Abstract
The eye of the Glacial Apollo butterfly, Parnassius glacialis, a ‘living fossil’ species of the family Papilionidae, contains three types of spectrally heterogeneous ommatidia. Electron microscopy reveals that the Apollo rhabdom is tiered. The distal tier is composed exclusively of photoreceptors expressing opsins of ultraviolet or blue-absorbing visual pigments, and the proximal tier consists of photoreceptors expressing opsins of green or red-absorbing visual pigments. This organization is unique because the distal tier of other known butterflies contains two green-sensitive photoreceptors, which probably function in improving spatial and/or motion vision. Interspecific comparison suggests that the Apollo rhabdom retains an ancestral tiered pattern with some modification to enhance its colour vision towards the long-wavelength region of the spectrum.
Keywords: vision, colour, motion, compound eye, photoreceptor, opsin
1. Introduction
Insect compound eyes are composed of units called ommatidia. In butterflies, an ommatidium bears nine photoreceptors R1–9, constructing a fused and tiered rhabdom. Typically, the distal and proximal tiers of the rhabdom are made up of the microvilli of R1–4 and R5–8 photoreceptors, respectively. R9 is a small basal photoreceptor adding a few microvilli at the base of the rhabdom [1–3]. The ommatidia are very similar in cellular arrangement, but they are in fact spectrally heterogeneous [4–7]. Since the first detailed report of the spectral heterogeneity of butterfly ommatidia in the Japanese yellow swallowtail, Papilio xuthus [8,9], similar heterogeneity has been demonstrated in four butterfly families: Papilionidae (Papilio glaucus [10]), Pieridae (Pieris rapae [11], Anthocharis scolymus [12], Colias erate [13]), Nymphalidae (Vanessa cardui [14], Danaus plexippus [15], Heliconius erato [16]) and Lycaenidae (Lycaena rubidus [17]). The accumulated evidence indicates that the eyes of most butterflies are composed of three randomly distributed types of ommatidia.
While the ommatidial heterogeneity itself appears to be a common feature, details of the physiological and molecular organization are quite diverse among species. The diversity includes the number of opsin genes, their expression pattern, the eye pigmentation and spectral sensitivities of the photoreceptors. In order to elucidate the origin of the various butterfly eye designs, we have initiated a study on the Glacial Apollo butterfly, Parnassius glacialis, a ‘living fossil’ species in the family Papilionidae [18]. The Apollo belongs to the Parnassini, the oldest tribe in the subfamily Parnassiinae, which diverged from the other papilionid subfamilies in the Cretaceous period [19] and rapidly radiated in the late Tertiary period [20].
In a previous study [18], we found that the eye of Parnassius, like all other butterfly eyes investigated so far, has three types of ommatidia (table 1). It expresses four mRNAs encoding visual pigment opsins, one ultraviolet- (UV) (PgUV), one blue- (PgB) and two long (PgL2, L3) wavelength-absorbing types. The R3–4 photoreceptors of type I and II ommatidia express PgL2, a green-absorbing visual pigment. PgL3, a presumptive red-absorbing visual pigment, exists in R3–4 photoreceptors of type III ommatidia whose rhabdom is surrounded by a red screening pigment [18]. This expression pattern is quite unexpected because it indicates that R3 and R4 are spectrally heterogeneous, whereas these photoreceptors in all studied butterflies as well as in other insects have been found to express the same visual pigment. To address the question of how such an organization functions in vision, detailed anatomical information of the visual system is indispensable. We therefore carried out an anatomical study of the compound eye retina of P. glacialis.
Table 1.
A summary of the characteristics of ommatidial types in P. glacialis. (n.a., data not available.)
| opsin/direction of microvilli |
|||
|---|---|---|---|
| photoreceptor/tier | type Ia | type II | type III |
| R1/distal | PgB/mostly straighta | ||
| R2/distal | PgUV/curve, ±30°a | PgUV + PgB/curve, 90° | PgUV + PgB/curve, 90° |
| R3–4/proximal | PgL2/straight, 90° | PgL2/straight, 90° | PgL3/straight, 90° |
| R5–8/proximal | PgL2/straight, 45° or 135° | PgL2/straight, 45° or 135° | PgL3/straight, 45° or 135° |
| R9/basal | n.a./variable | n.a./variable | n.a./variable |
| rhabdom shape | bean | dumbbell | dumbbell |
| fluorescence | yes/particles | no | no |
| pigment | no | no | yes |
| fraction (%)b | 45 | 23 | 32 |
aIn type Ib, R1 and R2 are exchanged.
bSee Awata et al. [18].
2. Material and methods
(a). Animals
Males of the Glacial Apollo, P. glacialis (Butler) were captured around Lake Tsukui, Kanagawa, Japan. We also used, for comparison, the common bluebottle, Graphium sarpedon (Linnaeus) and the Japanese yellow swallowtail, P. xuthus (Linnaeus), which were captured around the Sokendai-Hayama campus, Kanagawa, Japan.
(b). Histology
For light and electron microscopy, isolated eyes were pre-fixed in 2 per cent paraformaldehyde and 2 per cent glutaraldehyde in 0.1 mol l–1 sodium cacodylate buffer (CB; pH 7.3) for 120 min at 20–25°C. After a brief wash with CB, the eyes were postfixed in 2 per cent osmium tetroxide in CB for 2 h at 20–25°C. Following dehydration with acetone and infiltration with propylene oxide, eyes were embedded in Quetol 812 (Nisshin EM, Tokyo) or Spurr's resin (Polysciences, Warrington PA). For light microscopy, 5 µm-thick sections were observed with a light microscope (BX51, Olympus, Tokyo) equipped with a DP71 camera system (Olympus). Ultrathin sections double-stained with uranyl acetate and lead citrate were observed in a transmission electron microscope (H7650, Hitachi, Tokyo). In a series of electron micrographs obtained from a single eye, we quantified the cross-section of the rhabdom and its composing rhabdomeres at each depth using iTEM software (Soft Imaging System, Riverside, CA, USA).
(c). Fluorescence microscopy
We observed the ommatidial fluorescence in the intact eye of butterflies mounted on the stage of a fluorescence microscope (BX60, Olympus). The microscope was equipped with a dichroic cube U-MWU (band-pass filter at 350 and cut-off filter at 420 nm) and a DP71 camera (Olympus). To identify the fluorescing ommatidia, we put silvery ink around the illuminated region, which facilitated further anatomical processing for light and electron microscopy as described already.
To observe the ommatidial fluorescence in sections of the eyes of P. xuthus, isolated eyes were pre-treated in 2 per cent paraformaldehyde and 2 per cent glutaraldehyde in 0.1 mol l–1 sodium phosphate buffer (PB; pH 7.4) for 30 s in total (6 × 5 s) with a microwave oven to enhance fixative penetration. The eyes were further fixed for 20 min in the same fixative at 4°C. After being infiltrated in 30 per cent sucrose in PB for cryoprotection, the eyes were frozen in OCT compound (Sakura Finetek Japan, Tokyo), and cut with a cryostat (HM500 OM, Micro-edge Instruments, Tokyo).
3. Results
A compound eye of P. glacialis consists of about 5000 ommatidia, whose length varies between 200 and 500 µm, depending on the eye region. We investigated the ommatidia in the fronto-ventral region, where the thickness of the photoreceptor layer is about 400 µm and that of the dioptric apparatus (cornea and crystalline cone) is about 80 µm (figure 1a). The ommatidium contains nine photoreceptors, R1–9. The cell bodies of R1–8 stretch over the entire length of the ommatidium, while R9 appears only at the region immediately distal to the basement membrane. The rhabdomeral microvilli of all photoreceptors together form the fused and tiered rhabdom.
Figure 1.

Ommatidial structure of Parnassius glacialis. (a) Diagrammatic longitudinal (left) and transverse (right) views of an ommatidium, containing nine photoreceptors (R1–9). R1 and R2 have larger cell bodies than R3–9. (b) A transverse section through the distal tier (135 µm from the corneal surface), where three types of ommatidia, I–III, are recognized. The cell body of R1 photoreceptors in type Ia (Ib) ommatidia is smaller (larger) than that of R2. Type III has a red pigment around the rhabdom (arrowheads). (c) A transverse section of the identical ommatidia of those in (b) through the proximal tier (335 µm from the corneal surface). Scale bar, 10 µm.
(a). Heterogeneity of ventral ommatidia
Figure 1b shows a transverse section of the retina at a depth of about 180 µm from the corneal surface, that is, at about 100 μm from the rhabdom tip. The profiles of R1 and R2 cell bodies are evident at this level, whereas the cell bodies of the R3–8 are small and hardly distinguishable. As we described previously [18], the ommatidia can be divided into three types; I, II and III, based on two features: the size of R1 and R2 cell bodies and the perirhabdomal pigmentation (table 1). First, in type I ommatidia, the cross sections of R1 and R2 substantially differ. Either R1 is smaller than R2 (ommatidium Ia in figure 1b) or vice versa (ommatidium Ib in figure 1b). The ratio of the numbers of Ia and Ib ommatidia is about 1 : 1. The cell bodies of R1 and R2 cell are similar in size in ommatidial types II and III. Only type III has characteristic pigmentation around the rhabdom. The pigment colour is reddish; the colour is not obvious in figure 1b,c because these are sections from an osmicated tissue.
Figure 1c is a transverse section of the same eye region as that of figure 1b, but at a deeper level of about 300 µm from the corneal surface. The cell bodies of R3–8 are enlarged here, and the (reddish) pigment in type III ommatidia clearly extends to this level (arrowheads).
(b). Ultrastructure of the rhabdom
We analysed ultrathin transverse sections of ommatidia at every 20 µm along the entire length of the rhabdom. Figure 2 shows three representative sets of serial sections, each from a particular type of ommatidium. Actual depths at which each picture was taken are indicated at the bottom left-hand corner of the pictures. All three ommatidial types can be easily discriminated at any depth shown here. Figure 2a–d shows four consecutive images of the rhabdom of a type Ia ommatidium (figure 1b). As is clearly seen from the distance between belt desmosomes at the rhabdomere base, the rhabdomere of R2 is larger than that of R1 in the distal region (figure 2b, arrowheads). The microvilli of R1 are mostly straight, while the microvilli of R2 are curved and split into two halves, which fan out in the direction of +30° and −30° relative to the vertical axis of the eye. At a depth of about 330 µm, the R1 rhabdomere becomes larger, and R3–4 photoreceptors start to extend microvilli to form rhabdomeres, while the R5–8 do not yet have microvilli (figure 2c). At a depth of about 400 µm, the R5–8 contribute diagonally oriented microvilli to the rhabdom. The R2 rhabdomere disappears at this level (figure 2d).
Figure 2.
Electron micrographs of serial sections of type I (a–d), II (e–h) and III (i–l) ommatidia at ca 120 (a,e,i), 200 (b,f,j), 300 (c,g,k) and 400 µm (d,h,l) depth. Rh indicates the rhabdom. Scale bar, 1 µm.
Contrary to type I ommatidia, both type II (figure 2e–h) and type III (figure 2i–l) have R1 and R2 rhabdomeres of similar size. The most conspicuous difference between these two types is the red pigmentation as seen in light micrographs (figure 1b,c). The R3–8 photoreceptors of type III ommatidia contain pigment granules of irregular shape close to their rhabdomeres (figure 2i–k). Moreover, the rhabdom of type III is distally, immediately below the crystalline cone, much larger than the rhabdoms in types I and II (figure 2i). At 200–300 µm, the rhabdom exhibits a characteristic dumbbell shape in both types II and III. The distal rhabdom of types II and III is composed exclusively of the microvilli of R1 and R2, which fan out almost horizontally (figure 2f,g,j,k). R3 and R4 start to contribute their microvilli to the rhabdom at around 300 µm, and at 400 µm the rhabdom is made up of exclusively R3–8 microvilli in both types (figure 2h,l).
To quantify the organization of tiers and to assess the relative contribution to light sensitivity of photoreceptors, we measured the cross-sectional area of rhabdoms and estimated the fraction of each rhabdomere at various depths in electron micrographs. The size of the rhabdomeres was then calculated by multiplying the fraction of each by the total rhabdom area at each depth (figure 3). In type I, the rhabdom area increases from 1.5 µm2 at 100 µm depth to 4.5 µm2 at 250 µm. It slightly decreases at around 300 µm, and it again increases towards the basal region (figure 3a). The rhabdom area of type II peaks at 200–250 µm, decreases in the middle layer down to about 3.5 µm2 and increases again towards the basal region (figure 3b). The rhabdom area of type III peaks at 150–250 µm, then decreases in the middle layer to 2.5 µm2 and subsequently increases again (figure 3c). The rhabdoms of type II and III have a clear constriction around the border between the distal and proximal tier of the retina. The constriction is more pronounced in type III, which contains the red perirhabdomal pigment.
Figure 3.

Entire rhabdom areas (thick line) and rhabdomere areas of R1–8 in: (a) type I (open circles denote total Rh area; filled triangles, R1 (large at distal); inverted triangles, R2 (small at distal); open triangles, R3 + 4; filled circles, total R5–8); (b) type II (open circles denote total Rh area; filled triangles, R1 + 2; open triangles, R3 + 4; filled circles, total R5–8); and (c) type III (open circles denote total Rh area; filled triangles, R1 + 2; open triangles, R3 + 4; filled circles, total R5–8) ommatidia. The rhabdomere areas were calculated by multiplying rhabdom areas by rhabdomere fractions, which were estimated from typical electron micrographs at each depth. Data are means ± s.e.
(c). Ultraviolet induced autofluorescence
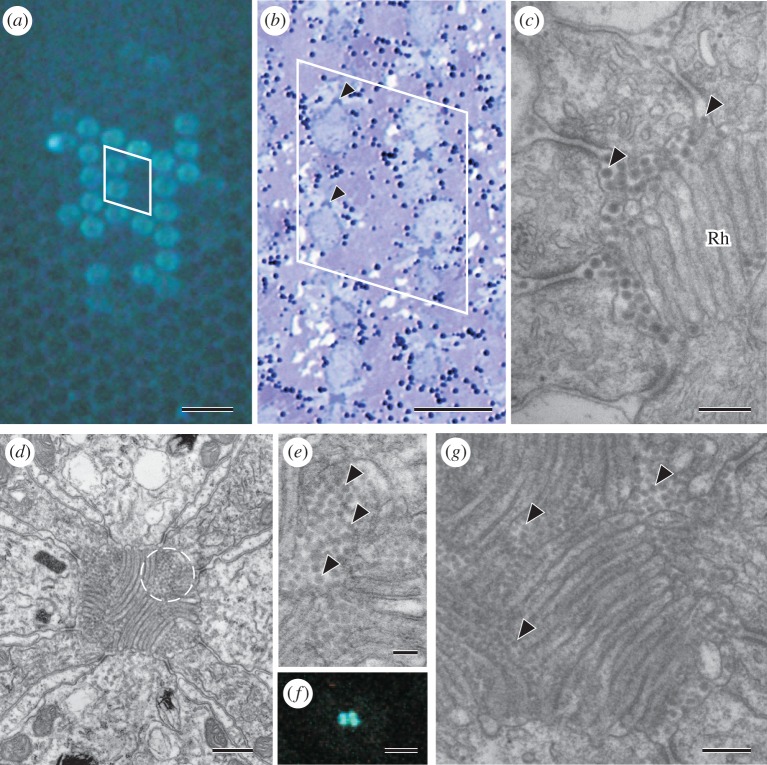
A number of butterfly eyes exhibit fluorescence depending on the ommatidial type [11,21]. We therefore investigated whether the Parnassius eye also exhibited fluorescence. We found a subset of ommatidia emitting strong fluorescence under UV epi-illumination (figure 4a). Figure 4b shows a transverse semi-thin section of the region shown in figure 4a, demonstrating that the fluorescing ommatidium is of type Ia (figure 4a,b, see also figure 1b). To uncover the physical basis of the fluorescence, we carefully inspected electron micrographs of rhabdoms. It thus appeared that the rhabdoms of type I ommatidia often contain particles of 30–40 nm in diameter. The particles have no membrane, and exist mostly in the distal 100 µm of the rhabdom, in the extracellular space within or around the rhabdom (figure 4c).
Figure 4.
Fluorescing ommatidia in the eye of P. glacialis. (a) UV epi-illumination reveals fluorescence in a subset of ommatidia. (b) A semi-thin, light-microscopical section of the eye observed in (a). The arrowheads indicate the same two fluorescing ommatidia in (a). The unequally sized cell bodies of R1 and R2 show that the ommatidia are of type I; in this particular case, type Ia. (c) The extracellular space of the rhabdom (Rh) of type I is filled with particles of diameter 30–40 nm. (d) A type II ommatidium of Papilio xuthus. Broken circle indicates one of four clusters of particles. (e) High magnification picture of the circled region in (d). Arrowheads indicate particles. (f) A transverse view of a section of an ommatidium with four fluorescing dots in the centre. (g) Part of a rhabdom of a fluorescing ommatidium of G. sarpedon. Particles are found within the rhabdom area. Scale bars: (a) 50 µm, (b) 20 µm, (c) 0.2 µm, (d) 0.50 µm, (e) 0.1 µm, (f) 5 µm, and (g) 0.2 µm.
The UV-induced fluorescence was first discovered in Papilio, in their type II ommatidia [9,21], but the origin of the fluorescence has not been identified so far. Because the anatomy of Parnassius suggested that the fluorescence is allocated in characteristic particles, we decided to re-examine the electron micrographs of Papilio eyes. We thus found particles accumulated in type II ommatidia as four clusters in the extracellular space in the most distal region of the rhabdom (figure 4d,e). A frozen section observed with a fluorescence microscope applying UV-excitation revealed four fluorescing spots, corresponding to the particle clusters (figure 4f) [22]. We also examined the eye of another papilionid species, G. sarpedon, and found fluorescing ommatidia, which contain particles similar to those of the eyes of Parnassius and Papilio in the extracellular space (arrowheads in figure 4g).
4. Discussion
(a). R3 and R4 are proximal, long-wavelength receptors
The most curious feature of Parnassius ommatidia is the organization of the tiered rhabdom. In all butterfly species studied so far, the distal tier of the rhabdom is formed by the microvilli extending from four photoreceptors, R1–4, while the R5–8 photoreceptors are usually contributing only to the proximal rhabdom [3,12,23–25]. In a few nymphalid species, R3 and R4 contribute microvilli to both the distal and proximal rhabdom [14,26–28]. However in Parnassius, the distal rhabdom is composed exclusively of the microvilli of R1 and R2 photoreceptors in all three ommatidial types, and R3 and R4 photoreceptors contribute their microvilli in the proximal tier, together with R5–8 photoreceptors (figures 2 and 3). In Parnassius, R3 and R4 are therefore proximal photoreceptors.
The organization of the rhabdom tiers is probably related to the localization of long-wavelength-absorbing visual pigments. In addition to a UV- and a blue-absorbing visual pigment, the eyes of Parnassius express two long-wavelength-absorbing visual pigments, PgL2 and PgL3, orthologues of Papilio PxL2 (515 nm-absorbing) and PxL3 (575 nm-absorbing), respectively [18]. In situ hybridization revealed that the PgL2 (presumptive green) mRNA is expressed in R3–4 and R5–8 of type I and II ommatidia, while the PgL3 (presumptive red) mRNA exists in R3–4 and R5–8 of the red-pigmented type III ommatidia [18]. In P. xuthus, the PxL3 is expressed in R5–8 proximal receptors of type I ommatidia. The spectral sensitivity of the PxL3-expressing receptors, which peaks at 600 nm, has a narrower profile than the absorption spectrum of a visual pigment peaking at 600 nm. This difference was explained by assuming that the perirhabdomal red pigment acts as a red filter in front of a 575 nm-peaking visual pigment [23].
The perirhabdomal pigment of P. xuthus effectively acts as a filter only for the proximal photoreceptors, as was also found in the Small White butterfly, P. rapae. The distal R3 and R4 as well as the proximal R5–8 photoreceptors in all ommatidia of Pieris express a 560 nm-peaking visual pigment, PrL [29]. Whereas the spectral sensitivity of the distal R3–4 well matches the predicted absorption spectrum of a 563 nm visual pigment, the peak sensitivity of the proximal photoreceptors is shifted to 620 nm in the orange-pigmented ommatidia, and to 640 nm in the red-pigmented ommatidia [30]. This indicates that the effect of the perirhabdomal pigment is negligible in the distal tier, but the filtering effect is prominent in the proximal tier. In our previous study on the opsin expression in Parnassius, it was therefore rather puzzling to find that the PgL3 mRNA was expressed in R3 and R4 in the red-pigmented type III ommatidia of Parnassius [18]. These cells were expected to be distal photoreceptors, but the present anatomy has revealed that R3 and R4 are in fact proximal receptors. Accordingly, we conclude that all R3–8 photoreceptors in the type III ommatidia are most likely red receptors. Interestingly, the rhabdom of type III ommatidia has a strong constriction between the distal and the proximal tiers (figure 3c). The constriction most probably enhances the filtering effect of the pigment on the proximal receptors. On the other hand, the PgL2-expressing R3–8 photoreceptors in type I and II ommatidia are predicted to be green sensitive.
(b). The distal photoreceptors, R1 and R2
The distal R1 and R2 photoreceptors are short wavelength-sensitive. In type I ommatidia, R1 and R2 form sub-tiers within the distal tier (figure 3a): in the most distal region, the rhabdom is dominated by the rhabdomere of the PgUV-expressing R1 (in type Ib, or R2 in type Ia), while the region deeper than 300 μm is dominated by the PgB-expressing R2 (or R1 in type Ia), which has microvilli down into the proximal tier. The microvilli of the PgB-expressing cells are straight and parallel to the animals' dorso-ventral axis at least in the distal and the proximal part of the ommatidia (figure 2a,d), suggesting the cell retains polarization sensitivity to some extent.
The type I ommatidia bear small non-membranous particles around the rhabdom (figure 4c), which fluoresce under UV epi-illumination (figure 4a), as in other papilionid species (figure 4d,e,g). The particles most likely contain 3-hydroxyretinol, the fluorescing material in the eye of Papilio [21]. We could not find similar particles in the subset of ommatidia of male Pieris that fluoresces under 420 nm epi-illumination [11].
The 3-hydroxyretinol in Papilio eyes acts as a far-UV-filter for the photoreceptors, which express a UV-absorbing visual pigment and thus become 400 nm-peaking violet receptors [21]. Therefore, the PgUV expressing photoreceptors in the Parnassius type I fluorescing ommatidia may be expected to have a narrower spectral sensitivity profile with peak wavelength shifted to a longer wavelength than that of the so-called UV receptors. In fact, we have encountered in our preliminary electrophysiological recordings photoreceptors with 380 nm-peaking, narrow sensitivity spectra and with maximal polarization sensitivity parallel to the animals' dorso-ventral axis.
In Papilio, coexpression of PxL2 (green) and PxL3 (red) visual pigment causes broad-band receptors [31], and therefore the R1 and R2 photoreceptors of Parnassius in type II and III ommatidia in the ventral region, which coexpress PgUV and PgB [18], may similarly be expected to have a broad sensitivity band, in the short wavelength region. We have detected some photoreceptors whose spectral sensitivity peaks at 360 nm with a broad sideband in the blue-green (450–550 nm) wavelength region with a reduced polarization sensitivity. These cells are yet to be localized by dye injection, but they are most likely the R1 and R2 of type II and III ommatidia whose microvilli are strongly curved (figure 2f,g,j,k).
(c). Evolution of rhabdom tiers
The present study on Parnassius provides some important implications about the evolution of tiered rhabdoms. Figure 5 illustrates several forms of tiered rhabdoms of butterflies, where the R1–9 photoreceptors contribute in different ways to the rhabdom, together with the rhabdom organization of flies, which have eight photoreceptors (figure 5a). The photoreceptor numbering system is different in butterflies and flies, but the numbering can be correlated based on the photoreceptor structures and differentiation processes [33]. Briefly, R1–6 in flies and R3–8 in butterflies have short axons terminating in the first optic ganglion, the lamina, and therefore are called short visual fibres (SVFs; short arrows in figure 5). The R1 and R2 of butterflies correspond to the fly R7, while the butterfly R9 corresponds to the fly R8. These photoreceptors are called long visual fibres (LVFs) because they extend their axons to the second optic ganglion, the medulla (long arrows in figure 5). The butterfly R3/4 is the ‘first photoreceptor pair’, which corresponds to the R2/5 pair in flies, which differentiates early in the developmental process, and the butterfly R5–8 quartet is the counterpart of the fly R1/3/4/6 quartet. The compartments in figure 5 depict the different photoreceptor sets: the R1/2 pair, the R3/4 pair, the R5–8 quartet and R9. The colours of the compartments indicate the visual pigment opsins expressed in the group of photoreceptors, although those for R9 are not necessarily clear. Coloured circles indicate the perirhabdomal pigments expressed in the respective set of photoreceptors.
Figure 5.
Evolution of the tiered rhabdom. The numbering systems of photoreceptors in (a) Drosophila and in (b–g) butterflies are not consistent. The butterfly R1/2 pair and R9 correspond to the fly R7 and R8, respectively, with long axons terminating in the medulla (long arrows). Similarly, butterfly R3/4 pair and R5–8 set correspond to the fly R2/5 pair and the set of R1,3,4 and 6, all with short axons terminating in the lamina (short arrows). Each compartment indicates either R1/2 (fly-R7), R3/4 (fly-R2/5), R5–8 (fly-R1/3/4/6) or R9 (fly-R8), whose colour indicates opsin(s) it expresses: grey colours are UV opsins. R3/4 and R5–8 are merged when the opsins and locations in tiers are identical. Different colours in a single compartment indicate that the photoreceptors in the same category express different opsins in different ommatidia. (a) Drosophila rhabdomeres. Unlike butterflies, the rhabdomeres are not fused in flies. R1–6 are divided into two groups, the R2/5 pair and R1/3/4/6 quartet according to their differentiation process. The rhabdomeres of R1–6 form a trapezoid, whose centre is occupied by the rhabdomeres of R7 and R8 where the R7 rhabdomere sits on the top of the R8 rhabdomere. (b) Ancestral type of ommatidium with a bundle of short wavelength (UV and/or blue) sensitive R1/2 and green sensitive R3–8, not tiered except for the basal R9 photoreceptor. (c) Ancestral tiered rhabdom with short wavelength receptors located distally. (d) Rhabdom with modified proximal tier, as in Parnassius, with two opsins (green and red). The red perirhabdomal pigment is found in the ommatidia where R3–8 express the red opsin. See Awata et al. [18] for details. (e) Tiered rhabdom with an independent R3/4 green receptor system as in the painted lady, Vanessa cardui (Nymphalidae). (f) Rhabdom of the Japanese yellow swallowtail, P. xuthus (Papilionidae). Distally, it has specialized R3/4, expressing two duplicated green opsins. The proximal R5–8 express either one of green or red opsin, or coexpress both, which makes three spectrally heterogeneous ommatidia together with the red or yellow perirhabdomal pigments. See Arikawa [8] for details. (g) Rhabdom of the Small White, Pieris rapae (Pieridae). Three short wavelength opsins (UV, blue and violet) are expressed in R1/2 in the distal tier. Both the R3/4 pair and the R5–8 set express identical green opsin, but the spectral sensitivities of R5–8 are strongly modified by the red or orange perirhabdomal pigments. See Wakakuwa et al. [32] for details.
The ancestral form of the fused rhabdom, with short wavelength sensitive R1/2 and long-wavelength sensitive R3–8, was probably not tiered (figure 5b): this basic organization is found in some contemporary species, including honeybees [34,35] and moths [36–40]. From an optical point of view, it is sensible to allocate the short wavelength receptors in the distal region of the rhabdom. First, this is because of the relatively low amount of UV light in the environment, and second, because the UV-absorbing visual pigment in the distal receptors can function as a UV filter for the proximal receptors. The latter then causes a narrower spectral sensitivity of the proximal receptors, thus improving spectral discrimination. In its extreme case, two LVFs-expressing, short wavelength-absorbing opsins form the distal tier, whereas six SVFs-expressing, long-wavelength-absorbing opsins form the proximal tier (figure 5c). This organization is basically retained in P. glacialis. More generally, the relationship between the axon length and visual pigments seems quite basic and therefore ancestral, because this is widely found among insects, including higher flies [41] that have acquired additional unique features such as an open rhabdom and neural superposition during evolution [42].
The tiered structure in butterflies further evolved at least in two ways to enhance visual capacity. One is towards better colour vision, and another is for spatial and motion vision. For improving colour vision, duplication of long-wavelength-absorbing visual pigments occurred in the lineage of Papilionidae and Riodinidae [18,43–45]. New L opsins are expressed in proximal photoreceptors in a subset of ommatidia, shifting their spectral sensitivity towards the long-wavelength direction. This is often accompanied by the addition of perirhabdomal pigments, which further sharpens the spectral sensitivity [23,29]. Type III ommatidia of Parnassius probably have evolved as a result of this process (figure 5d and table 1). The ommatidia of Papilio are even more strongly modified by coexpressing two L opsins in a set of proximal photoreceptors (figure 5f) [8,31]. Opsin duplication occurred also in the lineage of Pieridae and Lycaenidae, but B opsins in these families (figure 5g) [32]. In the genus Heliconius (Nymphalidae), UV opsins duplicated [46].
Note that in general, the R3/4 first photoreceptor pair [33] has rhabdomeres in the distal tier (figure 5e,f,g). Specialization of these receptors is probably related to improved spatial and motion vision, visual modalities that are often based on an achromatic system that is ‘green’ sensitive [47–49]. The prominence of green sensitivity seems reasonable because the natural environment for flower-visiting insects is dominated by greenish light. For the best spatial resolution, the green receptors should exist in all ommatidia, thus completely filling the ommatidial lattice, and should also preferably occur in the distal tier where perirhabdomal pigments are uncapable of modifying the spectral sensitivities. The R3/4 pair matches these criteria. In Vanessa, which has no perirhabdomal pigment, R3/4 bear rhabdomeres along the entire length of the ommatidium (figure 5e).
The structure of the axons of R3/4 photoreceptors implicates their involvement in motion vision. In Papilio, the thick and smooth R3/4 axons make direct contacts with second-order visual interneurons in the lamina, which is probably an adaptation for fast signal processing. The four SVFs, R5–8, in contrast, have fine collaterals in the lamina with which they make mutual synaptic contacts, which indicates that at this level some degree of colour processing occurs [50,51]. Terminal specialization of the fast photoreceptor pair has also been found in honeybees [33,52], implicating its specialized function as well.
Taken together, the eye of P. glacialis is apparently unique because it lacks the monochromatic R3/4 system in the distal tier. Instead, the receptor set is enriched in terms of spectral sensitivities owing to L opsin duplication. Presumably for the slow-flying, ancestral group of butterflies, acute spatial and motion vision had a lower priority than extreme colour discrimination.
Acknowledgements
We thank Dr D. G. Stavenga and Dr T. W. Cronin for critical reading of the manuscript. This work was supported in part by the JSPS (Japan Society for the Promotion of Science) Grants-in-Aid for Scientific Research no. 22570073 to A.M. and no. 21247009 to K.A., the MAFF (Ministry of Agriculture, Forestry and Fisheries of Japan) grant (Elucidation of biological mechanisms of photoresponse and development of advanced technologies using light) no. INSECT-1101 to K.A., and by a grant from the CPIS (Sokendai Center for the Promotion of Integrated Sciences) to H.A.
References
- 1.Ribi W. A. 1978. Ultrastructure and migration of screening pigments in the retina of Pieris rapae L. (Lepidoptera, Pieridae). Cell Tissue Res. 191, 57–73 10.1007/BF00223215 (doi:10.1007/BF00223215) [DOI] [PubMed] [Google Scholar]
- 2.Kolb G. 1977. The structure of the eye of Pieris brassicae L. (Lepidoptera). Zoomorphology 87, 123–146 10.1007/BF01007602 (doi:10.1007/BF01007602) [DOI] [Google Scholar]
- 3.Bandai K., Arikawa K., Eguchi E. 1992. Localization of spectral receptors in the ommatidium of butterfly compound eye determined by polarization sensitivity. J. Comp. Physiol. A 171, 289–297 10.1007/BF00223959 (doi:10.1007/BF00223959) [DOI] [Google Scholar]
- 4.Bernard G. D., Miller W. H. 1970. What does antenna engineering have to do with insect eyes? IEEE Student J. 8, 2–8 [Google Scholar]
- 5.Stavenga D. G., Kinoshita M., Yang E. C., Arikawa K. 2001. Retinal regionalization and heterogeneity of butterfly eyes. Naturwissenschaften 88, 477–481 10.1007/s001140100268 (doi:10.1007/s001140100268) [DOI] [PubMed] [Google Scholar]
- 6.Stavenga D. G. 2002. Colour in the eyes of insects. J. Comp. Physiol. A 188, 337–348 10.1007/s00359-002-0307-9 (doi:10.1007/s00359-002-0307-9) [DOI] [PubMed] [Google Scholar]
- 7.Stavenga D. G. 2002. Reflections on colourful ommatidia of butterfly eyes. J. Exp. Biol. 205, 1077–1085 [DOI] [PubMed] [Google Scholar]
- 8.Arikawa K. 2003. Spectral organization of the eye of a butterfly, Papilio. J. Comp. Physiol. A 189, 791–800 10.1007/s00359-003-0454-7 (doi:10.1007/s00359-003-0454-7) [DOI] [PubMed] [Google Scholar]
- 9.Arikawa K., Stavenga D. G. 1997. Random array of colour filters in the eyes of butterflies. J. Exp. Biol. 200, 2501–2506 [DOI] [PubMed] [Google Scholar]
- 10.Briscoe A. D. 2008. Reconstructing the ancestral butterfly eye: focus on the opsins. J. Exp. Biol. 211, 1805–1813 10.1242/jeb.013045 (doi:10.1242/jeb.013045) [DOI] [PubMed] [Google Scholar]
- 11.Arikawa K., Wakakuwa M., Qiu X., Kurasawa M., Stavenga D. G. 2005. Sexual dimorphism of short-wavelength photoreceptors in the Small White butterfly, Pieris rapae crucivora. J. Neurosci. 25, 5935–5942 10.1523/JNEUROSCI.1364-05.2005 (doi:10.1523/JNEUROSCI.1364-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takemura S. Y., Stavenga D. G., Arikawa K. 2007. Absence of eye shine and tapetum in the heterogeneous eye of Anthocharis butterflies (Pieridae). J. Exp. Biol. 210, 3075–3081 10.1242/jeb.002725 (doi:10.1242/jeb.002725) [DOI] [PubMed] [Google Scholar]
- 13.Awata H., Wakakuwa M., Arikawa K. 2009. Evolution of color vision in pierid butterflies: blue opsin duplication, ommatidial heterogeneity and eye regionalization in Colias erate. J. Comp. Physiol. A 195, 401–408 10.1007/s00359-009-0418-7 (doi:10.1007/s00359-009-0418-7) [DOI] [PubMed] [Google Scholar]
- 14.Briscoe A. D., Bernard G. D., Szeto A. S., Nagy L. M., White R. H. 2003. Not all butterfly eyes are created equal: rhodopsin absorption spectra, molecular identification and localization of UV- blue- and green-sensitive rhodopsin encoding mRNA in the retina of Vanessa cardui. J. Comp. Neurol. 458, 334–349 10.1002/cne.10582 (doi:10.1002/cne.10582) [DOI] [PubMed] [Google Scholar]
- 15.Sauman I., Briscoe A. D., Zhu H., Shi D., Froy O., Stalleicken J., Yuan Q., Casselman A., Reppert S. M. 2005. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 46, 457–467 10.1016/j.neuron.2005.03.014 (doi:10.1016/j.neuron.2005.03.014) [DOI] [PubMed] [Google Scholar]
- 16.Zaccardi G., Kelber A., Sison-Mangus M. P., Briscoe A. D. 2006. Color discrimination in the red range with only one long-wavelength sensitive opsin. J. Exp. Biol. 209, 1944–1955 10.1242/jeb.02207 (doi:10.1242/jeb.02207) [DOI] [PubMed] [Google Scholar]
- 17.Sison-Mangus M. P., Bernard G. D., Lampel J., Briscoe A. D. 2006. Beauty in the eye of the beholder: the two blue opsins of lycaenid butterflies and the opsin gene-driven evolution of sexually dimorphic eyes. J. Exp. Biol. 209, 3079–3090 10.1242/jeb.02360 (doi:10.1242/jeb.02360) [DOI] [PubMed] [Google Scholar]
- 18.Awata H., Matsushita A., Wakakuwa M., Arikawa K. 2010. Eyes with basic dorsal and specific ventral regions in the glacial Apollo, Parnassius glacialis (Papilionidae). J. Exp. Biol. 213, 4023–4029 10.1242/jeb.048678 (doi:10.1242/jeb.048678) [DOI] [PubMed] [Google Scholar]
- 19.Omoto K., Yonezawa T., Shinkawa T. 2009. Molecular systematics and evolution of the recently discovered ‘Parnassian’ butterfly (Parnassius davydovi Churkin, 2006) and its allied species (Lepidoptera, Papilionidae). Gene 441, 80–88 10.1016/j.gene.2008.10.030 (doi:10.1016/j.gene.2008.10.030) [DOI] [PubMed] [Google Scholar]
- 20.Omoto K., Katoh T., Chichvarkhin A., Yagi T. 2004. Molecular systematics and evolution of the ‘Apollo’ butterflies of the genus Parnassius (Lepidoptera: Papilionidae) based on mitochondrial DNA sequence data. Gene 326, 141–147 10.1016/j.gene.2003.10.020 (doi:10.1016/j.gene.2003.10.020) [DOI] [PubMed] [Google Scholar]
- 21.Arikawa K., Mizuno S., Scholten D. G., Kinoshita M., Seki T., Kitamoto J., Stavenga D. G. 1999. An ultraviolet absorbing pigment causes a narrow-band violet receptor and a single-peaked green receptor in the eye of the butterfly Papilio. Vision Res. 39, 1–8 10.1016/S0042-6989(98)00070-4 (doi:10.1016/S0042-6989(98)00070-4) [DOI] [PubMed] [Google Scholar]
- 22.Kitamoto J., Ozaki K., Arikawa K. 2000. Ultraviolet and violet receptors express identical mRNA encoding an ultraviolet-absorbing opsin: identification and histological localization of two mRNAs encoding short-wavelength-absorbing opsins in the retina of the butterfly Papilio xuthus. J. Exp. Biol. 203, 2887–2894 [DOI] [PubMed] [Google Scholar]
- 23.Arikawa K., Scholten D. G. W., Kinoshita M., Stavenga D. G. 1999. Tuning of photoreceptor spectral sensitivities by red and yellow pigments in the butterfly Papilio xuthus. Zool. Sci. 16, 17–24 10.2108/zsj.16.17 (doi:10.2108/zsj.16.17) [DOI] [Google Scholar]
- 24.Arikawa K., Pirih P., Stavenga D. G. 2009. Rhabdom constriction enhances filtering by the red screening pigment in the eye of the Eastern pale clouded yellow butterfly, Colias erate (Pieridae). J. Exp. Biol. 212, 2057–2064 10.1242/jeb.030692 (doi:10.1242/jeb.030692) [DOI] [PubMed] [Google Scholar]
- 25.Qiu X., Vanhoutte K. A., Stavenga D. G., Arikawa K. 2002. Ommatidial heterogeneity in the compound eye of the male Small White butterfly, Pieris rapae crucivora. Cell Tissue Res. 307, 371–379 10.1007/s00441-002-0517-z (doi:10.1007/s00441-002-0517-z) [DOI] [PubMed] [Google Scholar]
- 26.Gordon W. C. 1977. Microvillar orientation in the retina of the nymphalid butterfly. Z. Naturforsch. 32c, 662–664 [Google Scholar]
- 27.Kolb G. 1985. Ultrastructure and adaptation in the retina of Aglais urticae (Lepidoptera). Zoomorphology 105, 90–98 10.1007/BF00312143 (doi:10.1007/BF00312143) [DOI] [Google Scholar]
- 28.Kinoshita M., Sato M., Arikawa K. 1997. Spectral receptors of nymphalid butterflies. Naturwissenschaften 84, 199–201 10.1007/s001140050377 (doi:10.1007/s001140050377) [DOI] [Google Scholar]
- 29.Wakakuwa M., Stavenga D. G., Kurasawa M., Arikawa K. 2004. A unique visual pigment expressed in green, red and deep-red receptors in the eye of the Small White butterfly, Pieris rapae crucivora. J. Exp. Biol. 207, 2803–2810 10.1242/jeb.01078 (doi:10.1242/jeb.01078) [DOI] [PubMed] [Google Scholar]
- 30.Stavenga D. G., Arikawa K. 2011. Photoreceptor spectral sensitivities of the Small White butterfly Pieris rapae crucivora interpreted with optical modeling. J. Comp. Physiol. A 197, 373–385 10.1007/s00359-010-0622-5 (doi:10.1007/s00359-010-0622-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arikawa K., Mizuno S., Kinoshita M., Stavenga D. G. 2003. Coexpression of two visual pigments in a photoreceptor causes an abnormally broad spectral sensitivity in the eye of a butterfly, Papilio xuthus. J. Neurosci. 23, 4527–4532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakakuwa M., Stavenga D. G., Arikawa K. 2007. Spectral organization of ommatidia in flower-visiting insects. Photochem. Photobiol. 83, 27–34 10.1562/2006-03-03-IR-831 (doi:10.1562/2006-03-03-IR-831) [DOI] [PubMed] [Google Scholar]
- 33.Friedrich M., Wood E. J., Wu M. 2011. Developmental evolution of the insect retina: insights from standardized numbering of homologous photoreceptors. J. Exp. Zool. B 316B, 484–499 10.1002/jez.b.21424 (doi:10.1002/jez.b.21424) [DOI] [PubMed] [Google Scholar]
- 34.Gribakin F. G. 1975. Functional morphology of the compound eye of the bee. In The compound eye and vision of insects (ed. Horridge G. A.), pp. 154–176 Oxford, UK: Clarendon Press [Google Scholar]
- 35.Wakakuwa M., Kurasawa M., Giurfa M., Arikawa K. 2005. Spectral heterogeneity of honeybee ommatidia. Naturwissenschaften 92, 464–467 10.1007/s00114-005-0018-5 (doi:10.1007/s00114-005-0018-5) [DOI] [PubMed] [Google Scholar]
- 36.Meinecke C. C. 1981. The fine structure of the compound eye of the African armyworm moth, Spodoptera exempta Walk (Lepidoptera, Noctuidae). Cell Tissue Res. 216, 333–347 10.1007/BF00233623 (doi:10.1007/BF00233623) [DOI] [PubMed] [Google Scholar]
- 37.White R. H., Brown P. K., Hurley A. K., Bennett R. R. 1983. Rhodopsins, retinula cell ultrastructure, and receptor potentials in the developing pupal eye of the moth Manduca sexta. J. Comp. Physiol. A 150, 153–163 10.1007/BF00606365 (doi:10.1007/BF00606365) [DOI] [Google Scholar]
- 38.White R. H., Xu H., Munch T., Bennett R. R., Grable E. A. 2003. The retina of Manduca sexta: rhodopsin-expression, the mosaic of green- blue- and UV-sensitive photoreceptors and regional specialization. J. Exp. Biol. 206, 3337–3348 10.1242/jeb.00571 (doi:10.1242/jeb.00571) [DOI] [PubMed] [Google Scholar]
- 39.Welsch B. 1977. Ultrastruktur und funktionelleMorphologie der Augen des Nachtfalters Deilephila elpenor (Lepidoptera, Sphingidae). Cytobiologie 14, 378–400 [Google Scholar]
- 40.Kelber A., Balkenius A., Warrant E. J. 2003. Colour vision in diurnal and nocturnal hawkmoths. Integr. Comp. Biol. 43, 571–579 10.1093/icb/43.4.571 (doi:10.1093/icb/43.4.571) [DOI] [PubMed] [Google Scholar]
- 41.Hardie R. C. 1985. Functional organization of the fly retina. In Progress in sensory physiology, vol. 5 (ed. Ottoson D.), pp. 1–79 Berlin, Germany: Springer [Google Scholar]
- 42.Osorio D. 2007. Spam and the evolution of the fly's eye. Bioessays 29, 111–115 10.1002/bies.20533 (doi:10.1002/bies.20533) [DOI] [PubMed] [Google Scholar]
- 43.Briscoe A. D. 2000. Six opsins from the butterfly Papilio glaucus: molecular phylogenetic evidence for paralogous origins of red-sensitive visual pigments in insects. J. Mol. Evol. 51, 110–121 [DOI] [PubMed] [Google Scholar]
- 44.Kitamoto J., Sakamoto K., Ozaki K., Mishina Y., Arikawa K. 1998. Two visual pigments in a single photoreceptor cell: identification and histological localization of three mRNAs encoding visual pigment opsins in the retina of the butterfly Papilio xuthus. J. Exp. Biol. 201, 1255–1261 [DOI] [PubMed] [Google Scholar]
- 45.Frentiu F. D., Bernard G. D., Sison-Mangus M. P., Brower A. V., Briscoe A. D. 2007. Gene duplication is an evolutionary mechanism for expanding spectral diversity in the long-wavelength photopigments of butterflies. Mol. Biol. Evol. 24, 2016–2028 10.1093/molbev/msm132 (doi:10.1093/molbev/msm132) [DOI] [PubMed] [Google Scholar]
- 46.Briscoe A. D., Bybee S. M., Bernard G. D., Yuan F., Sison-Mangus M. P., Reed R. D., Warren A. D., Llorente-Bousquets J., Chiao C. C. 2010. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl Acad. Sci. USA 107, 3628–3633 10.1073/pnas.0910085107 (doi:10.1073/pnas.0910085107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horridge G. A., Marcelja L., Jahnke R. 1984. Color vision in butterflies. 1. Single colour experiments. J. Comp. Physiol. A 155, 529–542 10.1007/BF00611917 (doi:10.1007/BF00611917) [DOI] [Google Scholar]
- 48.Kaiser W. 1974. The spectral sensitivity of the honeybee's optomotor walking response. J. Comp. Physiol. A 90, 405–408 10.1007/BF00694179 (doi:10.1007/BF00694179) [DOI] [Google Scholar]
- 49.Singarajah K. V. 1988. Spectral sensitivity of motion-sensitive units of the butterfly ventral nerve cord. J. Insect Physiol. 34, 1005–1012 10.1016/0022-1910(88)90199-0 (doi:10.1016/0022-1910(88)90199-0) [DOI] [Google Scholar]
- 50.Takemura S., Kinoshita M., Arikawa K. 2005. Photoreceptor projection reveals heterogeneity of lamina cartridges in the visual system of the Japanese yellow swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 483, 341–350 10.1002/cne.20446 (doi:10.1002/cne.20446) [DOI] [PubMed] [Google Scholar]
- 51.Takemura S. Y., Arikawa K. 2006. Ommatidial type-specific interphotoreceptor connections in the lamina of the swallowtail butterfly, Papilio xuthus. J. Comp. Neurol. 494, 663–672 10.1002/cne.20830 (doi:10.1002/cne.20830) [DOI] [PubMed] [Google Scholar]
- 52.Ribi W. A. 1975. The first optic ganglion of the bee. I. Correlation between visual cell types and their terminals in the lamina and medulla. Cell Tissue Res. 165, 103–111 [DOI] [PubMed] [Google Scholar]