Abstract
Egg mimicry by obligate avian brood parasites and host rejection of non-mimetic eggs are well-known textbook examples of host–parasite coevolution. By contrast, reciprocal adaptations and counteradaptations beyond the egg stage in brood parasites and their hosts have received less attention. The screaming cowbird (Molothrus rufoaxillaris) is a specialist obligate brood parasite whose fledglings look identical to those of its primary host, the baywing (Agelaioides badius). Such a resemblance has been proposed as an adaptation in response to host discrimination against odd-looking young, but evidence supporting this idea is scarce. Here, we examined this hypothesis by comparing the survival rates of young screaming cowbirds and non-mimetic shiny cowbirds (Molothrus bonariensis) cross-fostered to baywing nests and quantifying the similarity in plumage colour and begging calls between host and cowbird fledglings. Shiny cowbirds suffered higher post-fledging mortality rates (83%) than screaming cowbirds (0%) owing to host rejection. Visual modelling revealed that screaming cowbirds, but not shiny cowbirds, were indistinguishable from host young in plumage colour. Similarly, screaming cowbirds matched baywings' begging calls more closely than shiny cowbirds. Our results strongly support the occurrence of host fledgling mimicry in screaming cowbirds and suggest a role of visual and vocal cues in fledgling discrimination by baywings.
Keywords: brood parasitism, mimicry, coevolution, chick rejection, visual modelling, cowbird
1. Introduction
Exploitation of parental care by obligate avian brood parasites typically entails fitness costs to host parents. Parasitized nests may suffer partial or total brood losses owing to the removal or destruction of host eggs by parasitic females [1–3], killing or eviction of host young by parasitic chicks [4,5], or increased mortality of host chicks owing to competition within parasitized broods [6,7]. These interactions between parasites and their hosts may result in a coevolutionary arms race in which hosts evolve defences against parasitism that, in turn, select for counterdefences in parasite populations [8,9]. A paradigmatic example is the evolution of egg mimicry in parasitic females in response to host rejection of alien eggs, which has been extensively studied in the common cuckoo (Cuculus canorus) [4,10–12].
Beyond the egg stage, however, the occurrence of coevolved adaptations between parasite and hosts has received less attention [13,14]. Some recent studies in bronze-cuckoos (Chalcites spp. and Chrysococcyx minutillus) and their hosts have shown that host parents were able to reject parasitic chicks either through the desertion of parasitized nests soon after hatching [15] or active eviction of parasitic young out of the nest [16,17], and later work suggests that host rejection behaviour, in turn, may have selected for reciprocal host chick mimicry in bronze-cuckoos [15,18]. These findings have challenged prior theoretical arguments that learned chick discrimination would be maladaptive in hosts of evictor brood parasites such as cuckoos [19] (see also [20]) and suggest that a coevolutionary arms race similar to that observed at the egg stage may also occur at the nestling stage [15–17] (but see [21]). Visual mimicry of host chicks has also been reported in parasitic finches (Vidua sp.), whose young match precisely elaborate gape markings of host chicks [22,23]. Nevertheless, whether such a resemblance evolved in response to host rejection of alien chicks or through competition between host and parasitic young is not yet clear [23,24].
Reciprocal adaptations and counteradaptations beyond the nestling stage are even less documented [14]. In the screaming cowbird (Molothrus rufoaxillaris), host fledgling mimicry has been proposed to explain the close resemblance between parasitic fledglings and those of its primary host, the baywing (Agelaioides badius) [25]. Newly hatched screaming cowbirds and baywings differ minimally in skin and bill coloration but as parasitic chicks grow older, they become increasingly similar to host chicks [26]. By the time they leave the nest, screaming cowbirds and baywings look nearly identical to the human eye, and this resemblance persists until cowbird juveniles begin to moult into the adult black plumage, about five weeks after hatching [27,28]. The appearance of screaming cowbird juveniles cannot be attributed to common descent [29], thus it has been proposed as a case of visual mimicry driven by host rejection of odd-looking young soon after fledging [25]. This idea was based on the observation that non-mimetic juveniles of another brood parasite, the shiny cowbird (Molothrus bonariensis), fledged from baywing nests but experienced high post-fledging mortality rates, presumably because baywings no longer feed them once they leave the nest [25]. Shiny cowbird fledglings resemble hosts and screaming cowbirds in size and body shape but lack the rufous plumage coloration characteristic of baywings [28]. However, further evidence in support of host discrimination against odd-looking juveniles is lacking, as are objective assessments of the degree of similarity between screaming cowbird and host young. Avian visual systems differ markedly from that of humans [30], thus quantifying plumage similarity between hosts and parasites from an avian perspective is necessary to better understand the evolution of mimicry in host–parasite systems [11,12,18,31,32]. In addition, hosts may use vocal cues to discriminate between their own and alien young, which may select for mimetic begging calls in brood parasite chicks [15]. Although prior observations suggest that vocal mimicry may occur in screaming cowbirds [25], the similarity in begging call structure between screaming cowbird and host nestlings and fledglings has not yet been analysed.
Our aim in this study was to evaluate the occurrence of host fledgling mimicry in screaming cowbirds. We conducted a cross-fostering experiment in order to test whether post-fledging survival rates differ between ‘non-mimetic’ (shiny cowbird) and ‘mimetic’ (screaming cowbird) fledglings reared by baywings, and quantified how well screaming cowbirds match host fledglings in plumage colour and begging calls using avian visual modelling and multivariate techniques.
2. Material and methods
(a). Study site and field methods
The study was conducted at ‘Reserva El Destino’ (35°08′ S, 57°23′ W) in the province of Buenos Aires, Argentina, between 2002 and 2007. Baywings breed in the area from December to February using domed nests of other bird species (e.g. Annumbius annumbis, Phacellodomus spp., Synallaxis spp.), secondary cavities and nest-boxes (see the electronic supplementary material for details). Screaming cowbirds and shiny cowbirds are year-round residents in the area and annually parasitize roughly 91–97% and 7–25% of baywing nests, respectively [33]. The screaming cowbird is the most specialized parasitic cowbird with only three known host species so far; it parasitizes almost exclusively the baywing in most of its geographical range [25,34]. By contrast, the shiny cowbird parasitizes more than 200 species throughout its distribution and uses baywings as secondary hosts [4,25].
To assess baywings' response towards young that do not resemble their own, we artificially parasitized 53 baywing nests (from a sample of 193 nests) with a single shiny cowbird egg (n = 50) or hatchling (n = 3) collected from nearby nests of chalk-browed mockingbirds (Mimus saturninus). Artificially parasitized broods were not further manipulated because we aimed to determine survival rates of shiny cowbird chicks under the most realistic conditions in baywing nests. Shiny cowbird and screaming cowbird nestlings typically hatched one day before host chicks (incubation periods: 13 days for baywings, and 12 days for shiny and screaming cowbirds). We estimated survival rates of host and parasite chicks from 34 nests (10 unparasitized, 12 parasitized by screaming cowbird only, six parasitized by screaming/shiny cowbirds and six parasitized by shiny cowbird only) that either survived to fledging (n = 33) or failed owing to causes other than nest predation (the entire brood died as a result of heavy ectoparasite infestation, n = 1).
Nests were checked every 1–2 days. We marked chicks with permanent ink and assigned them to baywing, screaming or shiny cowbird using skin and bill coloration as diagnostic cues [26]. We weighed all chicks daily or every other day in order to build growth curves, which are published elsewhere [33]. These data show that shiny cowbirds in baywing nests grew faster than host young and reached a higher asymptotic body mass, comparable to that of shiny cowbird chicks reared by their primary hosts in the same study area [33]. At the age of 10–11 days, host and parasite chicks were banded with a unique combination of colour plastic leg bands and a numbered aluminium band. We monitored nests from a distance daily or every other day to determine the date at which chicks fledged (average fledging age: 14 days post-hatching [33]).
We estimated survival rates of host and parasitic fledglings during the breeding seasons of 2005–2006 and 2006–2007 by following eight groups of adults with colour-banded fledglings over a week after fledging (table 1). From these, six were artificially parasitized with a single shiny cowbird egg (n = 4) or hatchling (n = 2), whereas the remaining two were naturally parasitized by screaming cowbirds and left unmanipulated. All groups parasitized by shiny cowbirds and one of those parasitized solely by screaming cowbirds had at least one helper at the nest in addition to the breeding pair. These groups remained in the vicinity (less than 200 m) of their nesting sites and were located through alarm calls of adults and begging calls of young. We conducted focal observations on each group once a day until every fledgling within the group was located and its identity recorded using 8 × 40 binoculars. Fledglings that were not found by the end of the first week after fledging despite exhaustive searching within the group's territory were considered to be dead. Shiny cowbird fledglings gave loud and distinctive begging calls that made them conspicuous and easy to locate even when hidden in the surrounding vegetation. Thus, we are confident that all shiny cowbird fledglings still alive were sighted during our observations.
Table 1.
Brood composition at fledging of eight breeding groups of baywings observed over a week after fledging. (Figures indicate number of fledglings of each species; bw, baywing; scr, screaming cowbird; sh, shiny cowbird. The last column summarizes the observations of non-mimetic shiny cowbird fledglings. All baywing (n = 21) and screaming cowbird fledglings (n = 6) were found alive by the end of the observation period.)
| group | bw | scr | sh | fate of shiny cowbird fledgling |
|---|---|---|---|---|
| 1 | 1 | 0 | 1 | survived, fed by individuals other than the foster parents |
| 2 | 5 | 0 | 1 | not fed by baywings, found dead on the ground near the nest on day 2 after fledging |
| 3 | 4 | 1 | 1 | not fed by baywings on day 1 post-fledging, ‘vanished’ on day 2 |
| 4 | 2 | 2 | 0 | |
| 5 | 0 | 0 | 1 | fed by a baywing on day 1 post-fledging, found alone on day 2, ‘vanished’ on day 3 |
| 6 | 4 | 1 | 1 | not fed by baywings on days 1 and 2 post-fledging, ‘vanished’ on day 3 |
| 7 | 4 | 1 | 1 | not fed by baywings on day 1 post-fledging, ‘vanished’ on day 2 |
| 8 | 1 | 1 | 0 |
(b). Sampling of plumage reflectance and visual modelling
We analysed the degree of similarity in plumage coloration among 14 screaming cowbird, 25 shiny cowbird and 15 baywing fledglings born in the study area during the breeding season 2009–2010. Baywing and cowbird chicks were removed from nests at the age of 8–10 days, taken to the laboratory and hand-reared to nutritional independence (see the electronic supplementary material for details).
We measured fledglings’ plumage reflectance on eight body regions (crown, throat, breast, upper and lower back, rump, outer wing coverts and primary feathers; see the electronic supplementary material) between days 13 and 20 post-hatching (average age: 16.1 ± 0.4 days). Reflectance measurements were taken indoors using an Ocean Optics S2000 spectrometer with a PX-2 pulsed xenon light source and a bifurcated fibreoptic probe (Ocean Optics, Inc.). The probe was housed in a black plastic tube to minimize incident ambient light and hold the probe tip at a constant distance (19.0 ± 0.1 mm) and angle (90°) from the body surface. Reflectance measures were calibrated against a white standard of barium sulphate [35], and against a black standard with the light source off. Calibration was performed immediately before measuring each individual in order to minimize any error owing to light source or sensor drift. We obtained data via spectral acquisition software package OOIBase32 (Ocean Optics, Inc.).
We used the Vorobyev–Osorio colour discrimination model [36,37] to calculate discriminability for each pair of homologous body regions of baywing, screaming and shiny cowbird fledglings. The model calculates a distance in avian colour space (ΔS) defined by the quantum catches of each cone cell type in the avian retina. Calculation of quantum catches requires data on spectral sensitivities and the relative number of each cone cell type in the receiver's retina. These data are unavailable for baywings, but current evidence suggests that spectral sensitivities of photoreceptors are highly conserved among non-corvid songbirds [30,38]. Therefore, we used data from the blue tit (Cyanistes caeruleus) as representative of the baywing's visual system [18,30]. Cone cell type ratios, however, vary among species [30]. Thus, in order to assess sensitivity of our results to different single cone ratios, we calculated quantum catches using data from the blue tit, blackbird (Turdus merula) and European starling (Sturnus vulgaris), which encompasses known variation among passerines [30,39]. Results were qualitatively identical, thus we reported ΔS values generated using blue tit data. Calculations were performed using the SPEC package in R [40,41] for standard d65 daylight illumination. The output of the model is in terms of just noticeable differences (jnds), where 1.0 jnd means that two spectra are barely distinguishable under ideal viewing conditions. Values of ΔS less than 1.0 mean that two spectra would be indistinguishable, and values of more than 1.0 indicate that two spectra would be increasingly easy to discriminate [42,43].
(c). Sampling of begging calls
We recorded begging calls of 13 baywing, 13 screaming cowbird and 15 shiny cowbird fledglings in captivity between 13 and 20 days post-hatching (average: 16.1 ± 0.4 days). Recordings were made using a directional microphone Sennheiser ME 66 connected to a digital audio recorder Edirol R-09, at a sampling rate of 44.1 kHz stereo (16 bits), and saved as standard wav files. Prior to recording, fledglings were fed to satiation and then deprived of food for 60 min to standardize their hunger level. Subsequently, we isolated them visually and acoustically from other fledglings and recorded their begging calls during 3–5 min. Sound files were converted to sonograms using Raven Pro v. 1.3 (Cornell Bioacoustics Research Program, Ithaca, NY, USA) with a Hanning window of 256 samples and 248 Hz filter bandwidth, a time grid with hop size of 128 samples and 50 per cent overlap, and a frequency grid with discrete Fourier transform size of 256 samples and grid spacing of 172 Hz. Call bouts were defined as repetitions of syllables with a discrete beginning and end in the sonogram. For each host and cowbird fledgling, we measured the maximum and minimum frequency (kHz), peak frequency (kHz), frequency bandwidth (kHz), call duration (s) and number of syllables from sonograms (see the electronic supplementary material for details). Measurements were taken of each of the first 10 call bouts following the initial 60 s of recording and then averaged for each individual. The shiny cowbird sample included fledglings reared by baywings and other host species (see the electronic supplementary material for details). However, a prior study failed to find significant differences in begging call structure between shiny cowbirds reared by different host species in our study area (R. Gloag 2010, unpublished data). These results suggest a minor role of learning in shaping the begging call structure of shiny cowbird young that could have obscured the interpretation of our results.
(d). Statistical analysis
We ran two-tailed one-sample t-tests to analyse whether mean chromatic differences between each pair of species significantly differ from the discrimination threshold of 1 jnd. To remove the problem of non-independence of pairwise chromatic distances in statistical tests, we first compared each individual host and parasitic fledgling with all fledglings of a given species to calculate a mean chromatic distance, unique to each host or parasitic fledgling [18]. These means were the unit of analyses in t-tests.
We performed a discriminant function analysis to assess how the parameters extracted from begging calls discriminate between baywing, screaming and shiny cowbird fledglings. The analysis followed a stepwise procedure in which the variable that minimizes the overall Wilk's lambda was entered at each step. Statistical analyses were performed using SPSS v. 19 (IBM, Chicago, IL, USA) and StatView v. 5.0 (SAS Institute). Tests were two-tailed with an alpha level of 0.05.
(e). Ethical considerations
Experimental cross-fostering of shiny cowbird chicks was necessary to conduct this study given the low rates of natural parasitism by shiny cowbirds in baywing nests. Failure rates of baywing nests are typically high (approx. 80%) in the study area, mainly owing to nest predation or abandonment in response to multiple parasitism. Thus, we needed to parasitize a relatively large number of nests in order to get estimates of post-fledging survival rates. However, as we observed that most cross-fostered chicks died after leaving the nest we discontinued the experiment, limiting our sample to six artificially parasitized nests that fledged shiny cowbird young.
3. Results
(a). Survival of mimetic and non-mimetic young
Chick survival rates in nests that were not depredated were 91 per cent for baywings (n = 33 nests), 94 per cent for screaming cowbird (n = 18 nests) and 92 per cent for shiny cowbird chicks (n = 12 nests). ‘Non-mimetic’ (shiny cowbird) and ‘mimetic’ (screaming cowbird) chicks fledged successfully in all but one nest where the entire brood died as a result of heavy infestation with nest mites. Overall, 11 of 12 shiny and 30 of 31 screaming cowbird chicks survived to fledging in baywing nests.
By contrast, post-fledging survival rates differed markedly between ‘mimetic’ and ‘non-mimetic’ young. Although all host and screaming cowbird fledglings were found alive a week after nest departure, only one of six shiny cowbird fledglings survived the same period (table 1). We did not see baywings escorting or feeding this one shiny cowbird, but we repeatedly observed two individuals of another species (Cychlaris gujanensis) delivering food to it. From the remaining five shiny cowbird fledglings, one was found dead on the ground near the nest on day 2 post-fledgling and the other four were not longer seen after 48–72 h outside the nest. These missing fledglings were alone and begging repeatedly perched on trees or on the ground within their natal territories the last day we observed them alive.
(b). Visual and vocal similarity between host and parasitic fledglings
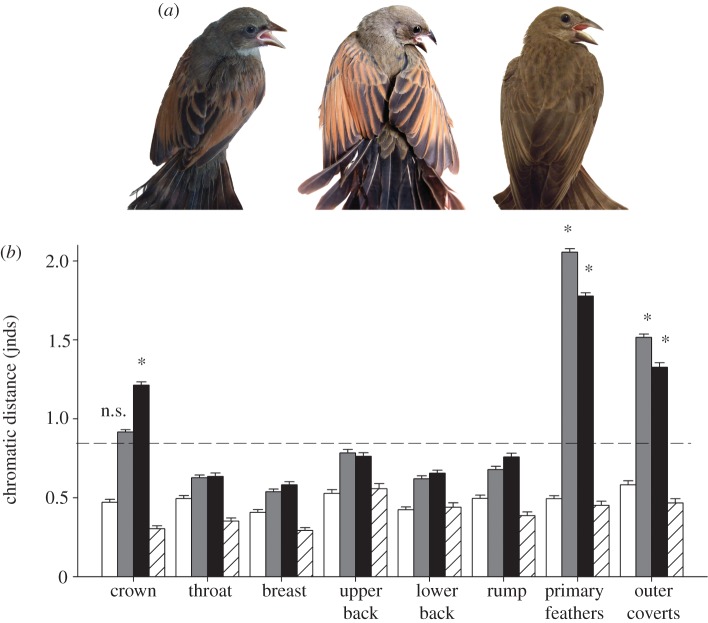
Screaming cowbird and baywing fledglings were almost identical in appearance to human eyes (figure 1a), and results of visual modelling indicated that they were also indistinguishable from an avian perspective. Mean chromatic distances between plumage spectra of screaming cowbird and host juveniles were significantly below the discrimination threshold (figure 1b). Interestingly, screaming cowbird fledglings were visually not different from host fledglings than host fledglings were from each other in most plumage patches (figure 1b). Shiny cowbird fledglings, on the other hand, varied in appearance from both screaming cowbirds and baywings (figure 1a). Mean chromatic distances between shiny cowbirds and the other two species were consistently larger than the corresponding distances between screaming cowbirds and baywings and exceeded the discrimination threshold in primary feathers and outer wing coverts (figure 1b). Mean chromatic distance between shiny and screaming cowbirds for the crown plumage patch was also above the threshold (figure 1b).
Figure 1.
(a) Baywing, screaming cowbird and shiny cowbird fledglings (from left to right), illustrating juvenile plumage coloration. (b) Mean ± s.e. chromatic distances (ΔS) in terms of just noticeable differences (jnds) between baywings and screaming cowbirds (white bars), baywings and shiny cowbirds (grey bars), and screaming and shiny cowbirds (black bars). Intraspecific contrasts among host fledglings are given for comparison (stripped white bars). The dashed line indicates the discrimination threshold of 1 jnd. Asterisks indicate mean chromatic distances significantly larger than 1 jnd after one-sample t-tests (p < 0.05); n.s. indicates mean contrasts not significantly different from 1 jnd. Sample sizes were: 15 baywing, 14 screaming cowbird and 25 shiny cowbird juveniles.
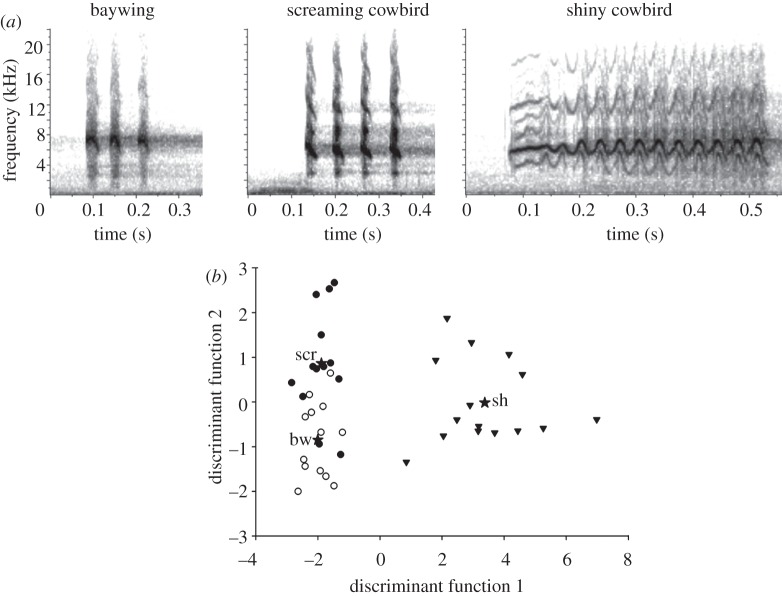
Screaming cowbirds matched baywing's begging calls more closely than shiny cowbirds (table 2 and figure 2a,b). The discriminant function analysis classified the begging calls of baywings, screaming cowbirds and shiny cowbirds with 86 per cent, 86 per cent and 100 per cent accuracy respectively, using the number of syllables, peak frequency and call duration (Wilks’ λ = 0.082, exact F: F6,72 = 29.8, p < 0.001; figure 2b). The number of syllables and call duration distinguished between shiny cowbirds and the other two species, whereas peak frequency distinguishes between baywing and screaming cowbirds (table 2 and figure 2b). The latter resemble baywing begging calls in number of syllables and duration but uttered lower pitched calls.
Table 2.
Begging call parameters (mean ± 1 s.e.) of baywing (bw), screaming cowbird (scr) and shiny cowbird (sh) fledglings. (The last two columns show the standardized coefficients for each parameter for each discriminant function (see §2). Only peak frequency, number of syllables and call duration entered the discriminant analysis.)
| call parameter | bw (n = 13) | scr (n = 13) | sh (n = 15) | function 1 | function 2 |
|---|---|---|---|---|---|
| min frequency (kHz) | 5.71 ± 0.25 | 4.67 ± 0.12 | 5.19 ± 0.33 | ||
| max frequency (kHz) | 8.48 ± 0.35 | 7.66 ± 0.21 | 8.96 ± 0.21 | ||
| bandwidth (kHz) | 2.78 ± 0.23 | 2.99 ± 0.17 | 3.77 ± 0.37 | ||
| peak frequency (kHz) | 7.26 ± 0.20 | 6.23 ± 0.14 | 7.67 ± 0.15 | 0.15 | 1.00 |
| number of syllables | 3.18 ± 0.27 | 3.32 ± 0.28 | 10.2 ± 0.59 | 1.90 | −0.71 |
| call duration (s) | 0.18 ± 0.02 | 0.18 ± 0.02 | 0.37 ± 0.02 | −1.22 | 0.60 |
Figure 2.
(a) Representative sonograms of begging calls of host and parasite fledglings and (b) canonical plots from discriminant function analysis separating the begging calls of baywing (open circles), screaming cowbird (filled circles) and shiny cowbird (black triangles). Black stars indicate the centroid of each group labelled bw, scr and sh, respectively. Sample sizes were: 13 baywing, 13 screaming cowbird and 15 shiny cowbird fledglings.
4. Discussion
This study supports the idea that screaming cowbirds mimic the juveniles of its primary host, the baywing. Cross-fostering experiments of shiny cowbird chicks showed that baywings successfully reared non-mimetic chicks, but they stopped providing parental care to them after fledging. Furthermore, our observations indicated that both host parents refused to feed shiny cowbirds fledglings, suggesting that female and male baywings use similar rejection rules. This contrasts with prior studies on the superb fairy-wren (Malurus cyaneus) parasitized by Horsfield's bronze-cuckoos (Chalcites basalis) and the shining bronze-cuckoo (Chalcites lucidus), where apparently only host females showed discrimination behaviour against parasitic young [15,44].
Increased post-fledging mortality as a consequence of host discrimination seems the most likely explanation for shiny cowbird fledglings ‘vanishing’ after leaving the nest, as they have limited flying skills and are nutritionally dependent on their host parents for several weeks. Although adoption of parasitic fledglings by individuals other than the foster parents is possible ([45], this study), this is a rare phenomenon that cannot account for the consistent disappearance of shiny cowbirds fledged from baywing nests. Likewise, it seems unlikely that cross-fostering per se had an effect on host's behaviours towards screaming and shiny cowbird fledglings given that this manipulation was performed early in the egg or nestling stages; well in advance hosts began to show rejection behaviour towards cross-fostered young. In addition, the results of visual modelling indicated that, from an avian perspective, screaming cowbird but not shiny cowbird fledglings were indistinguishable from those of baywings on the basis of plumage coloration. These findings, in agreement with prior observations [25], strongly suggest that the host-like appearance of screaming cowbird juveniles is adaptive and evolved in response to host discrimination against odd-looking young after fledging.
Coevolved adaptations beyond the egg stage between brood parasites and their hosts have been investigated relatively little, partly because theory predicts evolutionary constraints on learned chick discrimination in the hosts of evictor brood parasites such as cuckoos [19]. This traditional view, however, has been challenged by recent evidences of a coevolutionary arms race at the nestling stage between bronze-cuckoos and their hosts [15–17]. On the other hand, there are no theoretical constraints to the possibility of nestling rejection when hosts are exploited by non-evictor or ‘nest-mate tolerant’ brood parasites such as cowbirds [19]. Nevertheless, clear-cut examples of host discrimination against non-evictor parasites are still relatively scarce [13]. Our findings that baywings discriminate against non-mimetic parasitic young after they leave the nest extend these prior results and show that host–parasite coevolution may occur even at the fledgling stage.
The evolution of parasite chick rejection so late in the breeding cycle is puzzling as, by the time of fledging, host parents have already paid much of the costs of parasitism [25]; at this late stage, the potential costs of erroneously rejecting one's own offspring (recognition errors) might be expected to outweigh any benefit of rejection behaviour [46]. Furthermore, during the late nestling and fledgling periods, baywings often have helpers at the nest that contribute to young provisioning and may reduce the costs to host parents of raising parasitized broods to independence [47,48]. In theory, hosts may evolve defences against parasitic young either when parasites have broken down earlier lines of defence [15] or when they are constrained from evolving defences against parasite eggs [17]. In baywings, the evolution of fledgling discrimination in the absence of rejection behaviour against parasite eggs and nestlings suggests that they might be prevented from reliably discriminating against parasitic eggs or chicks [25]. Baywings breed in close, dark nests where host and parasitic chicks are often crowded and might be difficult to distinguish from each other on the basis of visual or vocal cues. Under this scenario, rejection of parasitic fledglings may still be selectively advantageous to host parents if they avoid prolonged parental care of unrelated offspring and improve their prospects for future survival or reproduction [5]. An alternative explanation is that rejection behaviour in baywings is the expression of host's pre-existing preferences for fledglings exhibiting visual or vocal traits that signal species identity, rather than an adaptation in response to fitness costs of parasitism at the fledging stage. From this perspective, host fledgling mimicry in screaming cowbirds would not be the outcome of a coevolutionary arms race, but the result of parasites tuning into host's sensory preferences to elicit adequate parental care and effectively competing for food with their brood-mates [24,49]. Both alternatives are compatible with our results, and more data are needed to assess their relative importance as selective forces driving the resemblance of screaming cowbirds to baywing young.
Screaming cowbirds and baywings also bore a closer vocal resemblance than baywings and shiny cowbirds. Screaming cowbirds matched the begging calls of host juveniles in all parameters analysed but gave lower frequency calls, a difference that might be partly owing to the parasite's larger body size [33,50]. These results support the early suggestion that mimicry of host fledglings by screaming cowbirds may extend also to vocal cues [25], though similarity does not necessarily imply mimicry [51]. Vocal resemblance by parasitic chicks has been previously reported in various cuckoo species [15,52,53], and available evidence indicates that it may be socially acquired, as cross-fostered parasite chicks modified the structure of their begging calls to match more closely that of the host that raised them [52,53]. Whether host-like begging calls in screaming cowbirds are innate or socially acquired remains an open question. Pilot observations, however, showed that screaming cowbird fledglings experimentally cross-fostered to chalk-browed mockingbird nests displayed similar begging calls to those raised by baywings and were able to attract the attention of adult baywings from nearby territories (M. C. De Mársico 2011, unpublished data). These observations suggest that the begging call structure of screaming cowbirds might be at least partially genetically determined and may play a role in attracting host attention after leaving the nest.
Our results support the hypothesis that screaming cowbird fledglings exhibit both visual and vocal similarity to their primary host, the baywing, and strongly suggest that such a resemblance makes them mimetic to host fledglings, probably driven by host discrimination against non-mimetic juveniles. Future research should aim to assess the fitness payoffs of post-fledging parental care to baywings and the relative importance of visual and vocal cues in host discrimination in order to improve our understanding of the evolution of this last line of host defence.
Acknowledgements
The manipulations performed in this work comply with the current laws of Argentina.
We thank Fundación Elsa Shaw de Pearson for permitting us to conduct this study at Reserva El Destino, and C. Ursino, V. Fiorini and A. de la Colina for helping us with chick collection and hand rearing. We are grateful to C. Facchinetti, R. Gloag and D. Lijtmaer for advice in data analysis; R. Gloag, B. Mahler, J. Sapp and two anonymous reviewers for helpful comments on earlier drafts. M.C.D.M. was supported by a fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). J.C.R. is a Research Fellow of CONICET. This work was supported by grants of Agencia Nacional de Promoción Científica y Tecnológica and University of Buenos Aires.
References
- 1.Davies N. B., Brooke M. de L. 1988. Cuckoos versus reed warbler hosts: adaptations and counteradaptations. Anim. Behav. 36, 262–284 10.1016/S0003-3472(88)80269-0 (doi:10.1016/S0003-3472(88)80269-0) [DOI] [Google Scholar]
- 2.Soler M., Soler J. J., Martínez J. G. 1997. Great spotted cuckoos improve their reproductive success by damaging magpie host eggs. Anim. Behav. 54, 1227–1233 10.1006/anbe.1997.0524 (doi:10.1006/anbe.1997.0524) [DOI] [PubMed] [Google Scholar]
- 3.Astié A. A., Reboreda J. C. 2006. Costs of egg punctures and parasitism by shiny cowbirds (Molothrus bonariensis) at creamy-bellied thrush (Turdus amaurochalinus) nests. Auk 123, 23–32 10.1642/0004-8038(2006)123[0023:COEPAP]2.0.CO;2 (doi:10.1642/0004-8038(2006)123[0023:COEPAP]2.0.CO;2) [DOI] [Google Scholar]
- 4.Davies N. B. 2000. Cuckoos, cowbirds and other cheats. London, UK: T & A. D. Poyser [Google Scholar]
- 5.Spottiswoode C. N., Koorevaar J. 2012. A stab in the dark: chick killing by brood parasitic honeyguides. Biol. Lett. 8, 241–244 10.1098/rsbl.2011.0739 (doi:10.1098/rsbl.2011.0739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoover J. P. 2003. Multiple effects of brood parasitism reduce the reproductive success of prothonotary warblers, Protonotaria citrea. Anim. Behav. 65, 923–934 10.1006/anbe.2003.2155 (doi:10.1006/anbe.2003.2155) [DOI] [Google Scholar]
- 7.Duré Ruiz N. M., Mermoz M. E., Fernández G. J. 2008. Effect of cowbird parasitism on brood reduction in the brown-and-yellow marshbird. Condor 110, 507–513 10.1025/cond.2008.8428 (doi:10.1025/cond.2008.8428) [DOI] [Google Scholar]
- 8.Dawkins R., Krebs J. R. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511 10.1098/rspb.1979.0081 (doi:10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 9.Rothstein S. I. 1990. A model system for coevolution: avian brood parasitism. Ann. Rev. Ecol. Syst. 21, 481–508 10.1146/annurev.es.21.110190.002405 (doi:10.1146/annurev.es.21.110190.002405) [DOI] [Google Scholar]
- 10.Brooke M. de L., Davies N. B. 1988. Egg mimicry by cuckoos, Cuculus canorus, in relation to discrimination by hosts. Nature 335, 630–632 10.1038/335630a0 (doi:10.1038/335630a0) [DOI] [Google Scholar]
- 11.Avilés J. M. 2008. Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc. R. Soc. B 275, 2345–2352 10.1098/rspb.2008.0720 (doi:10.1098/rspb.2008.0720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassey P., Honza M., Grim T., Hauber M. E. 2008. The modelling of avian visual perceptions predicts behavioural rejection responses to foreign egg colours. Biol. Lett. 4, 515–517 10.1098/rsbl.2008.0279 (doi:10.1098/rsbl.2008.0279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grim T. 2006. The evolution of nestling discrimination by hosts of parasitic birds: why is rejection so rare? Evol. Ecol. Res. 8, 785–802 [Google Scholar]
- 14.Grim T. 2011. Ejecting chick cheats: a changing paradigm? Front. Zool. 8, 14. 10.1186/1742-9994-8-14 (doi:10.1186/1742-9994-8-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmore N. E., Hunt S., Kilner R. M. 2003. Escalation of a coevolutionary arms race through host rejection of brood parasitic young. Nature 422, 157–160 10.1038/nature01460 (doi:10.1038/nature01460) [DOI] [PubMed] [Google Scholar]
- 16.Sato N. J., Tokue K., Noske R. A., Mikami O. K., Ueda K. 2010. Evicting cuckoo nestlings from the nest: a new anti-parasitism behaviour. Biol. Lett. 6, 67–69 10.1098/rsbl.2009.0540 (doi:10.1098/rsbl.2009.0540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tokue K., Ueda K. 2010. Mangrove gerygones Gerygone laevigaster eject little bronze-cuckoo Chalcites minutillus hatchlings from parasitized nests. Ibis 152, 835–839 10.1111/j.1474-919X.2010.01056.x (doi:10.1111/j.1474-919X.2010.01056.x) [DOI] [Google Scholar]
- 18.Langmore N. E., Stevens M., Maurer G., Heinsohn R., Hall M. L., Peters A., Kilner R. M. 2011. Visual mimicry of host nestlings by cuckoos. Proc. R. Soc. B 278, 2455–2463 10.1098/rspb.2010.2391 (doi:10.1098/rspb.2010.2391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotem A. 1993. Learning to recognize nestlings is maladaptive for cuckoo Cuculus canorus hosts. Nature 362, 743–745 10.1038/362743a0 (doi:10.1038/362743a0) [DOI] [Google Scholar]
- 20.Shizuka D., Lyon B. E. 2010. Coots use hatch order to learn to recognize and reject conspecific brood parasitic chicks. Nature 463, 223–226 10.1038/nature08655 (doi:10.1038/nature08655) [DOI] [PubMed] [Google Scholar]
- 21.Anderson M. G., Ross H. A., Brunton D. H., Hauber M. E. 2009. Begging call matching between a specialist brood parasite and its host: a comparative approach to detect coevolution. Biol. J. Linn. Soc. 98, 208–216 10.1111/j.1095-8312.2009.01256.x (doi:10.1111/j.1095-8312.2009.01256.x) [DOI] [Google Scholar]
- 22.Payne R. B., Woods J. L., Payne L. L. 2011. Parental care in estrildid finches: experimental tests of a model of Vidua brood parasitism. Anim. Behav. 62, 473–483 10.1006/anbe.2001.1773 (doi:10.1006/anbe.2001.1773) [DOI] [Google Scholar]
- 23.Schuetz J. 2005. Reduced growth but not survival of chicks with altered gape patterns: implications for the evolution of nestling similarity in a parasitic finch. Anim. Behav. 70, 839–848 10.1016/j.anbehav.2005.01.007 (doi:10.1016/j.anbehav.2005.01.007) [DOI] [Google Scholar]
- 24.Hauber M. E., Kilner R. M. 2007. Coevolution, communication, and host chick mimicry in parasitic finches: who mimics whom? Behav. Ecol. Sociobiol. 61, 497–503 10.1007/s00265-006-0291-0 (doi:10.1007/s00265-006-0291-0) [DOI] [Google Scholar]
- 25.Fraga R. M. 1998. Interactions of the parasitic screaming and shiny cowbirds (Molothrus rufoaxillaris and M. bonariensis) with a shared host, the bay-winged cowbird (M. badius). In Parasitic birds and their hosts: studies in coevoluti on (eds Rothstein S. I., Robinson S. K.), pp. 173–193 New York, NY: Oxford University Press [Google Scholar]
- 26.Fraga R. M. 1979. Differences between nestlings and fledglings of screaming and bay-winged cowbirds. Wilson Bull. 91, 151–154 [Google Scholar]
- 27.Hudson W. H. 1874. Notes on the procreant instincts of three species of Molothrus found in Buenos Ayres. Proc. Zool. Soc. Lond. 42, 153–174 10.1111/j.1096-3642.1874.tb02466.x (doi:10.1111/j.1096-3642.1874.tb02466.x) [DOI] [Google Scholar]
- 28.Ursino C. A., Facchinetti C., Reboreda J. C. In press Plumage maturation in brood parasitic screaming and shiny cowbirds. Ornithol. Neotrop. [Google Scholar]
- 29.Lanyon S. M. 1992. Interspecific brood parasitism in blackbirds (Icterinae): a phylogenetic perspective. Science 225, 77–79 10.1126/science.1553533 (doi:10.1126/science.1553533) [DOI] [PubMed] [Google Scholar]
- 30.Hart N. S. 2001. The visual ecology of avian photoreceptors. Prog. Ret. Eye Res. 20, 675–703 10.1016/S1350-9462(01)00009-X (doi:10.1016/S1350-9462(01)00009-X) [DOI] [PubMed] [Google Scholar]
- 31.Spottiswoode C. N., Stevens M. 2010. Visual modeling shows that avian host parents use multiple visual cues in rejecting parasitic eggs. Proc. Natl Acad. Sci. USA 107, 8672–8676 10.1073/pnas.0910486107 (doi:10.1073/pnas.0910486107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Igic B., Cassey P., Grim T., Greenwood D. R., Moskát C., Rutila J., Hauber M. E. 2012. A shared chemical basis of avian host–parasite egg colour mimicry. Proc. R. Soc. B 279, 1068–1076. 10.1098/rspb.2011.1718 (doi:10.1098/rspb.2011.1718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Mársico M. C., Mahler B., Reboreda J. C. 2010. Reproductive success and nestling growth of the baywing parasitized by screaming and shiny cowbirds. Wilson J. Ornithol. 122, 417–431 10.1676/09-140.1 (doi:10.1676/09-140.1) [DOI] [Google Scholar]
- 34.De Mársico M. C., Mahler B., Chomnalez M., Di Giacomo A. G., Reboreda J.C. 2010. Host use by generalist and specialist brood parasitic cowbirds at population and individual levels. Adv. Stud. Behav. 42, 83–121 10.1016/S0065-3454(10)42003-3 (doi:10.1016/S0065-3454(10)42003-3) [DOI] [Google Scholar]
- 35.Osorio D., Ham A. D. 2002. Spectral reflectance and directional properties of structural coloration in bird plumaje. J. Exp. Biol. 205, 2017–2027 [DOI] [PubMed] [Google Scholar]
- 36.Vorobyev M., Osorio D. 1998. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358 10.1098/rspb.1998.0302 (doi:10.1098/rspb.1998.0302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorobyev M., Osorio D., Bennett A. T. D., Marshall N. J., Cuthill I. C. 1998. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A 183, 621–633 10.1007/s003590050286 (doi:10.1007/s003590050286) [DOI] [PubMed] [Google Scholar]
- 38.Renoult J. P., Courtiol A., Kjellberg F. 2010. When assumptions on visual system evolution matter: nestling colouration and parental visual performance in birds. J. Evol. Biol. 23, 220–225 10.1111/j.1420-9101.2009.01885.x (doi:10.1111/j.1420-9101.2009.01885.x) [DOI] [PubMed] [Google Scholar]
- 39.Benites P., Eaton M. D., Lijtmaer D. A., Lougheed S. C., Tubaro P. L. 2010. Analysis from avian visual perspective reveals plumage colour differences among females of capuchino seedeaters (Sporophila). J. Avian Biol. 41, 597–602 10.1111/j.1600-048X.2010.05205.x (doi:10.1111/j.1600-048X.2010.05205.x) [DOI] [Google Scholar]
- 40.Hadfield J. D., Owens I. P. F. 2006. Strong environmental determination of a carotenoid-based plumage trait is not mediated by carotenoid availability. J. Evol. Biol. 19, 1104–1114 10.1111/j.1420-9101.2006.01095.x (doi:10.1111/j.1420-9101.2006.01095.x) [DOI] [PubMed] [Google Scholar]
- 41.R Development Core Team 2008. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.r.project.org. [Google Scholar]
- 42.Siddiqi A., Cronin T. W., Loew E. R., Vorobyev M., Summers K. 2004. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J. Exp. Biol. 207, 2471–2485 10.1242/jeb.01047 (doi:10.1242/jeb.01047) [DOI] [PubMed] [Google Scholar]
- 43.Eaton M. D. 2005. Human vision fails to distinguish widespread sexual dichromatism among sexually ‘monochromatic’ birds. Proc. Natl Acad. Sci. USA 102, 10 936–10 942 10.1073/pnas.0501891102 (doi:10.1073/pnas.0501891102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langmore N. E., Cockburn A., Russell A. F., Kilner R. M. 2009. Flexible cuckoo chick-rejection rules in the superb fairy-wren. Behav. Ecol. 20, 978–984 10.1093/beheco/arp086 (doi:10.1093/beheco/arp086) [DOI] [Google Scholar]
- 45.Sealy S. G., Lorenzana J. C. 1997. Feeding of nestling and fledgling brood parasites by individuals other than the foster parents: a review. Can. J. Zool. 75, 1739–1752 10.1139/z97-804 (doi:10.1139/z97-804) [DOI] [Google Scholar]
- 46.Lawes M. J., Marthews T. R. 2003. When will rejection of parasite nestlings by hosts of nonevicting brood parasites be favored? A misimprinting-equilibrium model. Behav. Ecol. 14, 757–770 10.1093/beheco/arg068 (doi:10.1093/beheco/arg068) [DOI] [Google Scholar]
- 47.Fraga R. M. 1991. The social system of a communal breeder, the bay-winged cowbird Molothrus badius . Ethology 89, 195–210 10.1111/j.1439-0310.1991.tb00304.x (doi:10.1111/j.1439-0310.1991.tb00304.x) [DOI] [Google Scholar]
- 48.Ursino C. A., De Mársico M. C., Sued M., Farall A., Reboreda J. C. 2011. Brood parasitism disproportionately increases nest provisioning and helper recruitment in a cooperatively breeding bird. Behav. Ecol. Sociobiol. 12, 2279–2286 10.1007/s00265-011-1238-7 (doi:10.1007/s00265-011-1238-7) [DOI] [Google Scholar]
- 49.Davies N. B. 2011. Cuckoo adaptations: trickery and tuning. J. Zool. 284, 1–14 10.1111/j.1469-7998.2011.00810.x (doi:10.1111/j.1469-7998.2011.00810.x) [DOI] [Google Scholar]
- 50.Ryan M. J., Brenowitz E. A. 1985. The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am. Nat. 126, 87–100 10.1086/284398 (doi:10.1086/284398) [DOI] [Google Scholar]
- 51.Grim T. 2005. Mimicry versus similarity: which resemblances between brood parasites and their hosts are mimetic and which are not? Biol. J. Linn. Soc. 84, 69–78 10.1111/j.1095-8312.2005.00414.x (doi:10.1111/j.1095-8312.2005.00414.x) [DOI] [Google Scholar]
- 52.Madden J. R., Davies N. B. 2006. A host-race difference in begging calls of nestling cuckoos Cuculus canorus develops through experience and increases host provisioning. Proc. R. Soc. B 273, 2343–2351 10.1098/rspb.2006.3585 (doi:10.1098/rspb.2006.3585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langmore N. E., Maurer G., Adcock G. J., Kilner R. M. 2008. Socially acquired host-specific mimicry and the evolution of host races in Horsfield's bronze-cuckoo Chalcites basalis. Evolution 62, 1689–1699 10.1111/j.1558-5646.2008.00405.x (doi:10.1111/j.1558-5646.2008.00405.x) [DOI] [PubMed] [Google Scholar]