Abstract
NUT midline carcinomas (NMC) comprise a group of highly aggressive tumors that have been reported primarily in the head, neck, and mediastinum of younger individuals. These tumors overexpress the nuclear protein in testis (NUT), most commonly due to a chromosomal translocation that fuses the NUT gene on chromosome 15 with the BRD4 gene on chromosome 19. Although the earliest recognized cases were described in the thymus or mediastinum, an extensive survey for NMC among malignant thymic or other mediastinal neoplasms has not been reported. We examined NUT expression in 114 cases of poorly differentiated carcinomas or unclassified mediastinal malignancies using a clinically validated NUT-specific monoclonal antibody. Four of 114 (3.5%) cases showed nuclear NUT expression. A NUT translocation was confirmed by fluorescence in situ hybridization (FISH) in 3 of these cases. These tumors arose in two male and two female adults with a median age of 50 (range 28 to 68). Three of the tumors were originally diagnosed as undifferentiated epithelioid or round cell malignant neoplasms; one tumor contained focal squamous differentiation and was originally diagnosed as a poorly differentiated squamous carcinoma of probable thymic origin. We find that the incidence of NMC within the mediastinum, particularly amongst undifferentiated tumors, is similar to that reported at other anatomic sites. NMC should be considered in the differential diagnosis of any poorly-differentiated epithelioid mediastinal tumor, regardless of age.
BACKGROUND
Midline carcinomas with t(15;19) translocations were first described in the early 1990s in relatively young patients with mediastinal or intrathoracic carcinomas.11,12,14 Since that time, the majority of reported NUT-translocated carcinomas have been described in midline head, neck, and thoracic structures, although additional locations such as the abdomen and iliac bone have been reported.5,6 To date, the histopathology of at least 39 individual cases has been reported in the literature. In most cases, NUT midline carcinomas (NMCs) appear morphologically and imunohistochemically undifferentiated. In a subset of cases, focal and abrupt squamous differentiation can be seen adjacent to poorly differentiated areas of tumor.10,18
Patients with NMC typically present with a large tumor burden, mass-effect, and distant metastases at the time of diagnosis. Aside from single case reports, NMC is uniformly fatal with an average survival of 9.4 months from the time of diagnosis (unpublished data), consistent with its undifferentiated morphology and typically advanced stage at presentation.5,17 Initially thought to be a malignancy of the young, NMC has now been reported over a wide age range, from infancy up to 78 years old, with the frequency of newly diagnosed adult cases outpacing pediatric ones.5,17,18
NMCs are defined by rearrangements of the NUT locus at 15q14 that result in fusion with a member of the bromodomain-containing protein (BRD) family, usually BRD4 located on chromosome 19. Discovery of the BRD4-NUT fusion oncogene led to more rapid and reliable detection of NMC via FISH, as well as insights into the role of NUT in tumorigenesis.8,9,16 Nuclear overexpression of the resulting BRD4-NUT fusion protein is detectable by immunohistochemical staining with a highly sensitive and specific NUT monoclonal antibody; as a result, diagnosis is no longer predicated upon demonstrating specific chromosomal rearrangements with FISH. 10
Despite the original reports describing t(15;19) translocated tumors arising in the mediastinum, there are no published studies describing the clinicopathologic features of NMC arising at this location, nor are there any estimates available as to its relative incidence overall or compared to the few other anatomic sites that have been studied. In this ten year retrospective study, we perform NUT immunohistochemistry (IHC) to identify undiagnosed NMC among all thymic carcinomas and undifferentiated mediastinal malignancies available from the Brigham and Women's Hospital (BWH) Department of Pathology files (both adult and pediatric patients) covering the period (2001–2010) leading up to routine utilization of NUT IHC for diagnostic purposes at our institution. Newly diagnosed cases of NMC are further examined by FISH, and the clinicopathologic features of these NUT-expressing tumors are described.
METHODS
Case Selection
We queried the pathology database from BWH, including general and personal consult files from 2001–2010 using search terms “thymic”, “mediastinal”, and “carcinoma”. Consults directed to C.D.F. for general consultation were included. Consults directed to C.A.F. for a priori suspicion of NMC, as well as tumors classified as thymomas or representing metastatic disease or contiguous spread from another site, were excluded to avoid bias. Our database search identified 180 cases of poorly differentiated thymic carcinoma or unclassified mediastinal neoplasms (including undifferentiated epithelioid, round cell, and/or spindle cell morphologies). Of these, 114 cases had sufficient material available for testing. The clinical and primary pathologic features of these 114 cases are described in Tables 1 and 2, respectively.
Table 1.
Clinical characteristics of mediastinal neoplasms selected from Brigham and Women's Hospital, Department of Pathology (2001–2010).
| Clinicopathologic Parameter | n (%) |
|---|---|
| Total | 114 (100) |
| Age (years) | |
| Median Age [Range] | 54 [1–89] |
| 1 – 19, pediatric | 8 (7) |
| 20 – 29, young adult | 8 (7) |
| 30 – 59, adult | 52 (46) |
| 60 – 89, senior | 46 (40) |
| Sex | |
| Male | 62 (54) |
| Female | 52 (46) |
| Specimen type | |
| Surgical resection | 62 (54) |
| Biopsy (core or open) | 52 (46) |
| Specimen Origin | |
| In-House | 68 (60) |
| Consult | 46 (40) |
Table 2.
Histopathologic characterization of selected mediastinal neoplasms.
| Primary Diagnosis | n (%) | Reclassified as NMC n (%) |
|---|---|---|
| Total | 114 (100) | 4 (3.5) |
| Carcinoma † | 84 (74) | 1 (1) |
| Thymic Carcinoma | 24 (21) | 0 |
| Poorly Differentiated/Undifferentiated Carcinoma | 22 (19) | 0 |
| Squamous Cell Carcinoma | 13 (11) | 1 (8) |
| Non-small Cell Carcinoma | 12 (11) | 0 |
| Poorly Differentiated Neuroendocrine Carcinoma | 8 (7) | 0 |
| Sarcomatoid Carcinoma | 2 (2) | 0 |
| Carcinoma, possibly thymic | 3 (3) | 0 |
| Undifferentiated tumors # | 30 (26) | 3 (10) |
| Undifferentiated Malignant Epithelioid or Spindle Cell Neoplasm | 24 (21) | 2 (8) |
| Unclassified Round Cell Sarcoma | 3 (3) | 1 (33) |
| Undifferentiated Neoplasm | 2 (2) | 0 |
| Atypical Epithelioid Neoplasm | 1 (<1) | 0 |
Cases which exhibited keratin immunoreactivity or otherwise met standard histopathologic criteria for diagnosis of carcinoma.
Cases without diagnostic histopathologic features or immunohistochemical staining patterns.
Clinicopathologic review
Original diagnostic slides and associated immunohistochemical stains were reviewed by two pathologists (A.G.E. and L.M.S.). Patient demographic data was obtained from the medical records following approval by the BWH Institutional Review Board.
Immunohistochemistry
Immunohistochemistry for NUT was performed on 4 micron formalin-fixed, paraffin-embedded sections as previously described.10 Briefly, slides underwent heat-induced epitope retrieval in pH 6 citrate buffer using a pressure cooker. After washing in distilled water and treatment with Peroxidase Block (Dako) for 5 minutes to quench endogenous peroxidase activity, sections were incubated with primary rabbit monoclonal anti-NUT (clone C52, 1:200) in antibody diluent (Dako) for 1 hr, washed in 50 mM Tris-HCl (pH 7.4), and incubated with horseradish peroxidase-conjugated secondary antibodies (Envision detection kit, DAKO USA). Staining was developed through incubation with diaminobenzidine (DAB), and sections were counterstained. Immunohistochemical staining for NUT was interpreted by two or more pathologists (A.G.E., L.M.S., and C.A.F.). Cases with strong, speckled nuclear staining were considered positive.
Additional immunohistochemical tumor markers were performed at the time of primary diagnosis according to standard operating procedures in the BWH Department of Pathology Immunohistochemistry Laboratory.
FISH
Chromosomal translocation of the NUT gene locus was confirmed by FISH as previously described.7 Dual-color FISH assays evaluating chromosome 19p13.1 BRD4 and 15q13 NUT break points were performed on formalin-fixed, paraffin-embedded, unstained, 4-μm sections. Probes used for the 19p13.1 BRD4 break point included telomeric tandem bacterial artificial chromosome (BAC) clones RP11-319o10 and RP11-681d10 (digoxigenin-labeled, FITC anti-digoxigenin-detected, green) and centromeric BAC clones RP11-207i16, and CTD-3055m5 (biotin-labeled, rhodamine-streptavidin-detected, red). Probes used for the 15q13 NUT break point, flanking a 181-kb region, included telomeric BAC clones 1H8 and 64o3B (digoxigenin-labeled, FITC anti-digoxigenin-detected, green) and centromeric clones 1084a12 and 3d4 (biotin-labeled, rhodamine-streptavidin-detected, red). Patients with more than 80% hybridization efficiency in four areas (200 cells /area) of the tissue section were regarded as interpretable.
RESULTS
NUT Immunohistochemistry
Among the 114 mediastinal tumors selected (Table 2), a total of 4 (3.5%) showed positive NUT staining with the characteristic speckled nuclear pattern (Figure 1). NMC was more commonly diagnosed among consult cases (3 of 46 or 6.5%) as compared to in-house cases (1 of 68 or 1.5%).
Figure 1. Histopathology and NUT immunohistochemistry.
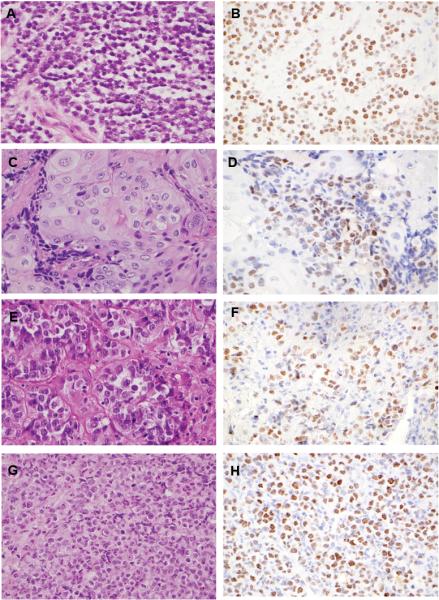
Representative histologic images and immunohistochemical staining patterns for all 4 newly diagnosed mediastinal NMC cases. Case numbers correspond to the clinicopathologic features outlined in Tables 3 and 4: panels A and B, Case 1; panels C and D, Case 2; panels E and F, Case 3; panels G and H, Case 4.
Clinicopathologic Correlations
All cases of NMC were identified in adults, including two men (ages 28 and 39) and two women (ages 61 and 68). Additional details regarding clinical presentation were available in three of the four patients (Table 3). Notably, no examples of NMC were discovered amongst 24 tumors originally diagnosed definitively as thymic carcinoma. The NUT-positive cases demonstrated nested to sheetlike growth of monomorphic, small to intermediate-sized tumor cells with vesicular to hyperchromatic nuclei and variable amounts of cytoplasm. One case showed focal, abrupt squamous differentiation, while another showed abundant granular eosinophilic cytoplasm (Figure 1). By IHC, three of four NMCs were negative for keratins (including a pan-keratin cocktail, Cam5.2, and/or AE1/AE3), and all were negative for sarcoma, melanoma, and lymphoma markers (for specific stains, see Table 4.). The one case with extensive but focally abrupt squamous differentiation showed multifocal cytokeratin7 (CK7) and scattered CD5-positive cells, thus leading to an original diagnosis of poorly differentiated squamous cell carcinoma of probable thymic origin (Figure 1, Tables 3 and 4). This was the only case out of 84 carcinomas (~1%) that was reclassified as NMC. In comparison, the rate of NMC detection among undifferentiated neoplasms without keratin expression was higher, but not by a statistically significant margin (3 of 30, or 10%; p = 0.06, Fisher's exact test) (Table 2).
Table 3.
Clinicopathologic characteristics of newly diagnosed NMC.
| Case | Age | Gender | Presentation | Primary Diagnosis |
|---|---|---|---|---|
| 1 | 28 | Male | Pleural effusion, left anterior mediastinal mass, and lymph node metastasis | Undifferentiated round cell sarcoma |
| 2 | 39 | Male | Mediastinal mass and multiple bone metastases | Poorly differentiated squamous cell carcinoma of probable thymic origin |
| 3 | 61 | Female | Mediastinal mass | Unclassified epithelioid malignant neoplasm |
| 4 | 68 | Female | Cough, weight loss, and a mediastinal mass at the mainstem bronchus and/or hilar region. | Poorly differentiated malignant neoplasm |
Table 4.
Histologic features, FISH, and additional IHC staining results of newly diagnosed NMC.
| Case | Histology | FISH | Additional IHC |
|---|---|---|---|
| 1 | Nests of small round cells Scant cytoplasm Hyperchromatic nuclei Smudgy to vesicular chromatin Indistinct nucleoli |
NUT-variant translocation | Negative: pan-K, AE1/AE3, CAM5.2, PLAP, S-100, CD30, CD99, CD34, LCA, synaptophysin, desmin |
| 2 | Heterogenous areas of tumor including: Pleomorphic epithelioid cells with squamous features Hyperchromatic small to intermediate-sized cells |
BRD4-NUT translocation |
Positive: focal CK7, rare CD 117, scattered CD5 Negative: TTF-1 |
| 3 | Intermediate-sized epithelioid cells Nested growth Abundant granular eosinophilic cytoplasm Vesicular chromatin Prominent nucleoli |
N.D. | Negative: pan-K, CK7, CK20, AE1/AE3, p63, TTF-1, chromogranin, synaptophysin, CEA, EMA, S-100, HMB-45, PLAP, CD30, CD31, CD34, LCA |
| 4 | Diffuse, sheet-like growth Small-to-intermediate sized cells Round to irregular nuclei Vesicular chromatin Small distinct nucleoli |
BRD4-NUT translocation | Negative: CK7, CK20, TTF-1, synaptophysin, chromogranin, AE1/AE3, CAM5.2, pan-K, LCA, CD30, CD34, CD56, CD117, S-100, desmin, MPO, lysozyme |
N.D. = no translocation detected
FISH Correlation
FISH studies demonstrated rearrangement of the NUT locus in three of the four NUT-positive cases (Table 4). Two cases showed rearrangements of both BRD4 and NUT, one showed rearrangement of NUT but lacked a demonstrable BRD4 rearrangement (i.e. a NUT-variant translocation), and one case failed to show rearrangement of either BRD4 or NUT by FISH. This case likely harbored a cryptic NUT translocation (as previously described, see discussion), however lacked sufficient material to perform more comprehensive FISH analysis.10
DISCUSSION
This study demonstrates that NMC represents a small but appreciable percentage of poorly or undifferentiated mediastinal malignancies, including examples of both epithelioid and round cell types. Using IHC, we detected overexpression of the NUT protein in 4 of 114 (3.5%) mediastinal tumors including thymic carcinomas and difficult-to-classify malignancies. The frequency of NMC was enriched among undifferentiated mediastinal tumors (10%). To confirm the mechanism of NUT overexpression, we performed FISH for NUT and BRD4; two cases contained a NUT-BRD4 translocation and one contained a NUT-variant translocation. One case lacked a demonstrable rearrangement using probes against NUT and BRD4 despite high levels of NUT protein expression; this case likely harbored a cryptic translocation, described in detail below.10
The incidence of mediastinal NMC is within the same order of magnitude as that found at other anatomic sites. Previous reports that relied on FISH for screening demonstrated that 7% of midline carcinomas in children and young adults and 18% of undifferentiated carcinomas of the upper aerodigestive tract harbor a NUT translocation.7,18 In contrast, only two new cases of NMC were detected amongst a series of >1000 neoplasms that were screened by NUT IHC without being selected for histologic type or anatomic location.10 The variable rate of detection is most likely influenced by case selection methodologies; in particular, the incidence reported in this and some other studies may be biased by the substantial number of cases drawn from personal consult files containing difficult-to-classify tumors. One additional previously unsuspected case of mediastinal NMC was identified in 2010 in consultation at BWH, following the introduction of routine IHC testing for NUT; this case is not included in this series. Ultimately, this study demonstrates that although NMC can be reproducibly detected by IHC among mediastinal tumors from a wide age range of patients, the incidence does not indicate a strong predilection for the thymus or the mediastinum in comparison to other sites.
The large number of consult cases in this study precluded our ability to systematically examine the clinical features of this patient cohort. However, all three NMC cases for which some clinical details were available presented with locally and/or systemically advanced disease, consistent with prior reports of aggressive behavior. While it is interesting to note that NMC was identified more frequently among cases with undifferentiated histology, our study was not powered to detect a statistically significant difference in incidence between different tumor subgroups. Histologically, all four mediastinal NMCs in this series were predominantly comprised of undifferentiated round-to-epithelioid cells growing in nests and sheets. With the exception of rare CK7 and CD5 immunoreactivity in one case, all NMCs in this study were negative for a broad panel of immunohistochemical markers. These results are consistent with previous reports, which have emphasized that NMCs typically exhibit the cytologic features of a poorly differentiated or undifferentiated carcinoma.1,10,19
Some cases of NMC show abrupt squamous differentiation, a feature that has been associated with NUT-variant translocations in prior reports, although this was not true of the single case with squamous differentiation in this series.7 The frequency of squamous differentiation in this study (1 of 4) is similar to that described in a series of NMC from the upper aerodigestive tract (2 of 5).18 In contrast, squamous differentiation was common (9 of 11 cases) in one study examining a cohort of poorly differentiated epithelial tumors from patients <40 years old.7
The presence of a NUT-translocation was confirmed in three of the four NUT-overexpressing tumors identified in this study. The single FISH-negative case that exhibited strong nuclear NUT protein expression is included in the series based on previous data demonstrating 100% specificity of the NUT C52 monoclonal antibody for tumors which harbor translocations involving NUT.10 As in our series, Haack et al. identified two cases of NMC by NUT IHC that were negative using a similar FISH assay. Upon further study, these cases did in fact contain a NUT-BRD4 rearrangement, secondary to a small interstitial deletion involving the NUT gene on chr15 that cannot be detected using the flanking probes employed in this study (a so-called cryptic NUT rearrangement). In fact, these authors concluded that they could only attain 100% sensitivity for diagnosis of NMC by employing a combined NUT IHC and “gold standard” FISH (i.e. flanking probes) approach.10 Because we did not employ a combined screening approach in our series, our cumulative data may slightly underestimate the true incidence of mediastinal NMC seen at a tertiary care referral center.
A strength of our study is that it has examined a large number of primary mediastinal malignancies for the presence of NUT protein expression using whole tissue slides. While most of the NUT-positive cases were diffusely positive, there was some variable expression that may have led to false negatives had this study been performed using a tissue microarray. This study was limited by access to corresponding clinical data, therefore it is difficult to comment on patient features associated with mediastinal NMC such as smoking status or other potential risk factors.
Studies of the molecular pathogenesis of NMC have also implicated the BRD-NUT fusion oncoprotein in squamous cell carcinogenesis. While the role of NUT in normal tissues remains unknown, inhibition of BRD-NUT fusion proteins in NMC cell lines arrests cell growth and promotes striking and irreversible squamous cell differentiation in vitro.6,9 Consistent with the known role of BRD family members in chromatin remodeling, the fusion oncoproteins appear to promote epigenetic reprogramming via globally decreased histone acetylation and transcriptional repression, thus leading to undifferentiated cell growth.16,20 In combination, the histopathologic features described above and the laboratory data on BRD-NUT fusion protein-mediated oncogenesis support the notion that NMC is a distinctive genetic variant of squamous cell carcinoma, or perhaps a relatively undifferentiated tumor arising from a common squamous cell precursor.
Rapid advances in the targeted treatment of other translocation-driven tumors, such as anaplastic lymphoma kinase (ALK)-rearranged lung carcinoma and BCR-ABL in chronic myelogenous leukemia, serve as exciting precedent for diagnosis and treatment of this under recognized tumor.3,13 In fact, insights into the oncogenic role of BRD-NUT fusion proteins have triggered research into novel, directed therapies with histone deacetylase inhibitors and bromodomain-ET protein family (BET) members, including small molecule inhibitors of BRD4.2,4,15,16 As with other models of targeted therapy, advancing therapeutic research beyond the preclinical stage will by necessity require accurate recognition of NMC. Until the clinical features of this tumor type are better clarified, the diagnosis is almost entirely dependent upon the pathologist. Our findings indicate that the diagnosis of NMC should not be overlooked in the differential of any difficult-to-classify epithelioid mediastinal malignancy. In this series examining poorly differentiated malignancies of the mediastinum, NMC is relatively frequent, occurring in approximately 10% of undifferentiated tumors. Therefore, use of NUT IHC to evaluate for NMC should be considered in any poorly differentiated or undifferentiated epithelioid malignancy (with or without focal keratinization), particularly in the setting of otherwise negative immunohistochemical studies. Further advances in the treatment of NMC will require more widespread recognition of this aggressive malignancy.
Acknowledgments
Source of Funding This work was supported by a Dana Farber/Harvard Cancer Center Nodal Award 5P30CA06516-44 (C.A. French), US National Institutes of Health grant 1R01CA124633 (C.A. French), the Samuel Waxman Cancer Research Foundation Reprogramming the Cancer Cell, and the American Association for Cancer Research grant 10-20-03-FREN (C.A. French).
Footnotes
Conflicts of Interest The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bellizzi AM, Bruzzi C, French CA, et al. The cytologic features of NUT midline carcinoma. Cancer. 2009;117:508–515. doi: 10.1002/cncy.20044. [DOI] [PubMed] [Google Scholar]
- 2.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–917. doi: 10.1016/j.cell.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 4.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French CA. Pathogenesis of NUT Midline Carcinoma. Annu Rev Pathol. 2012;7:247–65. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 6.French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2010;63:492–496. doi: 10.1136/jcp.2007.052902. [DOI] [PubMed] [Google Scholar]
- 7.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NUT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 8.French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 9.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 10.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19) Am J Pediatr Hematol Oncol. 1991;13:459–464. doi: 10.1097/00043426-199124000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51:3327–3328. [PubMed] [Google Scholar]
- 13.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee AC, Kwong YI, Fu KH, et al. Disseminated mediastinal carcinoma with chromosomal translocation (15;19). A distinctive clinicopathologic syndrome. Cancer. 1993;72:2273–2276. doi: 10.1002/1097-0142(19931001)72:7<2273::aid-cncr2820720735>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Muller S, Filippakopoulos P, Knapp S. Bromodomains as therapeutic targets. Expert Rev Mol Med. 2011;13:e29. doi: 10.1017/S1462399411001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz BE, Hofer MD, Lemieux ME, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–2696. doi: 10.1158/0008-5472.CAN-10-3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stelow EB. A Review of NUT Midline Carcinoma. Head Neck Pathol. 2011;5:31–35. doi: 10.1007/s12105-010-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 19.Stelow EB, French CA. Carcinomas of the upper aerodigestive tract with rearrangement of the nuclear protein of the testis (NUT) gene (NUT midline carcinomas) Adv Anat Pathol. 2009;16:92–96. doi: 10.1097/PAP.0b013e31819923e4. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Diaz J, Jiao J, et al. Perturbation of BRD4 protein function by BRD4-NUT protein abrogates cellular differentiation in NUT midline carcinoma. J Biol Chem. 2011;286:27663–27675. doi: 10.1074/jbc.M111.246975. [DOI] [PMC free article] [PubMed] [Google Scholar]


