADP increases, implicating a role for Ca2+ influx in metabolic control [113].
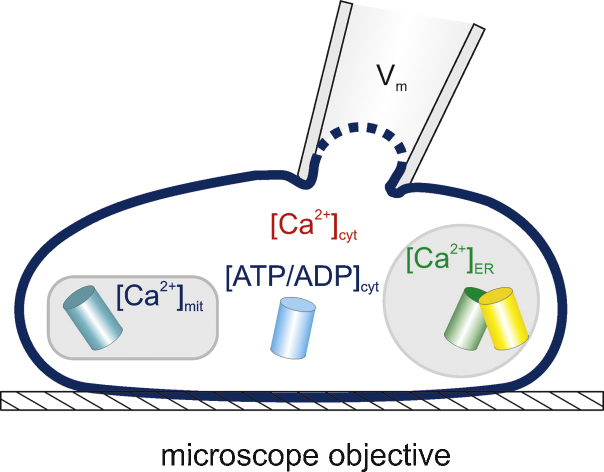
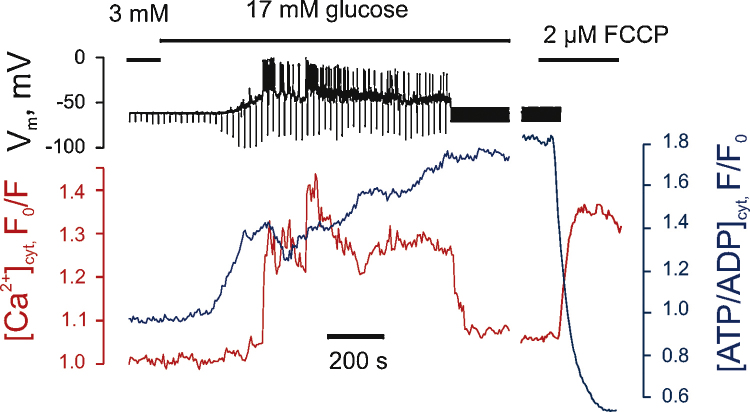
In light of the above limitations, we have recently established a technique whereby [Ca2+]m can be imaged in real time in single primary living β-cells using a mitochondrially targeted, adenovirally-delivered and GFP-based reporter (“Pericam”; 2mt8RP) simultaneously with [Ca2+]c measured with the trappable small molecule, Fura Red (Fig. 3). Importantly, after the formation of a perforated patch, imaging of the two probes can be performed simultaneously with recordings or manipulation (at will) of plasma membrane potential. This approach demonstrates that [Ca2+]m is unable to track fast oscillations of [Ca2+]c, imposed by single action potentials that have the duration of 50–200 ms in β-cells [115]. However, [Ca2+]m does follow slow changes in [Ca2+]c similar to those associated with slow electrical bursting [115]. This “accumulation” mechanism makes [Ca2+]m highly sensitive to the frequency of Ca2+ oscillations [115] (Fig. 4), such that low frequency spiking barely affects [Ca2+]m. This “Frequency-Amplitude” demodulation may thus allow filtering of low level stimulation (for example at low glucose concentrations) whilst amplifying the response as glucose levels rise – thus contributing to the well-known highly sigmoidal response of insulin secretion to blood glucose levels [95]. This positive feedback may play an important role in coordinating insulin secretion in pancreatic islets where β-cells are electrically coupled [116]. Using the same approach to combine imaging of [Ca2+]c and [ATP/ADP]c, using the genetically encoded fluorescent sensor Perceval [117], we have been able to demonstrate a biphasic increase in [ATP/ADP]c in response to high glucose, with Ca2+ entry into cytosol being the key factor for the second phase [115] (Fig. 5).
Fig. 3.
Principle of simultaneous patch-clamp recording and multiparameter imaging of compartmentalised Ca2+ and ATP/ADP in single cells.
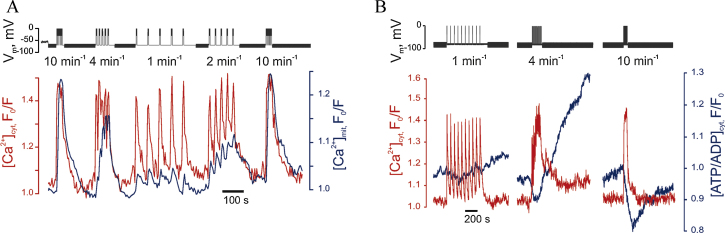
Fig. 4.
Mitochondrial Ca2+ (A) and [ATP/ADP]cyt (B) increases respond to the frequency of electrical bursting and Ca2+ oscillations in a single primary mouse β-cell. Depolarisations of the plasma membrane were applied at different frequency from 10 min−1 to 1 min−1[115].
Fig. 5.
Multiphasic increases in [ATP/ADP]c are prompted by high glucose in the β-cell. Recordings and image collection were as in Fig. 4; note the second phase of ATP/ADP increase ∼400 s after the increase in glucose [115].
These observations are consistent with a model for the β-cell in which elevation of [Ca2+]c leads firstly to the activation of a number of processes (e.g. Ca2+ pumping [118,119]) that elevate ATP consumption [101,113,115]. This is then matched by a Ca2+-dependent potentiation of mitochondrial metabolism [115,120]. The existence of these two phases thus generates a biphasic increase in [ATP/ADP]c. (Fig. 5). Whilst the role in stimulus-secretion coupling of the second phase is unclear, an intriguing possibility is that it is involved in the mobilisation of secretory granules from a “reserve pool” in the cell [95].
Consistent with these findings, silencing of MCU [115], or deployment of an intramitochondrial Ca2+ buffer [120], blocks both mitochondrial Ca2+ uptake and the second phase of ATP/ADP increase. Interestingly, glucolipotoxic conditions mimicking the diabetic milieu and known to affect mitochondrial integrity [121], suppress [Ca2+]m changes and selectively reduce the second phase of [ATP/ADP]c increase [115]. Defective mitochondrial Ca2+ handling may thus play a part in the defects in insulin secretion in type 2 diabetes.
6. Conclusions
There is no doubt that [Ca2+]m plays an important role in both myocardial energy production and in intracellular Ca2+ signalling in many other systems including the pancreatic β-cell. Future challenges will involve further dissection of the molecular mechanisms involved (including identification of the Ca2+ binding sites on the mitochondrial dehydrogenases) and exploration of the potential therapeutic use of new information flowing from the identification of the molecular players in Ca2+ transport.
Conflict of interest
None of the authors declares any conflict of interest.
Acknowledgements
GAR and AIT thank the Royal Society and the Juvenile Diabetes Research Foundation respectively for a Wolfson Research Merit Award and a post-doctoral Fellowship. Work in GAR's laboratory is supported by the Wellcome Trust (Programme 081958/Z/07/Z), DiabetesUK (Project 11/0004210) and the European Association for the Study of Diabetes (EFSD). EJG was supported by the British Heart Foundation, the Biotechnology and Biological Sciences Research Council, and the Medical Research Council (U.K.). We would like to thank Professor Richard M. Denton (University of Bristol) for critical comments on the text.
References
- 1.Berg J.M., Tymoczko J.L., Stryer L., Gatto G.J. W.H. Freeman; New York: 2002. Biochemistry. [Google Scholar]
- 2.Chance B., Williams G.R. Respiratory enzymes in oxidative phosphorylation. VI. The effects of adenosine diphosphate on azide-treated mitochondria. J. Biol. Chem. 1956;221:477–489. [PubMed] [Google Scholar]
- 3.Lehninger A.L., Carafoli E., Rossi C.S. Energy-linked ion movements in mitochondrial systems. Adv. Enzymol. Relat. Areas Mol. Biol. 1967;29:259–320. doi: 10.1002/9780470122747.ch6. [DOI] [PubMed] [Google Scholar]
- 4.Denton R.M., McCormack J.G. On the role of the calcium transport cycle in heart and other mammalian mitochondria. FEBS Lett. 1980;119:1–8. doi: 10.1016/0014-5793(80)80986-0. [DOI] [PubMed] [Google Scholar]
- 5.Hansford R.G., Castro F. Effects of micromolar concentrations of free calcium ions on the reduction of heart mitochondrial NAD(P) by 2-oxoglutarate. Biochem. J. 1981;198:525–533. doi: 10.1042/bj1980525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crompton M. The Regulation of Mitochondrial Calcium Transport in Heart. Curr. Top. Membr. Transport. 1985;25:231. [Google Scholar]
- 7.Rutter G.A., Denton R.M. The binding of Ca2+ ions to pig heart NAD+-isocitrate dehydrogenase and the 2-oxoglutarate dehydrogenase complex. Biochem. J. 1989;263:453–462. doi: 10.1042/bj2630453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter G.A. Ca2(+)-binding to citrate cycle dehydrogenases. Int. J. Biochem. 1990;22:1081–1088. doi: 10.1016/0020-711x(90)90105-c. [DOI] [PubMed] [Google Scholar]
- 9.McCormack J.G., Denton R.M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzuto R., Simpson A.W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 11.Rutter G.A., Theler J.M., Murgia M., Wollheim C.B., Pozzan T., Rizzuto R. Stimulated Ca2+ influx raises mitochondrial free Ca2+ to supramicromolar levels in a pancreatic beta-cell line. Possible role in glucose and agonist-induced insulin secretion. J. Biol. Chem. 1993;268:22385–22390. [PubMed] [Google Scholar]
- 12.Rutter G.A., Burnett P., Rizzuto R. Subcellular imaging of intramitochondrial Ca2+ with recombinant targeted aequorin: significance for the regulation of pyruvate dehydrogenase activity. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5489–5494. doi: 10.1073/pnas.93.11.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- 14.Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunter T.E., Pfeiffer D.R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 16.Luciani D.S., Misler S., Polonsky K.S. Ca2+ controls slow NAD(P)H oscillations in glucose-stimulated mouse pancreatic islets. J. Physiol. 2006;572:379–392. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholls D.G., Crompton M. Mitochondrial calcium transport. FEBS Lett. 1980;111:261–268. doi: 10.1016/0014-5793(80)80806-4. [DOI] [PubMed] [Google Scholar]
- 18.Miyata H., Silverman H.S., Sollott S.J., Lakatta E.G., Stern M.D., Hansford R.G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am. J. Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 19.Andrienko T.N., Picht E., Bers D.M. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J. Mol. Cell. Cardiol. 2009;46:1027–1036. doi: 10.1016/j.yjmcc.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baughman J.M., Perocchi F., Girgis H.S. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De S.D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palty R., Silverman W.F., Hershfinkel M. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. U.S.A. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wernette M.E., Ochs R.S., Lardy H.A. Ca2+ stimulation of rat liver mitochondrial glycerophosphate dehydrogenase. J. Biol. Chem. 1981;256:12767–12771. [PubMed] [Google Scholar]
- 24.MacDonald M.J., Brown L.J. Calcium activation of mitochondrial glycerol phosphate dehydrogenase restudied. Arch. Biochem. Biophys. 1996;326:79–84. doi: 10.1006/abbi.1996.0049. [DOI] [PubMed] [Google Scholar]
- 25.Rutter G.A., Pralong W.F., Wollheim C.B. Regulation of mitochondrial glycerol-phosphate dehydrogenase by Ca2+ within electropermeabilized insulin-secreting cells (INS-1) Biochim. Biophys. Acta. 1992;1175:107–113. doi: 10.1016/0167-4889(92)90016-5. [DOI] [PubMed] [Google Scholar]
- 26.Randle P.J. Metabolic fuel selection: general integration at the whole-body level. Proc. Nutr. Soc. 1995;54:317–327. doi: 10.1079/pns19950057. [DOI] [PubMed] [Google Scholar]
- 27.Sale G.J., Randle P.J. Occupancy of phosphorylation sites in pyruvate dehydrogenase phosphate complex in rat heart in vivo. Relation to proportion of inactive complex and rate of re-activation by phosphatase. Biochem. J. 1982;206:221–229. doi: 10.1042/bj2060221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fries M., Chauhan H.J., Domingo G.J., Jung H.I., Perham R.N. Site-directed mutagenesis of a loop at the active site of E1 (alpha2beta2) of the pyruvate dehydrogenase complex. A possible common sequence motif. Eur. J. Biochem. 2003;270:861–870. doi: 10.1046/j.1432-1033.2003.03444.x. [DOI] [PubMed] [Google Scholar]
- 29.Turkan A., Hiromasa Y., Roche T.E. Formation of a complex of the catalytic subunit of pyruvate dehydrogenase phosphatase isoform 1 (PDP1c) and the L2 domain forms a Ca2+ binding site and captures PDP1c as a monomer. Biochemistry. 2004;43:15073–15085. doi: 10.1021/bi048901y. [DOI] [PubMed] [Google Scholar]
- 30.Vassylyev D.G., Symersky J. Crystal structure of pyruvate dehydrogenase phosphatase 1 and its functional implications. J. Mol. Biol. 2007;370:417–426. doi: 10.1016/j.jmb.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton R.M., Randle P.J., Martin B.R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem. J. 1972;128:161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denton R.M., McCormack J.G., Edgell N.J. Role of calcium ions in the regulation of intramitochondrial metabolism. Effects of Na+, Mg2+ and ruthenium red on the Ca2+-stimulated oxidation of oxoglutarate and on pyruvate dehydrogenase activity in intact rat heart mitochondria. Biochem. J. 1980;190:107–117. doi: 10.1042/bj1900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormack J.G. Characterization of the effects of Ca2+ on the intramitochondrial Ca2+-sensitive enzymes from rat liver and within intact rat liver mitochondria. Biochem. J. 1985;231:581–595. doi: 10.1042/bj2310581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall S.E., McCormack J.G., Denton R.M. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat epididymal adipose tissue. Evidence against a role for Ca2+ in the activation of pyruvate dehydrogenase by insulin. Biochem. J. 1984;218:249–260. doi: 10.1042/bj2180249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramachandran N., Colman R.F. Chemical characterization of distinct subunits of pig heart DPN-specific isocitrate dehydrogenase. J. Biol. Chem. 1980;255:8859–8864. [PubMed] [Google Scholar]
- 36.Gabriel J.L., Plaut G.W. Inhibition of bovine heart NAD-specific isocitrate dehydrogenase by reduced pyridine nucleotides: modulation of inhibition by ADP, NAD+, Ca2+, citrate, and isocitrate. Biochemistry. 1984;23:2773–2778. doi: 10.1021/bi00307a037. [DOI] [PubMed] [Google Scholar]
- 37.Denton R.M., Richards D.A., Chin J.G. Calcium ions and the regulation of NAD+ -linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem. J. 1978;176:899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutter G.A., Midgley P.J., Denton R.M. Regulation of the pyruvate dehydrogenase complex by Ca2+ within toluene-permeabilized heart mitochondria. Biochim. Biophys. Acta. 1989;1014:263–270. doi: 10.1016/0167-4889(89)90222-x. [DOI] [PubMed] [Google Scholar]
- 39.McLain A.L., Szweda P.A., Szweda L.I. alpha-Ketoglutarate dehydrogenase: a mitochondrial redox sensor. Free Radic. Res. 2011;45:29–36. doi: 10.3109/10715762.2010.534163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawlis V.B., Roche T.E. Regulation of bovine kidney alpha-ketoglutarate dehydrogenase complex by calcium ion and adenine nucleotides. Effects on S0. 5 for alpha-ketoglutarate. Biochemistry. 1981;20:2512–2518. doi: 10.1021/bi00512a023. [DOI] [PubMed] [Google Scholar]
- 41.Lawlis V.B., Roche T.E. Effect of micromolar Ca2+ on NADH inhibition of bovine kidney alpha-ketoglutarate dehydrogenase complex and possible role of Ca2+ in signal amplification. Mol. Cell. Biochem. 1980;32:147–152. doi: 10.1007/BF00227441. [DOI] [PubMed] [Google Scholar]
- 42.Wan B., LaNoue K.F., Cheung J.Y., Scaduto R.C., Jr. Regulation of citric acid cycle by calcium. J. Biol. Chem. 1989;264:13430–13439. [PubMed] [Google Scholar]
- 43.Territo P.R., Mootha V.K., French S.A., Balaban R.S. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am. J. Physiol. Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 44.Mildaziene V., Baniene R., Nauciene Z. Ca2+ stimulates both the respiratory and phosphorylation subsystems in rat heart mitochondria. Biochem. J. 1996;320(Pt 1):329–334. doi: 10.1042/bj3200329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris D.A., Das A.M. Control of mitochondrial ATP synthesis in the heart. Biochem. J. 1991;280(Pt 3):561–573. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholz T.D., Balaban R.S. Mitochondrial F1-ATPase activity of canine myocardium: effects of hypoxia and stimulation. Am. J. Physiol. 1994;266:H2396–H2403. doi: 10.1152/ajpheart.1994.266.6.H2396. [DOI] [PubMed] [Google Scholar]
- 47.Hubbard M.J., McHugh N.J. Mitochondrial ATP synthase F1-beta-subunit is a calcium-binding protein. FEBS Lett. 1996;391:323–329. doi: 10.1016/0014-5793(96)00767-3. [DOI] [PubMed] [Google Scholar]
- 48.Hopper R.K., Carroll S., Aponte A.M. Mitochondrial matrix phosphoproteome: effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boerries M., Most P., Gledhill J.R. Ca2+-dependent interaction of S100A1 with F1-ATPase leads to an increased ATP content in cardiomyocytes. Mol. Cell. Biol. 2007;27:4365–4373. doi: 10.1128/MCB.02045-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bender E., Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 51.Kirichenko A.V., Pfitzner U., Ludwig B., Soares C.M., Vygodina T.V., Konstantinov A.A. Cytochrome c oxidase as a calcium binding protein. Studies on the role of a conserved aspartate in helices XI–XII cytoplasmic loop in cation binding. Biochemistry. 2005;44:12391–12401. doi: 10.1021/bi050376v. [DOI] [PubMed] [Google Scholar]
- 52.Contreras L., Gomez-Puertas P., Iijima M., Kobayashi K., Saheki T., Satrustegui J. Ca2+ Activation kinetics of the two aspartate-glutamate mitochondrial carriers, aralar and citrin: role in the heart malate-aspartate NADH shuttle. J. Biol. Chem. 2007;282:7098–7106. doi: 10.1074/jbc.M610491200. [DOI] [PubMed] [Google Scholar]
- 53.Palmieri L., Pardo B., Lasorsa F.M. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davidson A.M., Halestrap A.P. Inhibition of mitochondrial-matrix inorganic pyrophosphatase by physiological [Ca2+], and its role in the hormonal regulation of mitochondrial matrix volume. Biochem. J. 1989;258:817–821. doi: 10.1042/bj2580817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Otto D.A., Ontko J.A. Activation of mitochondrial fatty acid oxidation by calcium. Conversion to the energized state. J. Biol. Chem. 1978;253:789–799. [PubMed] [Google Scholar]
- 56.Traba J., Del A.A., Duchen M.R., Szabadkai G., Satrustegui J. SCaMC-1 promotes cancer cell survival by desensitizing mitochondrial permeability transition via ATP/ADP-mediated matrix Ca(2+) buffering. Cell Death Differ. 2011:10. doi: 10.1038/cdd.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neely J.R., Denton R.M., England P.J., Randle P.J. The effects of increased heart work on the tricarboxylate cycle and its interactions with glycolysis in the perfused rat heart. Biochem. J. 1972;128:147–159. doi: 10.1042/bj1280147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katz L.A., Swain J.A., Portman M.A., Balaban R.S. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am. J. Physiol. 1989;256:H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- 59.Das A.M., Harris D.A. Regulation of the mitochondrial ATP synthase in intact rat cardiomyocytes. Biochem. J. 1990;266:355–361. doi: 10.1042/bj2660355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCormack J.G., Halestrap A.P., Denton R.M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 61.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 62.Das A.M., Harris D.A. Control of mitochondrial ATP synthase in heart cells: inactive to active transitions caused by beating or positive inotropic agents. Cardiovasc. Res. 1990;24:411–417. doi: 10.1093/cvr/24.5.411. [DOI] [PubMed] [Google Scholar]
- 63.Yamada E.W., Huzel N.J. Ca2+-binding properties of a unique ATPase inhibitor protein isolated from mitochondria of bovine heart and rat skeletal muscle. Cell Calcium. 1985;6:469–479. doi: 10.1016/0143-4160(85)90022-3. [DOI] [PubMed] [Google Scholar]
- 64.Bell C.J., Bright N.A., Rutter G.A., Griffiths E.J. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J. Biol. Chem. 2006;281:28058–28067. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- 65.Robert V., Gurlini P., Tosello V. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. EMBO J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trollinger D.R., Cascio W.E., Lemasters J.J. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem. Biophys. Res. Commun. 1997;236:738–742. doi: 10.1006/bbrc.1997.7042. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths E.J., Stern M.D., Silverman H.S. Measurement of mitochondrial calcium in single living cardiomyocytes by selective removal of cytosolic indo 1. Am. J. Physiol. 1997;273:C37–C44. doi: 10.1152/ajpcell.1997.273.1.C37. [DOI] [PubMed] [Google Scholar]
- 68.Zhou Z., Matlib M.A., Bers D.M. Cytosolic and mitochondrial Ca2+ signals in patch clamped mammalian ventricular myocytes. J. Physiol. 1998;507(Pt 2):379–403. doi: 10.1111/j.1469-7793.1998.379bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spat A., Szanda G., Csordas G., Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008;44:51–63. doi: 10.1016/j.ceca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garcia-Perez C., Hajnoczky G., Csordas G. Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J. Biol. Chem. 2008;283:32771–32780. doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allen S.P., Stone D., McCormack J.G. The loading of fura-2 into mitochondria in the intact perfused rat heart and its use to estimate matrix Ca2+ under various conditions. J. Mol. Cell. Cardiol. 1992;24:765–773. doi: 10.1016/0022-2828(92)93390-6. [DOI] [PubMed] [Google Scholar]
- 72.Gellerich F.N., Gizatullina Z., Trumbeckaite S. The regulation of OXPHOS by extramitochondrial calcium. Biochim. Biophys. Acta. 2010;1797:1018–1027. doi: 10.1016/j.bbabio.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Balaban R.S. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J. Mol. Cell. Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 74.Heineman F.W., Balaban R.S. Effects of afterload and heart rate on NAD(P)H redox state in the isolated rabbit heart. Am. J. Physiol. 1993;264:H433–H440. doi: 10.1152/ajpheart.1993.264.2.H433. [DOI] [PubMed] [Google Scholar]
- 75.Kennedy H.J., Pouli A.E., Ainscow E.K., Jouaville L.S., Rizzuto R., Rutter G.A. Glucose generates sub-plasma membrane ATP microdomains in single islet beta-cells. Potential role for strategically located mitochondria. J. Biol. Chem. 1999;274:13281–13291. doi: 10.1074/jbc.274.19.13281. [DOI] [PubMed] [Google Scholar]
- 76.Griffiths E.J. Mitochondrial calcium transport in the heart: physiological and pathological roles. J. Mol. Cell. Cardiol. 2009;46:789–803. doi: 10.1016/j.yjmcc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 77.Brandes R., Bers D.M. Intracellular Ca2+ increases the mitochondrial NADH concentration during elevated work in intact cardiac muscle. Circ. Res. 1997;80:82–87. doi: 10.1161/01.res.80.1.82. [DOI] [PubMed] [Google Scholar]
- 78.Saks V., Kuznetsov A.V., Gonzalez-Granillo M. Intracellular Energetic Units regulate metabolism in cardiac cells. J. Mol. Cell. Cardiol. 2012;52:419–436. doi: 10.1016/j.yjmcc.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 79.Cortassa S., Aon M.A., Marban E., Winslow R.L., O’Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anmann T., Eimre M., Kuznetsov A.V. Calcium-induced contraction of sarcomeres changes the regulation of mitochondrial respiration in permeabilized cardiac cells. FASEB J. 2005;272:3145–3161. doi: 10.1111/j.1742-4658.2005.04734.x. [DOI] [PubMed] [Google Scholar]
- 81.Griffiths E.J., Halestrap A.P. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nazareth W., Yafei N., Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J. Mol. Cell. Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 83.Liu T., O’Rourke B. Regulation of mitochondrial Ca2+ and its effects on energetics and redox balance in normal and failing heart. J. Bioenerg. Biomembr. 2009;41:127–132. doi: 10.1007/s10863-009-9216-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carry M.M., Mrak R.E., Murphy M.L., Peng C.F., Straub K.D., Fody E.P. Reperfusion injury in ischemic myocardium: protective effects of ruthenium red and of nitroprusside. Am. J. Cardiovasc. Pathol. 1989;2:335–344. [PubMed] [Google Scholar]
- 85.Park Y., Bowles D.K., Kehrer J.P. Protection against hypoxic injury in isolated-perfused rat heart by ruthenium red. J. Pharmacol. Exp. Ther. 1990;253:628–635. [PubMed] [Google Scholar]
- 86.Griffiths E.J. Use of ruthenium red as an inhibitor of mitochondrial Ca(2+) uptake in single rat cardiomyocytes. FEBS Lett. 2000;486:257–260. doi: 10.1016/s0014-5793(00)02268-7. [DOI] [PubMed] [Google Scholar]
- 87.Griffiths E.J., Ocampo C.J., Savage J.S. Mitochondrial calcium transporting pathways during hypoxia and reoxygenation in single rat cardiomyocytes. Cardiovasc. Res. 1998;39:423–433. doi: 10.1016/s0008-6363(98)00104-7. [DOI] [PubMed] [Google Scholar]
- 88.Griffiths E.J., Rutter G.A. Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells. Biochim. Biophys. Acta. 2009;1787:1324–1333. doi: 10.1016/j.bbabio.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Liu T., O’Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ. Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rutter G.A. Visualising insulin secretion. The Minkowski lecture 2004. Diabetologia. 2004;47:1861–1872. doi: 10.1007/s00125-004-1541-1. [DOI] [PubMed] [Google Scholar]
- 91.Mari A., Tura A., Natali A. Impaired beta cell glucose sensitivity rather than inadequate compensation for insulin resistance is the dominant defect in glucose intolerance. Diabetologia. 2010;53:749–756. doi: 10.1007/s00125-009-1647-6. [DOI] [PubMed] [Google Scholar]
- 92.Tarasov A.I., Nicolson T., Riveline J.P. A rare mutation in ABCC8/SUR1 leading to altered KATP channel activity and {beta}-cell glucose sensing is associated with type 2 diabetes mellitus in adults. Diabetes. 2008;57:1595–1604. doi: 10.2337/db07-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Butler A.E., Janson J., Bonner-Weir S., Ritzel R., Rizza R.A., Butler P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 94.Rahier J., Guiot Y., Goebbels R.M., Sempoux C., Henquin J.C. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 2008;10(Suppl4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- 95.Rutter G.A. Nutrient-secretion coupling in the pancreatic islet b-cell: Recent advances. Mol. Aspects Med. 2001;22:247–284. doi: 10.1016/s0098-2997(01)00013-9. [DOI] [PubMed] [Google Scholar]
- 96.Sekine N., Cirulli V., Regazzi R. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogease in pancreatic β-cell. Potential role in nutrient sensing. J. Biol. Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 97.Schuit F., DeVos A., Farfari S. Metabolic fate of glucose in purified islet cells - Glucose- regulated anaplerosis in beta cells. J. Biol. Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 98.Ishihara H., Wang H., Drewes L.R., Wollheim C.B. Overexpression of monocarboxylate transporter and lactate dehydrogenase alters insulin secretory responses to pyruvate and lactate in beta cells. J. Clin. Invest. 1999;104:1621–1629. doi: 10.1172/JCI7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao C., Wilson C.M., Schuit F., Halestrap A.P., Rutter G.A. Expression and distribution of lactate/monocarboxylate transporter (MCT) isoforms in pancreatic islets and the exocrine pancreas. Diabetes. 2001;50:361–366. doi: 10.2337/diabetes.50.2.361. [DOI] [PubMed] [Google Scholar]
- 100.Sekine N., Cirulli V., Regazzi R. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic beta-cells. Potential role in nutrient sensing. J. Biol. Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 101.Detimary P., Gilon P., Henquin J.C. Interplay between cytoplasmic Ca2+ and the ATP/ADP ratio: a feedback control mechanism in mouse pancreatic islets. Biochem. J. 1998;333:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ashcroft F.M., Harrison D.E., Ashcroft S.J.H. Glucose induces closure of single potassium channels in isolated rat pancratic B-cells. Nat. Lond. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 103.Safayhi H., Haase H., Kramer U. L-type calcium channels in insulin-secreting cells: Biochemical characterization and phosphorylation in RINm5F cells. Mol. Endocrinol. 1997;11:619–629. doi: 10.1210/mend.11.5.9922. [DOI] [PubMed] [Google Scholar]
- 104.Wollheim C.B., Sharp G.W. Regulation of insulin release by calcium. Physiol. Rev. 1981;61:914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- 105.Sun G., Tarasov A.I., McGinty J. Ablation of AMP-activated protein kinase alpha1 and alpha2 from mouse pancreatic beta cells and RIP2.Cre neurons suppresses insulin release in vivo. Diabetologia. 2010;53:924–936. doi: 10.1007/s00125-010-1692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Richards S.K., Parton L.E., Leclerc I., Rutter G.A., Smith R.M. Over-expression of AMP-activated protein kinase impairs pancreatic {beta}-cell function in vivo. J. Endocrinol. 2005;187:225–235. doi: 10.1677/joe.1.06413. [DOI] [PubMed] [Google Scholar]
- 107.Henquin J.C. Regulation of insulin secretion: a matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–751. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 108.Eliasson L., Renstrom E., Ding W.G., Proks P., Rorsman P. Rapid ATP-dependent priming of secretory granules precedes Ca2+-induced exocytosis in mouse pancreatic B-cells. J. Physiol. London. 1997;503:399–412. doi: 10.1111/j.1469-7793.1997.399bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takahashi N., Kadowaki T., Yazaki Y., Ellis-Davies G.C., Miyashita Y., Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:760–765. doi: 10.1073/pnas.96.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsuboi T., da S X., Holz G.G., Jouaville L.S., Thomas A.P., Rutter G.A. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem. J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tarasov A.I., Girard C.A., Ashcroft F.M. ATP sensitivity of the ATP-sensitive K+ channel in intact and permeabilized pancreatic beta-cells. Diabetes. 2006;55:2446–2454. doi: 10.2337/db06-0360. [DOI] [PubMed] [Google Scholar]
- 112.Ainscow E.K., Rutter G.A. Mitochondrial priming modifies Ca2+ oscillations and insulin secretion in pancreatic islets. Biochem. J. 2001;353:175–180. doi: 10.1042/0264-6021:3530175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ainscow E.K., Rutter G.A. Glucose-stimulated oscillations in free cytosolic ATP concentration imaged in single islet b cells: evidnece for a Ca2+-dependent mechanism. Diabetes. 2002;51:S162–S170. doi: 10.2337/diabetes.51.2007.s162. [DOI] [PubMed] [Google Scholar]
- 114.Pralong W.F., Spat A., Wollheim C.B. Dynamic pacing of cell metabolism by intercellular Ca2+ transients. J. Biol. Chem. 1994;269:27310–27314. [PubMed] [Google Scholar]
- 115.A.I. Tarasov, G.A. Rutter, unpublished observations.
- 116.Ravier M.A., Guldenagel M., Charollais A. Loss of connexin36 channels alters beta-cell coupling, islet synchronization of glucose-induced Ca2+ and insulin oscillations, and basal insulin release. Diabetes. 2005;54:1798–1807. doi: 10.2337/diabetes.54.6.1798. [DOI] [PubMed] [Google Scholar]
- 117.Berg J., Hung Y.P., Yellen G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat. Methods. 2009;6:161–166. doi: 10.1038/nmeth.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gall D., Gromada J., Susa I., Rorsman P., Herchuelz A., Bokvist K. Significance of Na/Ca exchange for Ca2+ buffering and electrical activity in mouse pancreatic beta-cells. Biophys. J. 1999;76:2018–2028. doi: 10.1016/S0006-3495(99)77359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gilon P., Arredouani A., Gailly P., Gromada J., Henquin J.C. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J. Biol. Chem. 1999;274:20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- 120.Wiederkehr A., Szanda G., Akhmedov D. Mitochondrial matrix calcium is an activating signal for hormone secretion. Cell Metab. 2011;13:601–611. doi: 10.1016/j.cmet.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 121.Molina A.J., Wikstrom J.D., Stiles L. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58:2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]