Summary
The concept that gliomas comprise a heterogeneous group of diseases distinguished by their developmental origin raises the intriguing possibility that neural stem cells (NSCs) from different germinal zones have differential capacities to respond to glioma-causing genetic changes. We demonstrate that NSCs of subventricular zone of lateral ventricle are molecularly and functionally distinct from those of the third ventricle. Consistent with a unique origin for pediatric low-grade glioma, third ventricle, but not lateral ventricle, NSCs hyperproliferate in response to mutations characteristic of childhood glioma. Finally, we demonstrate that pediatric optic gliomas in Nf1 genetically-engineered mice arise from the third ventricle. Collectively, these observations establish the importance of innate brain region NSC heterogeneity in the patterning of gliomagenesis in children and adults.
Keywords: optic glioma, astrocytoma, ventricular zone, stem cell niche, neurofibromatosis type 1, subventricular zone, third ventricle
Introduction
The importance of the cell of origin in tumorigenesis and clinical behavior of brain tumors (Singh et al., 2004; Taylor et al., 2005) has been strengthened by the observation that histologically-identical brain tumors are composed of molecularly-distinct subtypes that reflect their progenitor cell of origin (Gibson et al., 2010; Johnson et al., 2010; Kalamarides et al., 2011; Sharma et al., 2007). These findings suggest that brain tumors with distinct cellular origins are unique diseases with different growth control regulatory networks, genetic changes, and responses to therapy. Consistent with this, we have shown that mouse NSCs from the brainstem, but not the neocortex, exhibit increased proliferation and gliogenesis following inactivation of the neurofibromatosis-1 (NF1) tumor suppressor gene (Lee et al., 2010). This differential sensitivity to Nf1 loss closely parallels the propensity for pilocytic astrocytomas (PAs) in children with NF1 to form within the optic pathway and brainstem but rarely in the cortex (Guillamo et al., 2003). A similar geographic pattern of gliomagenesis is observed for sporadic pediatric PAs harboring KIAA1549:BRAF fusions (Jacob et al., 2009), which predominantly form in the cerebellum.
Within the brain, there are several germinal zones potentially germane to brain tumorigenesis, including lv-SVZ, TVZ, and the fourth ventricle (Quinones-Hinojosa et al., 2006; Weiss et al., 1996; Xu et al., 2005). While the lv-SVZ is often considered to be the likely stem cell compartment for cerebral hemisphere glioma formation in mice following the introduction of genetic alterations observed in high-grade human adult gliomas (Alcantara Llaguno et al., 2009; Jacques et al., 2010; Wang et al., 2009), other populations, including NG2+ cells (Assanah et al., 2006; Masui et al., 2010) and oligodendrocyte precursors (Liu et al., 2011; Sugiarto et al., 2011) can serve as potential cells of origin for malignant glioma. However, the origin of optic glioma, the second most common low-grade pediatric gliomas, remains unresolved. Based on the proximity of the optic nerve/chiasm to the TVZ and that optic nerve oligodendrocyte precursors can originate from the TVZ (Ono et al., 1997), we hypothesized that TVZ may be the progenitor compartment for these pediatric brain tumors.
Results
NSCs from the lv-SVZ and TVZ are molecularly-distinct populations
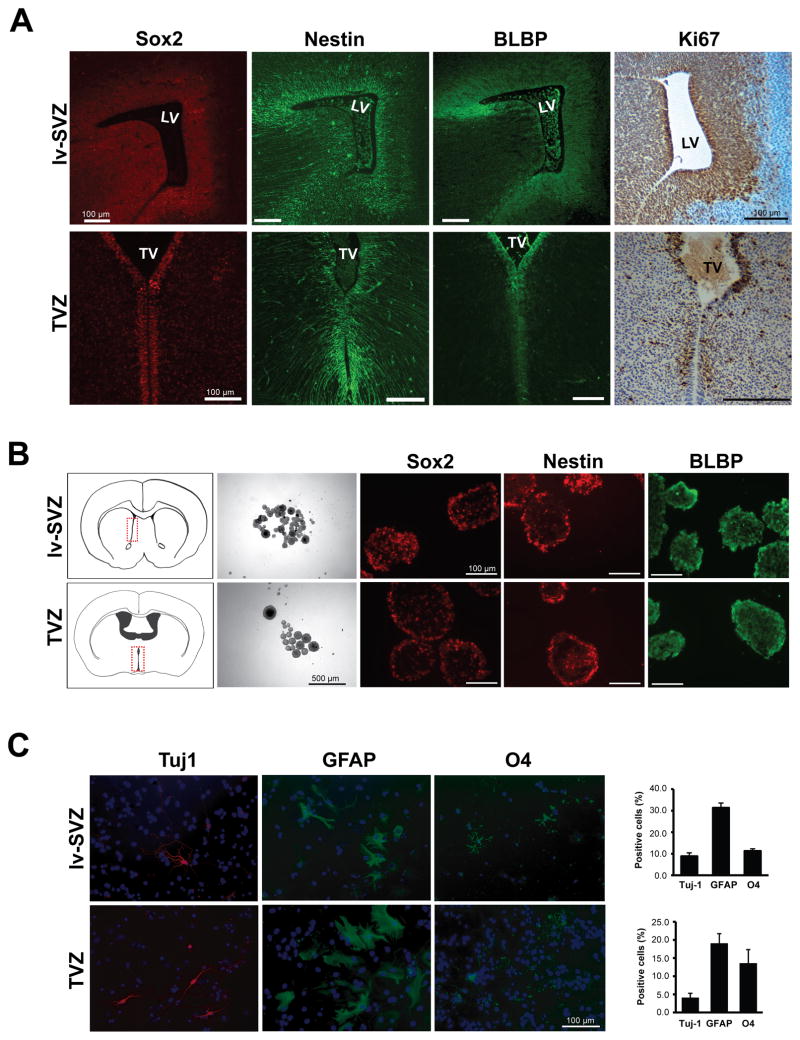
We obtain several lines of evidence supporting that TVZ is a true stem cell niche. First, cells lining the TVZ in the embryonic day 15.5 (E15.5) mouse brain express NSC markers Sox2, nestin and BLBP, and exhibit robust proliferation (Ki67 immunostaining) (Figure 1A). Second, TVZ NSCs can generate Sox2+, nestin+ and BLBP+ neurospheres and undergo self-renewal, similar to their lv-SVZ counterparts (Figure 1B). Third, dissociated single cells from TVZ neurospheres differentiate into neurons, astrocytes and oligodendrocytes in vitro (Figure 1C).
Figure 1. NSCs can be generated from the lv-SVZ and TVZ.
(A) Immunostaining of the mouse E15.5 TVZ cells express the Sox2, nestin, and BLBP NSC markers. (B) Single neurospheres from the lv-SVZ and TVZ form secondary neurospheres in vitro and express Sox2, nestin and BLBP. The diagrams denote the regions used for NSC cultures. (C) TVZ NSCs can differentiate into neurons (Tuj-1), astrocytes (GFAP) and oligodendrocytes (O4). Values denote the mean ± SEM.
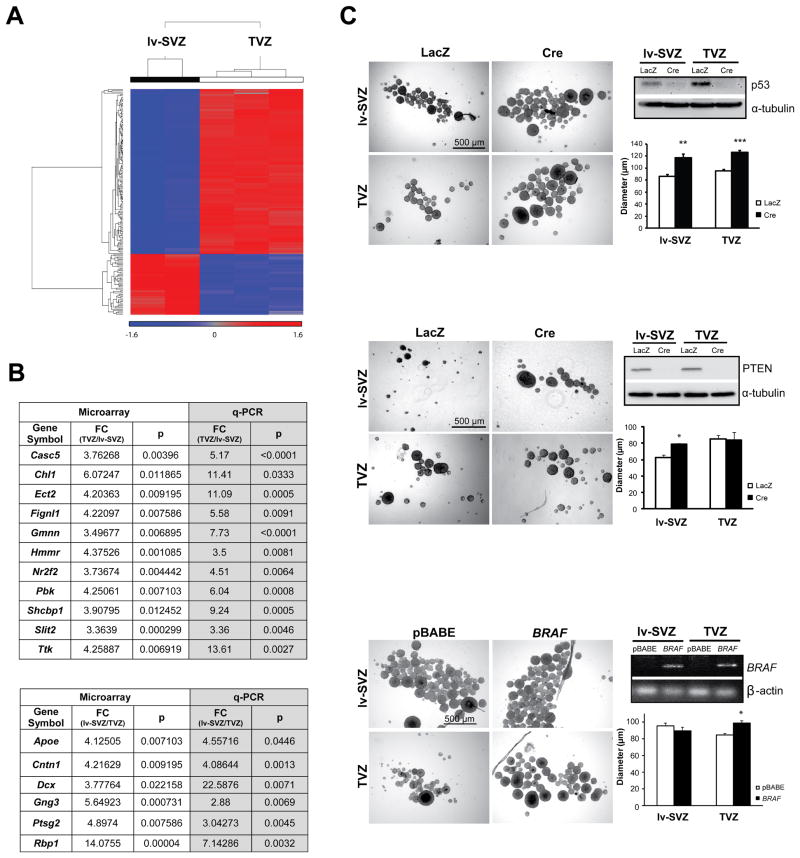
We then employed gene expression profiling to demonstrate that TVZ NSCs and lv-SVZ NSCs are molecularly distinct populations. Initially, E17.5 TVZ and lv-SVZ NSCs from three females were used for the profiling; however, one outlier lv-SVZ NSC sample, based on principal component analysis (PCA), was eliminated from the following analyses (Figure S1A). Using hierarchical clustering methods, lv-SVZ and TVZ NSCs were easily separable (Figure 2A and S1A). The differential expression of several genes were validated by quantitative RT-PCR (qRT-PCR) (Figure 2B) and by in situ hybridization (Allen Brain Atlas at http://www.brain-map.org/): Chl1 and Slit2 expression was higher in the hypothalamus/TVZ region compared to the anterior forebrain/lv-SVZ region, whereas Dcx and Cntn1 expression was higher in the anterior forebrain/lv-SVZ region relative to the hypothalamus/TVZ region (Figure S1B). A subset of these genes was also similarly differentially expressed in older mice (Figure S1B and C). Together, these data demonstrate that TVZ and lv-SVZ contain molecularly-distinct NSC populations.
Figure 2. NSCs from the lv-SVZ and TVZ are molecularly-distinct progenitor populations with unique cell-autonomous responses to glioma-causing genetic mutations.
(A) SAM separates lv-SVZ and TVZ NSCs with the expression level represented as standardized values from −1.6 (blue, <1-fold change) to 1.6 (red, >1-fold change). No change (0 value) is denoted by grey. (B) Validation of select differentially-expressed transcripts by qRT-PCR with fold changes (FC) and p values (p) shown. (C) Increased neurosphere diameters were observed in NSCs from both the lv-SVZ and TVZ following p53. Increased neurosphere diameters were found only in lv-SVZ NSCs following Pten loss. Increased neurosphere diameters were observed only in TVZ NSCs following KIAA1549:BRAF expression (RT-PCR). Values denote the mean ± SEM. p*<0.01, p**<0.001, p***<0.0001. See also Figure S1.
lv-SVZ and TVZ NSCs exhibit unique cell-autonomous responses to glioma-causing genetic mutations
To determine whether TVZ and lv-SVZ NSCs exhibit different responses to glioma-associated genetic events, we measured NSC proliferation in response to KIAA1549:BRAF expression, a representative pediatric glioma-causing genetic change (Jones et al., 2008), PTEN loss, a representative adult glioma-causing genetic change (Pollack et al., 2006), and p53 loss, which occurs in both adult and pediatric gliomas (Hayes et al., 1999; Kim et al., 2010). The KIAA1549:BRAF mutation is found in 62% of hypothalamus/optic pathway PAs, but is uncommon in histologically-identical tumors of the cerebral hemispheres (14%) (Jacob et al., 2009). p53 inactivation increased proliferation and decreased apoptosis of both lv-SVZ and TVZ NSCs (Figure 2C, top and Figure S1D). Pten inactivation increased proliferation of lv-SVZ, but not in TVZ, NSCs, whereas KIAA1549:BRAF overexpression increased proliferation of TVZ, but not in lv-SVZ, NSCs, with no effect in apoptosis (Figures 2C and S1D). Decreased apoptosis was observed in both NSC populations following Pten loss, while KIAA1549:BRAF overexpression resulted in no change. These differential responses do not reflect a failure to activate AKT following Pten loss in TVZ NSCs or MEK following KIAA1549:BRAF expression in lv-SVZ NSCs (Figure S1E).
Mouse Nf1 optic gliomas arise from TVZ
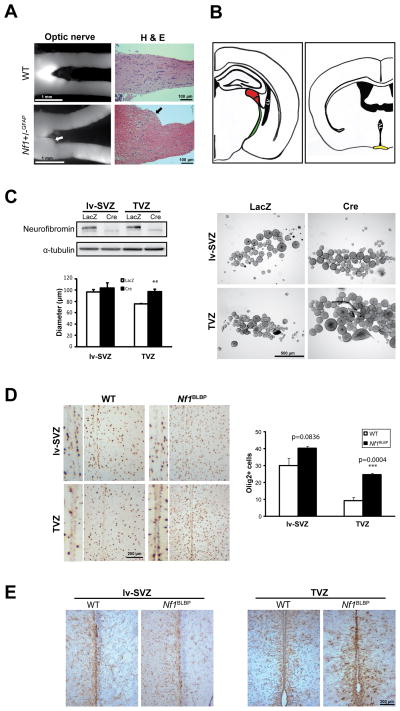
To identify the ventricular zone of origin for optic glioma, we chose NF1 as a model experimental system because gliomas predominate in the optic pathway of children with this syndrome (Guillamo et al., 2003). Similar to human NF1-associated gliomas, optic gliomas form in the prechiasmatic and chiasmal regions of Nf1+/− mice following complete Nf1 inactivation in glial progenitors (Figure 3A) (Bajenaru et al., 2003). These gliomas could arise from NSCs in the lv-SVZ, TVZ (Figure 3B), optic nerve, or retina.
Figure 3. TVZ NSCs are preferentially sensitive to Nf1 loss.
(A) Increased optic nerve volume (white arrow) and abnormal cell clusters (black arrow) were observed in optic gliomas from 3-month-old Nf1+/− GFAP mice. (B) The potential cellular origins (lv-SVZ and TVZ) of optic gliomas in Nf1 mutant mice are illustrated. LV: lateral ventricle, red: lateral geniculate nucleus, green: optic tract, TV: third ventricle, yellow: optic chiasm. (C) Increased TVZ neurosphere proliferation was seen following Nf1 loss, with little effect on lv-SVZ NSCs. (D) 3-fold more Olig2+ cells were found in the TVZ of Nf1BLBP mice (p=0.0004), but not in the lv-SVZ (p=0.0836), compared to controls. (E) Increased numbers of GFAP+ cells were found in the TVZ, but not in the lv-SVZ, of Nf1BLBP mouse compared to controls. Values denote the mean ± SEM. See also Figure S2.
We first excluded the retina and optic nerve as cell of origin of these gliomas because true NSCs capable of self-renewal and multi-lineage differentiation could not be generated from either E17.5 or postnatal day (PN) 1 retina cells or the optic nerve (Figure S2A, Cicero et al., 2009; Lee et al., 2010). Additionally, Cre transgene expression (LacZ+ cells) was not detected in the retina or optic nerve until after PN2 or E17.5, respectively (Figure S2B). We then show that Nf1−/− NSCs from the TVZ, but not the lv-SVZ, exhibit increased proliferation relative to wild-type NSCs, with no effect on apoptosis in vitro (Figure 3C and S2C). To provide in vivo support for these in vitro observations, we inactivated Nf1 in BLBP+ NSCs beginning at E9.5 and found that the numbers of Olig2+ glial progenitors (Figure 3D) and GFAP+ astrocytes (Figure 3E) were increased in the TVZ, but not in the lv-SVZ, of PN8 Nf1BLBP mice compared to control littermates in vivo.
To examine whether human hypothalamic/optic gliomas recapitulate the gene expression pattern of TVZ (Sharma et al., 2007), PCA and hierarchical clustering revealed that hypothalamic/optic pathway gliomas were separated from their supratentorial counterparts (Figure S2D). While some of differentially-expressed genes were not represented on the human Affymetrix Gene Chip, we found that one differentially-expressed TVZ transcript (Slit2) was significantly higher (3.7 fold; p=0.001) in hypothalamic/optic PAs compared to supratentorial PAs. Two other TVZ-overexpressed transcripts (Gmnn and Nr2f2) and two lv-SVZ-overexpressed transcripts (Cntn1 and Apoe) also exhibited increased expression in hypothalamic/optic gliomas and supratentorial gliomas, respectively, although not reaching statistical significance, likely due to the small sample size (p=0.08–0.1) (Figure S2D).
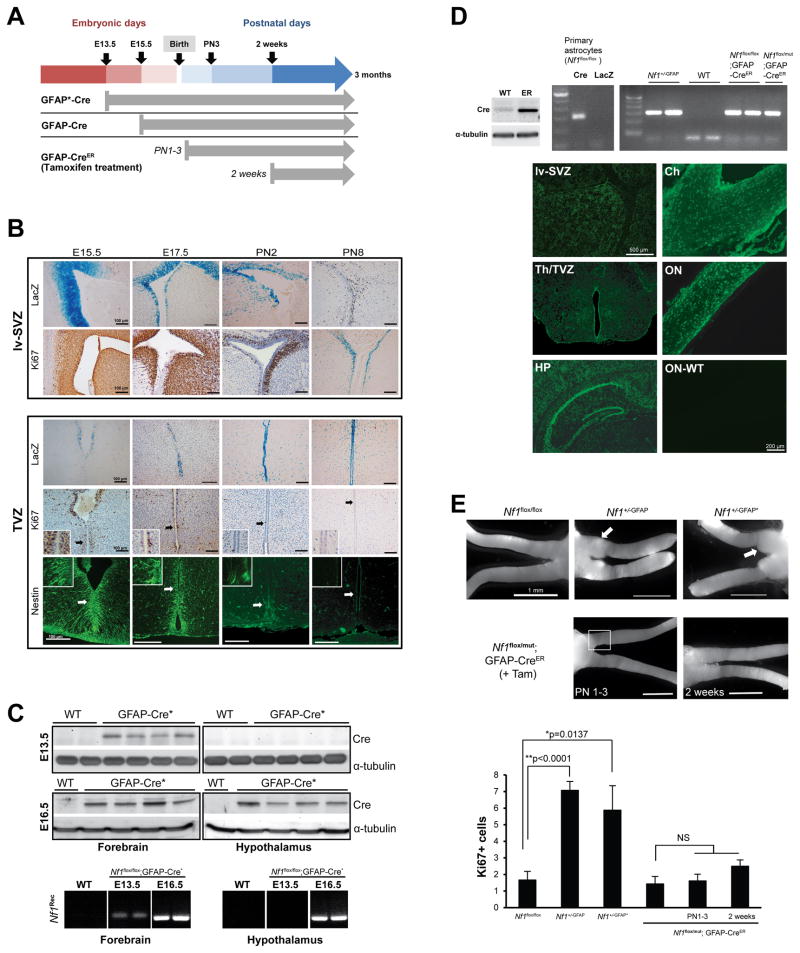
Finally, we sought to define a developmental window when optic glioma formation is favored by virtue of the selective proliferative activity of the TVZ and lv-SVZ using three different GFAP-Cre driver lines with distinct patterns of Cre-mediated Nf1 inactivation in vivo (Figures S3A and 4A). The GFAP-Cre:IRES-LacZ strain used to generate Nf1+/−GFAP mouse optic gliomas has detectable LacZ expression in the lv-SVZ and TVZ beginning at E15.5 (Figure 4B). The GFAP-Cre* strain (Zhuo et al., 2001) initiates Cre expression in the anterior part of forebrain by E13.5 (Figure 4A and 4C) and in the hypothalamus, which includes the TVZ, by E16.5 (Figure 4C). Nf1+/−GFAP* mice also develop optic glioma (Zhu et al., 2005) (Figure 4E). Analysis of the TVZ and lv-SVZ in these mice reveals that nestin+ and Ki67+ progenitor cells reside in both germinal zones at E15.5 (Figure 4B).
Figure 4. Embryonic Nf1 inactivation is required for optic glioma formation.
(A) The timing of Nf1 inactivation by Cre-mediated excision is shown for each strain. (B) X-gal staining reveals GFAP-Cre transgene expression in the lv-SVZ and TVZ beginning at E15.5. Whereas lv-SVZ cells are Ki67+ from E15.5 through PN8, scant numbers of Ki67+ or nestin+ cells are detected in the TVZ by PN2. (C) Cre expression and Nf1 gene recombination (Nf1Rec) is detected by E13.5 in the anterior forebrain/lv-SVZ (“forebrain”) and by E16.5 in the hypothalamus/TVZ (“hypothalamus”) of GFAP-Cre* mice. (D) Cre-mediated Nf1 gene recombination (PCR) in Nf1+/−GFAP mice and in tamoxifen-treated Nf1flox/flox; GFAP-CreER and Nf1flox/mut; GFAP-CreER mice was seen. Wild-type (WT) C57BL/6 mouse brain was used as a negative control, whereas Ad5Cre-infected (Cre) and Ad5LacZ-infected (LacZ) Nf1flox/flox astrocytes served as positive and WT controls, respectively. Cre-ER fusion protein (~70 KDa) expression was detected in GFAP-CreER (ER), but not in WT, mouse brains. EGFP was expressed in tamoxifen-treated ROSA-GREEN; GFAP-CreER mouse brains and optic nerves at 1 month of age, but not in a WT mouse optic nerve (ON-WT). HP: hippocampus, Th: thalamus, Ch: chiasm, ON: optic nerve. (E) Whereas optic gliomas develop in Nf1+/-GFAP and Nf1+/-GFAP* mice, no gliomas formed in Nf1flox/flox or Nf1flox/mut; GFAP-CreER treated with tamoxifen (+Tam) beginning at PN1 or PN14. Increased Ki67+ cells were found in the prechiasmatic and chiasmal regions (square) of Nf1+/−GFAP and Nf1+/−GFAP* mice. In contrast, the number of Ki67+ cells in Nf1flox/mut; GFAP-CreER postnatally-treated with tamoxifen is indistinguishable from control Nf1flox/flox mice. Values denote the mean ± SEM. See also Figure S3.
To distinguish between these two germinal zones, we employed the GFAP-CreER strain, which was similar to the first GFAP-Cre strain, but expressed a tamoxifen-regulatable Cre (CreER; Chow et al., 2008). Recombination and inactivation of the Nf1 gene was verified by recombination PCR (Figure 4D), while Cre activity in the brain, optic chiasm, and optic nerve following tamoxifen injection was demonstrated using ROSA-GREEN reporter mice (Figure 4D). Since lv-SVZ contained nestin+ Ki67+ cells at PN8–14 whereas these proliferating progenitors disappeared after PN2 in TVZ (Figure 4B, S3B and S3C), we inactivated Nf1 either during the first postnatal week of life (PN 1–3) or at 2 weeks of age when only the lv-SVZ harbors significant numbers of proliferating (Ki67+) progenitor (nestin+) cells (Figure S3B and S3C). Nf1 loss at these times did not result in glioma formation at 3 months of age (Figure 4E). As an internal control for the fidelity of the GFAP-CreER strain for inducing optic glioma, we treated >20 litters of pregnant females with tamoxifen at E16.5 (50 μg/g i.p.). The vast majority of pregnant dams did not deliver viable mice; however, the one embryonically-treated pup that survived to 3 months of age developed an optic glioma (Figure S3D) with increased numbers of Ki67+ cells and increased numbers of GFAP+ astrocytes (Figure S3E). Taken together, these data establish that optic gliomas arise from neural stem/progenitor cells in the proliferative TVZ during embryogenesis rather than from astrocytes at later postnatal stages.
Discussion
Our finding that NSCs from two different germinal zones are molecularly-distinct stem cell populations is consistent with previous reports examining mouse embryonic spinal cord and brain NSCs as well as human neural progenitor cells from the developing cortex and ventral 8 midbrain (Johnson et al., 2010; Kelly et al., 2009; Kim et al., 2009; Taylor et al., 2005). In each case, the unique genetic signature reflects the regional identity of the progenitors. Importantly, we show that the heterogeneity revealed at the molecular level translates into unique functional responses to glioma-causing genetic changes seen in children and adults. While the precise etiologies for these innate differences are unknown, they likely reflect transcriptional networks and signaling set-points unique to these brain regions. For example, we have previously shown that the expression of the mTOR component rictor underlies the ability of Nf1-deficient NSCs to increase their proliferation and glial differentiation (Lee et al., 2010), whereas basal cAMP levels in specific brain regions partly dictate the spatial pattern of gliomagenesis in NF1 (Warrington et al., 2010).
We also provide several lines of converging evidence that optic gliomas likely originate from stem/progenitor cells residing in the TVZ. While both TVZ and lv-SVZ germinal zones could provide cells of origin for these tumors, only TVZ, but not lv-SVZ, NSCs exhibit increased proliferation and gliogenesis following Nf1 inactivation. In addition, optic gliomas do not form in mouse strains following postnatal Nf1 inactivation when only lv-SVZ NSCs are proliferating. These latter experiments also demonstrate that Nf1 inactivation in GFAP-expressing astrocytes in young mice does not result in optic gliomagenesis. One report employing immunohistochemical and gene expression analysis similarly suggested that human optic gliomas might derive from third ventricle glial progenitors (Tchoghandjian et al., 2009). This result parallels the developmental origins of another optic nerve glial cell population in which oligodendrocyte precursor cells generated in the floor of the TVZ differentiate and migrate into the optic nerve in response to signaling molecules from retinal ganglion axons (Gao and Miller, 2006; Ono et al., 1997).
While our mouse experimental data argue that optic gliomas in children with NF1 arise from the TVZ, it is possible that we have modeled only one type of human optic glioma, and that other subtypes of optic glioma originate from different progenitor cells akin to other CNS cancers (Gibson et al., 2010; Johnson et al., 2010). Additional potential progenitors could be NG2+ oligodendrocyte precursor cells recently implicated in malignant gliomagenesis (Assanah et al., 2006; Liu et al., 2011; Masui et al., 2010; Sugiarto et al., 2011). However, Nf1 inactivation in NG2+ cells of Nf1+/− mice, similar to the Nf1 mouse models described here, is not sufficient for glioma formation (Solga A, manuscript in preparation). Future studies aimed at subdividing these common pediatric tumors into molecularly-distinct diseases will facilitate the development of brain tumor therapies targeted to the specific growth regulatory pathways that drive cell growth and differentiation in these distinct cancer-initiating cell populations.
Experimental Procedures
Mice
All strains were generated (Supplemental Experimental Procedures), maintained on a C57BL/6 background and used under an approved Animal Studies Committee protocol at Washington University.
NSC isolation and analysis
lv-SVZ and TVZ NSCs from N f1flox/flox, p53flox/flox and Ptenflox/flox PN1 mouse pups were infected with adenovirus containing LacZ or Cre, and protein loss confirmed by Western blotting (Lee et al., 2010). NSCs expressing KIAA1549:BRAF were generated following retrovirus infection (Peter Collins, University of Cambridge) and verified by RT-PCR (Supplemental Experimental Procedures). pBABE-puro retrovirus was used as control. NSC proliferation and multi-lineage differentiation assays were performed as described previously (Lee et al., 2010).
Immunohistochemistry and immunocytochemistry
Tissues and cells were prepared as previously reported (Hegedus et al., 2007) prior to staining with appropriate antibodies (Supplemental Experimental Procedures).
Microarray analysis
RNA from three independent litters of E17.5 C57BL/6 lv-SVZ and TVZ NSCs were subjected to microarray profiling (Supplemental Experimental Procedures), and differentially-expressed probe sets (p<0.05; fold change>3-fold increase or decrease) prioritized for validation.
qRT-PCR
mRNA expression was determined by qRT-PCR using NSCs from independently-generated litters as described previously (Yeh et al., 2009) (Supplemental Experimental Procedures).
X-gal staining
Six μm frozen sections were stained with X-Gal (Gold Biotechnology, St. Louis, MO) (Hegedus et al., 2007).
Tamoxifen injection and recombination PCR
Tamoxifen was injected into lactating females (1 mg/50 μL i.p.) at PN1–3 or PN14–18 (Supplemental Experimental Procedures), and Nf1 recombination determined by recombination PCR (Mayes et al., 2011).
Western blotting
Western blotting was performed as reported previously (Lee et al., 2010) (Supplemental Experimental Procedures).
Statistical analyses
Each experiment was performed with samples from at least three independent litters. Statistical significance (p<0.05) was determined (Student’s t-test) using GraphPad Prism 5.0 software (GraphPad, Inc.).
Supplementary Material
Significance.
Whereas some adult malignant cerebral hemispheric gliomas have been shown to arise from neural stem or progenitor cells residing in the subventricular zone of the lateral ventricle (lv-SVZ), the cellular origin of pediatric low-grade gliomas is unknown. Consistent with the propensity for childhood gliomas to develop in the optic nerve and chiasm, we demonstrate that third ventricle (TVZ) NSCs are molecularly and functionally distinct from their lv-SVZ counterparts and are the likely cell of origin for murine low-grade optic gliomas. These findings establish brain region NSC heterogeneity as a major determinant underlying the patterning of gliomagenesis in children and adults.
Highlights.
lv-SVZ and TVZ neural stem cells (NSCs) are molecularly-distinct populations
TVZ and lv-SVZ NSCs differentially respond to glioma-associated mutations
Third ventricle NSCs are the likely cell of origin for NF1 optic gliomas
Innate brain region NSC heterogeneity partly dictates the pattern of gliomagenesis
Acknowledgments
We thank Crystal White-Worsena and Madelyn Reynolds for technical assistance and Suzanne Baker (St. Jude Children’s Research Hospital, Memphis TN) for the GFAP-CreER mice. This work was funded by grants from the NIH (NS065547-01 to DHG), NCI (CA141549-01 to DHG) and the NEI (EY02687).
Footnotes
Accession number of microarray data. Human PA (GSE5675) and mouse NSC (GSE37832) microarray data were deposited in the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenaru ML, Hernandez MR, Perry A, Zhu Y, Parada LF, Garbow JR, Gutmann DH. Optic nerve glioma in mice requires astrocyte Nf1 gene inactivation and Nf1 brain heterozygosity. Cancer Res. 2003;63:8573–8577. [PubMed] [Google Scholar]
- Chow LM, Zhang J, Baker SJ. Inducible Cre recombinase activity in mouse mature astrocytes and adult neural precursor cells. Transgenic Res. 2008;17:919–928. doi: 10.1007/s11248-008-9185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero SA, Johnson D, Reyntjens S, Frase S, Connell S, Chow LM, Baker SJ, Sorrentino BP, Dyer MA. Cells previously identified as retinal stem cells are pigmented ciliary epithelial cells. Proc Natl Acad Sci U S A. 2009;106:6685–6690. doi: 10.1073/pnas.0901596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Miller RH. Specification of optic nerve oligodendrocyte precursors by retinal ganglion cell axons. J Neurosci. 2006;26:7619–7628. doi: 10.1523/JNEUROSCI.0855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, Kranenburg TA, Hogg T, Poppleton H, Martin J, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillamo JS, Creange A, Kalifa C, Grill J, Rodriguez D, Doz F, Barbarot S, Zerah M, Sanson M, Bastuji-Garin S, Wolkenstein P. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain. 2003;126:152–160. doi: 10.1093/brain/awg016. [DOI] [PubMed] [Google Scholar]
- Hayes VM, Dirven CM, Dam A, Verlind E, Molenaar WM, Mooij JJ, Hofstra RM, Buys CH. High frequency of TP53 mutations in juvenile pilocytic astrocytomas indicates role of TP53 in the development of these tumors. Brain Pathol. 1999;9:463–467. doi: 10.1111/j.1750-3639.1999.tb00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Jacob K, Albrecht S, Sollier C, Faury D, Sader E, Montpetit A, Serre D, Hauser P, Garami M, Bognar L, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101:722–733. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, COM, Naumann H, Alvarez-Buylla A, Brandner S. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 2010;29:222–235. doi: 10.1038/emboj.2009.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, Rand V, Leary SE, White E, Eden C, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamarides M, Stemmer-Rachamimov AO, Niwa-Kawakita M, Chareyre F, Taranchon E, Han ZY, Martinelli C, Lusis EA, Hegedus B, Gutmann DH, Giovannini M. Identification of a progenitor cell of origin capable of generating diverse meningioma histological subtypes. Oncogene. 2011;30:2333–2344. doi: 10.1038/onc.2010.609. [DOI] [PubMed] [Google Scholar]
- Kelly TK, Karsten SL, Geschwind DH, Kornblum HI. Cell lineage and regional identity of cultured spinal cord neural stem cells and comparison to brain-derived neural stem cells. PLoS One. 2009;4:e4213. doi: 10.1371/journal.pone.0004213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, McMillan E, Han F, Svendsen CN. Regionally specified human neural progenitor cells derived from the mesencephalon and forebrain undergo increased neurogenesis following overexpression of ASCL1. Stem Cells. 2009;27:390–398. doi: 10.1634/stemcells.2007-1047. [DOI] [PubMed] [Google Scholar]
- Kim YH, Nobusawa S, Mittelbronn M, Paulus W, Brokinkel B, Keyvani K, Sure U, Wrede K, Nakazato Y, Tanaka Y, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177:2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Yeh TH, Emnett RJ, White CR, Gutmann DH. Neurofibromatosis-1 regulates neuroglial progenitor proliferation and glial differentiation in a brain region-specific manner. Genes Dev. 2010;24:2317–2329. doi: 10.1101/gad.1957110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Sage JC, Miller MR, Verhaak RG, Hippenmeyer S, Vogel H, Foreman O, Bronson RT, Nishiyama A, Luo L, Zong H. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146:209–221. doi: 10.1016/j.cell.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui K, Suzuki SO, Torisu R, Goldman JE, Canoll P, Iwaki T. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia. 2010;58:1050–1065. doi: 10.1002/glia.20986. [DOI] [PubMed] [Google Scholar]
- Mayes DA, Rizvi TA, Cancelas JA, Kolasinski NT, Ciraolo GM, Stemmer-Rachamimov AO, Ratner N. Perinatal or adult Nf1 inactivation using tamoxifen-inducible PlpCre each cause neurofibroma formation. Cancer Res. 2011;71:4675–4685. doi: 10.1158/0008-5472.CAN-10-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Yasui Y, Rutishauser U, Miller RH. Focal ventricular origin and migration of oligodendrocyte precursors into the chick optic nerve. Neuron. 1997;19:283–292. doi: 10.1016/s0896-6273(00)80939-3. [DOI] [PubMed] [Google Scholar]
- Pollack IF, Hamilton RL, James CD, Finkelstein SD, Burnham J, Yates AJ, Holmes EJ, Zhou T, Finlay JL. Rarity of PTEN deletions and EGFR amplification in malignant gliomas of childhood: results from the Children’s Cancer Group 945 cohort. J Neurosurg. 2006;105:418–424. doi: 10.3171/ped.2006.105.5.418. [DOI] [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, Mirzadeh Z, Gil-Perotin S, Romero-Rodriguez R, Berger MS, Garcia-Verdugo JM, Alvarez-Buylla A. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J Comp Neurol. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Sharma MK, Mansur DB, Reifenberger G, Perry A, Leonard JR, Aldape KD, Albin MG, Emnett RJ, Loeser S, Watson MA, et al. Distinct genetic signatures among pilocytic astrocytomas relate to their brain region origin. Cancer Res. 2007;67:890–900. doi: 10.1158/0008-5472.CAN-06-0973. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Hanecker P, Ayers-Ringler J, Phillips J, Siu J, et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011;20:328–340. doi: 10.1016/j.ccr.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Tchoghandjian A, Fernandez C, Colin C, El Ayachi I, Voutsinos-Porche B, Fina F, Scavarda D, Piercecchi-Marti MD, Intagliata D, Ouafik L, et al. Pilocytic astrocytoma of the optic pathway: a tumour deriving from radial glia cells with a specific gene signature. Brain. 2009;132:1523–1535. doi: 10.1093/brain/awp048. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15:514–526. doi: 10.1016/j.ccr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington NM, Gianino SM, Jackson E, Goldhoff P, Garbow JR, Piwnica-Worms D, Gutmann DH, Rubin JB. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of neurofibromatosis-1. Cancer Res. 2010;70:5717–5727. doi: 10.1158/0008-5472.CAN-09-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16:7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Tamamaki N, Noda T, Kimura K, Itokazu Y, Matsumoto N, Dezawa M, Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Yeh TH, Lee da Y, Gianino SM, Gutmann DH. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia. 2009;57:1239–1249. doi: 10.1002/glia.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Harada T, Liu L, Lush ME, Guignard F, Harada C, Burns DK, Bajenaru ML, Gutmann DH, Parada LF. Inactivation of NF1 in CNS causes increased glial progenitor proliferation and optic glioma formation. Development. 2005;132:5577–5588. doi: 10.1242/dev.02162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.