Abstract
Indirect genetic effects (IGEs) occur when genes expressed in one individual affect the phenotype of a conspecific. Theoretical models indicate that the evolutionary consequences of IGEs critically depend on the genetic architecture of interacting traits, and on the strength and direction of phenotypic effects arising from social interactions, which can be quantified by the interaction coefficient Ψ. In the context of sexually selected traits, strong positive Ψ tends to exaggerate evolutionary change, whereas negative Ψ impedes sexual trait elaboration. Despite its theoretical importance, whether and how Ψ varies among geographically distinct populations is unknown. Such information is necessary to evaluate the potential for IGEs to contribute to divergence among isolated or semi-isolated populations. Here, we report substantial variation in Ψ for a behavioural trait involved in sexual selection in the field cricket Teleogryllus oceanicus: female choosiness. Both the strength and direction of Ψ varied among geographically isolated populations. Ψ also changed over time. In a contemporary population of crickets from Kauai, experience of male song increased female choosiness. In contrast, experience of male song decreased choosiness in an ancestral population from the same location. This rapid change corroborates studies examining the evolvability of Ψ and demonstrates how interpopulation variation in the interaction coefficient might influence sexual selection and accelerate divergence of traits influenced by IGEs that contribute to reproductive isolation in nascent species or subspecies.
Keywords: indirect genetic effect, mate choice plasticity, psi, sexual selection, social environment
1. Introduction
The social environment provides one of the most dynamic sources of environmental variation an individual animal is likely to experience in its lifetime [1]. Unlike the physical environment, social environments can generate indirect genetic effects (IGEs) when genes expressed in one individual affect the phenotype of an interacting conspecific [2]. The social environment itself can therefore evolve and generate additional selection that acts on interacting traits. This feedback can dramatically alter the rate and direction of selection, with consequent impacts on higher level evolutionary processes such as population divergence, reproductive isolation, sexual selection and speciation [3–5]. The importance of IGEs and social environments is that they can modulate evolutionary change, suggesting that traits showing particularly labile expression in response to social cues may play a key role in shaping evolutionary dynamics.
The classic view of IGEs describes how the phenotype of a focal individual, 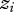 , is modified via an interaction with another individual, whose phenotype we denote as
, is modified via an interaction with another individual, whose phenotype we denote as 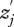 . The prime indicates that the phenotype is of an interacting individual, not the focal individual. The focal individual's phenotype can be partitioned as:
. The prime indicates that the phenotype is of an interacting individual, not the focal individual. The focal individual's phenotype can be partitioned as:
| 1.1 |
with contributions from additive genetic effects, 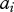 ; general environmental effects,
; general environmental effects, 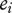 and the social environment,
and the social environment, 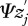 [2]. The interaction coefficient Ψ scales the effect of the interacting partner's phenotype on the focal individual's phenotype, and IGEs arise from the genetic component of the interacting partner's phenotype [2] (figure 1). Genes expressed in interacting individuals can thereby influence phenotypic expression in other individuals, meaning that the environmental component of trait expression can have a genetic basis, vary and evolve. The magnitude of Ψ describes the degree to which
[2]. The interaction coefficient Ψ scales the effect of the interacting partner's phenotype on the focal individual's phenotype, and IGEs arise from the genetic component of the interacting partner's phenotype [2] (figure 1). Genes expressed in interacting individuals can thereby influence phenotypic expression in other individuals, meaning that the environmental component of trait expression can have a genetic basis, vary and evolve. The magnitude of Ψ describes the degree to which 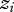 changes as a result of the interaction; in other words, the relative impact of the social environment on expression of the focal trait. The sign of Ψ describes whether the focal individual's trait value increases or decreases as a result of the interaction. When
changes as a result of the interaction; in other words, the relative impact of the social environment on expression of the focal trait. The sign of Ψ describes whether the focal individual's trait value increases or decreases as a result of the interaction. When 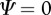 , the social environment has no effect (figure 1).
, the social environment has no effect (figure 1).
Figure 1.
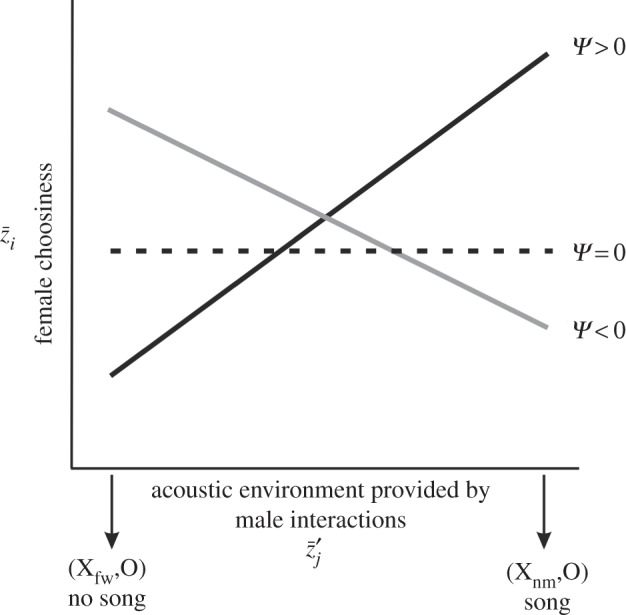
The quantification and interpretation of the interaction coefficient Ψ in the present study. The y-axis indicates the focal female trait (choosiness, 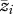 ) averaged among a sample of females. The x-axis indicates the average acoustic environment experienced by those females (
) averaged among a sample of females. The x-axis indicates the average acoustic environment experienced by those females (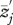 ). The prime (
). The prime ( ) indicates that the male trait is expressed in an interacting partner or partners. The male genotypes that determine the interacting phenotype are indicated below the x-axis. The flatwing trait segregates as a sex-linked Mendelian trait, and T. oceanicus has an (X,X)/(X,O) sex determination system. Thus, the intensity of the acoustic environment scales with the proportion of flatwing alleles in a population. Hypothetical values of Ψ are indicated by the lines. A positive Ψ means that average female choosiness increases as the acoustic environment becomes noisier. In contrast, a negative Ψ describes a situation where females become less choosy with a noisier acoustic environment. When
) indicates that the male trait is expressed in an interacting partner or partners. The male genotypes that determine the interacting phenotype are indicated below the x-axis. The flatwing trait segregates as a sex-linked Mendelian trait, and T. oceanicus has an (X,X)/(X,O) sex determination system. Thus, the intensity of the acoustic environment scales with the proportion of flatwing alleles in a population. Hypothetical values of Ψ are indicated by the lines. A positive Ψ means that average female choosiness increases as the acoustic environment becomes noisier. In contrast, a negative Ψ describes a situation where females become less choosy with a noisier acoustic environment. When 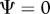 , the acoustic environment has no effect on female choosiness.
, the acoustic environment has no effect on female choosiness.
Quantitative genetic models of indirect genetic effects assume that Ψ is constant [2,3,5–8]. However, those models also indicate that the importance of IGEs in affecting evolution is critically dependent on Ψ. The coefficient Ψ represents an intuitive method for quantifying social effects to better understand the evolutionary dynamics of IGEs that those social effects generate. In the context of sexual selection, quantitative genetic models incorporating IGEs predict that Fisherian runaway sexual selection will depend on Ψ when female preference is affected by the social environment provided by male traits; positive Ψ increases the likelihood of runaway and enlarges the range of trait values to which populations can evolve via the Fisher process, whereas a negative Ψ will hinder evolutionary change [9]. Experience of male sexual signals has been found to alter female choice in many taxa, for example, through mate preference learning in damselflies [10], mate choice copying in fish [11], experience effects in bugs [12] and sexual imprinting in birds [13].
Despite its theoretical importance, we are aware of only three studies that have quantified Ψ. The first study estimated Ψ for traits involved in chemical communication in Drosophila melanogaster [14]. The second quantified Ψ for components of antipredator behaviour using inbred lines of the guppy Poecilia reticulata [15], and the third used artificial selection on Drosophila serrata to demonstrate that Ψ can evolve [16]. These studies provide empirical support for the importance of Ψ in shaping evolutionary outcomes when IGEs are implicated, thereby confirming theoretical predictions, but the extent to which Ψ varies among wild populations and across geographical barriers is unknown.
Variability in Ψ can have a large impact on evolution. If Ψ fluctuates over evolutionary time, then the influence of IGEs on selection will fluctuate accordingly. Similarly, if Ψ varies among populations, the rate and direction of evolutionary change within each population will differ even if direct selection and the genetic architecture of the traits of interest are uniform across populations. Such variation could be particularly important in nascent species or subspecies, because isolated but geographically proximate populations are likely to experience similar habitat and climate conditions. Inter-population variation in Ψ could have the effect of accelerating the accumulation of reproductive incompatibilities mediated by traits expressed in the context of social interactions such as mate choice. Empirical information establishing whether and how much Ψ varies among populations would therefore be helpful for predicting whether and how IGEs contribute to variation in selection on traits in natural populations, in particular, traits involved in reproductive isolation. We address these topics by capitalizing on an insect system in which the phenotype of focal individuals can be easily measured, interacting genotypes can be manipulated and Ψ can be compared among populations.
Our study organism is the field cricket Teleogryllus oceanicus. Teleogryllus oceanicus is distributed across northern Australia and Oceania, providing a natural laboratory in which relatively isolated populations can be screened using established behavioural assays to quantify components of female choice [17,18]. Males ordinarily sing to attract females for mating, but in 2003, a mutation, flatwing, arose on the Hawaiian island of Kauai that obligately silences males that carry it by erasing the sound-producing structures on their wings [19]. The mutation segregates as a single-locus, sex-linked trait [20] and it rapidly spread to near-fixation, with fewer than 10 per cent of males on Kauai now bearing normal wing morphologies [19]. As a result, the acoustic environment experienced by females on Kauai has dramatically shifted from an environment dense in male calling song to almost total silence. Since then, the flatwing phenotype has appeared on the neighbouring island of Oahu, albeit at a much lower frequency (ca 50% of males; J. T. Rotenberry and M. Zuk 2012, unpublished data). A number of studies have demonstrated that experience of male calling song in the environment mediates flexibility in female choice in this species [21–24]. The present study takes advantage of the flatwing mutation to evaluate female choosiness as the focal trait 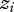 and the acoustic environment provided by male sexual signals as the interacting trait
and the acoustic environment provided by male sexual signals as the interacting trait 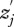 . Knowledge of the underlying genetics of the male trait allowed us to manipulate female interactions to precisely relate changes in the female phenotype—choosiness—to the genes present in interacting males comprising the social environment. In this case, Ψ describes how female choosiness changes as a result of experience of male sexual signals (figure 1). When Ψ is positive, female choosiness increases with acoustic experience, and vice versa.
. Knowledge of the underlying genetics of the male trait allowed us to manipulate female interactions to precisely relate changes in the female phenotype—choosiness—to the genes present in interacting males comprising the social environment. In this case, Ψ describes how female choosiness changes as a result of experience of male sexual signals (figure 1). When Ψ is positive, female choosiness increases with acoustic experience, and vice versa.
Treatments were set up to manipulate females' acoustic environments during rearing, and we then assessed the effect on the choosiness of females from different T. oceanicus populations. Variation in Ψ is indicated by the acoustic environment × population interaction [16] (figure 1). This rationale is developed further in §2. We evaluated the interaction in two experiments to test the hypotheses that (i) Ψ varies among geographically isolated populations of T. oceanicus, and (ii) Ψ can evolve over time within a population. Our results support both and provide insight into the impact that variation in Ψ is likely to have on the evolution of interacting traits potentially contributing to reproductive isolation, and of the potential influence of behavioural flexibility on population divergence.
2. Material and methods
(a). Cricket collection and rearing
Laboratory populations of crickets were established from five localities: Mission Beach, Australia; Samoa; and Hilo, Oahu and Kauai in Hawaii. Two populations of Kauai crickets were used. One was established prior to the emergence of the flatwing mutation (‘Ancestral Kauai’), and the other was established after the emergence of the mutation (‘Contemporary Kauai’). Ancestral Kauai has never contained flatwing male crickets, whereas flatwing males now comprise nearly 95 per cent of all males in Contemporary Kauai. Dates of population origin are given in Tinghitella & Zuk [25], and no population except Ancestral Kauai persisted unsupplemented in the laboratory for more than six generations at the time of testing. Ancestral Kauai could not be supplemented because the aim was to prevent the introduction of flatwing alleles. Population sizes exceeded 100 individuals at all times to minimize the loss of rare alleles through laboratory inbreeding or drift. Crickets were reared at 25°C on a 12 L:12 D cycle in separate 15 l containers with egg cartons for shelter, and they were provided Purina Rabbit Chow, Fluker's Cricket Chow and water ad libitum. All crickets were at least F2 individuals at the time of testing, which minimized the potential for field-based maternal effects.
(b). Treatment groups
Males and females were separated as soon as sex differences became apparent. Males were reared in single-sex groups of approximately 30 individuals in 15 l containers as above, and females were isolated in individual 118 ml containers with Purina Rabbit Chow, food and egg carton for shelter. The factor of interest in this study was the two-way interaction between population and acoustic treatment, so isolated females from each population were randomly assigned to a ‘no song’ or a ‘song’ treatment. No song females were reared in silence in a Precision 818 incubator under the same conditions as above. Song females were reared in a Precision 818 incubator in which male calling song was played back to simulate a high density of sexually signalling males. The protocol was identical to previous studies that successfully manipulated the acoustic environment in this species [21,24,26]. Briefly, male song was broadcast using sony SRS-m30 speakers at 80–85 dB measured at the lid of the females' containers, which yielded an intensity of approximately 70–75 dB inside the containers. This mimicked conditions that would be experienced in a wild population of singing T. oceanicus males [17].
Teleogryllus oceanicus calling song starts with a trill-like ‘long chirp’, which is followed by a series of lower amplitude paired pulses. The proportion of song occupied by long chirp influences the attractiveness of the song to females [17,18]. Following Bailey & Zuk [21], six male calling song models containing 0, 20, 40, 60, 80 or 100 per cent long chirp were simultaneously broadcast from separate speakers in the song incubator. Other song traits were parameterized to mean values of the Kauai population. The position of female containers in both the song and no song treatments was randomly rotated throughout the experiment, and a prior study that specifically tested for incubator effects found no evidence for them, using the same set of incubators as in the present study [26]. Thus, in all respects, the song and no song rearing conditions were identical, except for the acoustic environment experienced by females.
(c). Mating trials
To minimize the age-related variation in mating behaviour, all females were tested 6–10 days post-eclosion, and males used for testing were 6–15 days old. All females were paired with a randomly chosen flatwing male during their mating trial to evaluate female choosiness [25]. Flatwing males were used so that male acoustic signals would be controlled for, and no acoustic signals were present during mating trials. The assay thus estimated females' baseline willingness to mount, and allowed us to examine the effect of acoustic experience on their evaluation of male attractiveness. Each female was placed into a 1 l container and allowed to acclimate for 5–10 min, whereupon the male was introduced into the centre of the container and timing began. The latency of the male to court was recorded, plus the latency of the female to mount him. In some cases, females mounted males who had not stridulated. Trials lasted 10 min and if a female failed to mount a male during that time the trial was not included in the analysis.
Ordinarily, male crickets produce a courtship song once physical contact has been made with a female. Courtship song increases the likelihood of female mounting [18,27], but flatwing males are incapable of producing it. Nevertheless, they still raise their wings and attempt to stridulate, which enabled us to visually determine when males began to court during mating trials. Courtship stridulation is distinguishable from calling song stridulation because of the distinct visual pattern of wing movements and the presence of other close-range courtship behaviours such as antennation [27,28].
(d). Analysis
A previous study in a sister species of T. oceanicus found that female mounting latency reliably indicates male attractiveness [29]. As such, we quantified the latency of females to mount flatwing males starting from the time male courtship began, as indicated by the onset of stridulation movements of the elytra. Shorter latencies indicated greater female attraction [29].
The usual approach to estimating Ψ is to regress the phenotype of a focal individual or strain of known genotype on the phenotype of an interacting partner. The resulting regression coefficient indicates the strength and direction of Ψ [8]. Our estimates of Ψ are based on a population-level approach, much the same as has been used to estimate population-level female preference functions in this species [17]. The underlying assumption is that by testing multiple females from each population, we can estimate how Ψ varies among populations on average. Neutral marker genetic distances (FST) between these populations range from 0.027 (between Kauai and Hilo) to 0.240 (between Kauai and Samoa) [30], and considerable variation in other behavioural traits has been documented across a similar range of populations in T. oceanicus [17,25,31,32].
We adapted the analytical approaches of Bleakley & Brodie [15] and Chenoweth et al. [16] to detect differences in Ψ by evaluating whether the interaction between social treatment and population is significant. A significant interaction would indicate that crickets in different populations respond differently, on average, to variation in the social environment, and therefore that Ψ is different. We performed a general linear model (GLM) on natural log-transformed mounting latencies with ‘population’ as a categorical factor and ‘acoustic environment’ as a continuous variable, plus their interaction. The rationale for these factors is further developed below. Male and female mass were included as covariates. Consistent with a prior study [26], acoustic exposure affected the mass attained by developing females (see §3), so it was important to correct for the effects of female mass by including it as a covariate in the models. Latency data were standardized prior to analysis so that estimates of Ψ would be comparable across studies [5].
The social environments that we modelled were the song and no song acoustic environments that females experienced. These represent discrete intervals of a social environment that varies continuously in nature. The proportion of males carrying the flatwing allele(s) will be directly related to the degree of acoustic signalling that females experience in their environment (figure 1), and in our treatments, females were either exposed to constant silence or constant calling song during their dark cycles. The population × acoustic environment interaction was the primary variable of interest because it indicated whether the strength and/or sign of Ψ differed among populations. Treating the acoustic environment as a continuous variable in the model allowed us to generate estimates of Ψ based on regression coefficients and their associated p-values. These indicated whether Ψ differed significantly from zero within each population.
Two GLMs were performed. The first model examined population-level variation in Ψ, and it included data from the five recently collected populations and excluded data from Ancestral Kauai. In contrast, the second analysis included only Ancestral Kauai and Contemporary Kauai, which were collected from the same physical location but at different times. The latter analysis tested the possibility that the strength or direction of Ψ has changed in that population. The GLMs only included data from trials in which males produced courtship stridulations and females mounted them, to ensure that we only analysed cases where males could be verified as sexually receptive. However, females sometimes mounted males in the absence of any male stridulation (n = 92). We ran separate analyses in which these occurrences were included as latencies of zero. The results were qualitatively the same, so we present the first analysis only.
Post hoc tests were performed on the first analysis to examine which population comparisons contributed to the significant interaction. Interaction terms from all 10 population-pairwise GLMs provided an approximate guide to indicate which slopes significantly differed from one another; i.e. which populations differed in the strength and/or direction of Ψ.
Finally, the effect of acoustic experience on female body mass was analysed in all females from contemporary populations to compare with a previous study that found that males reared in the presence of song developed to be more massive and invested more in reproductive tissues [26]. This latter analysis was a two-way ANOVA with ‘acoustic experience’, ‘population’ and their interaction as factors, as before. Female body mass was natural-log transformed and standardized prior to analysis. Analyses were run in SAS v. 9.1 and Minitab v. 12.21.
3. Results
A total of 655 female crickets were tested. Consistent with previous studies [18,25], the rate at which females mount flatwing males was low (approx. 39%). Nevertheless, estimates of Ψ based on female mounting latencies varied significantly in both strength and direction among the different populations studied, as indicated by a significant population × acoustic environment interaction in the analysis of female response latencies (table 1). Figure 2 indicates for which population-pairwise comparisons Ψ differed significantly, and for which populations Ψ was significantly different from zero. Whether positive or negative, a departure from zero indicates that the acoustic environment had an impact on female choosiness. For example, Ψ was strong and negative in Hilo, meaning that choosiness was lower when females experienced male song. In contrast, it was positive in Mission Beach and Contemporary Kauai, where choosiness was higher when females experienced male song. In Samoa and Oahu, Ψ was not significantly different from zero, suggesting that on average, acoustic experience had little or no impact on the component of female choice measured in this study. It is also possible that Ψ was variable among individuals within those populations, such that Ψ was positive for some individuals, negative for others, and therefore approximately zero on a mean population level.
Table 1.
Results from a GLM of female latency to mount silent males in contemporary populations. Significant p-values are indicated in bold.
| d.f. | F | p | |
|---|---|---|---|
| population | 4 | 0.47 | 0.754 |
| treatment | 1 | 0.40 | 0.530 |
| population × treatment | 4 | 4.13 | 0.004 |
| female mass | 1 | 0.08 | 0.784 |
| male mass | 1 | 0.22 | 0.640 |
| error | 125 |
Figure 2.
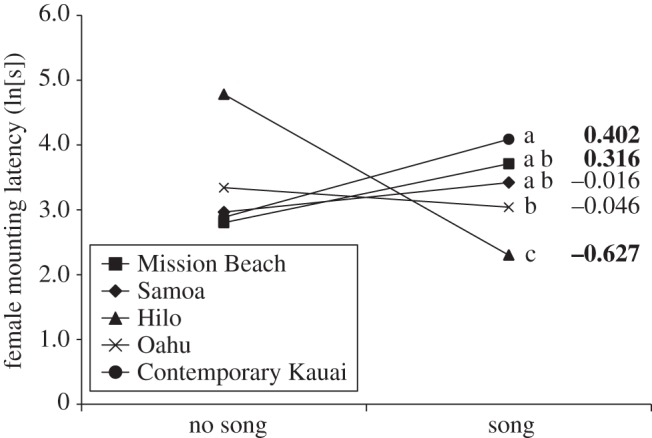
The influence of acoustic experience on female choosiness in contemporary T. oceanicus populations. Choosiness is indicated on the y-axis by natural log-transformed female mounting latency, with greater latency indicating greater choosiness. Error bars are omitted for ease of interpretation, but populations that differed significantly from one another in Ψ do not share one of the small letters to the right. Values of Ψ estimated from regression coefficients (see text for details) are indicated on the right, and those populations for which Ψ differed significantly from zero are indicated in bold. Squares with continuous line, Mission Beach; diamonds with continuous line, Samoa; triangles with continuous line, Hilo; crosses with continuous line, Oahu; circles with continuous line, Contemporary Kauai.
Females from Ancestral Kauai and Contemporary Kauai differed in their mounting latencies overall (table 2), but above and beyond that, the direction of Ψ was reversed in the two populations (figure 3). The difference between Ψ in each population was significant, and Ψ was also significantly different from zero in both populations (table 2). Contemporary Kauai females were more choosy after developing in the presence of male song, but Ancestral Kauai females were less choosy after developing in the presence of male song (figure 3). Females showed similar responses in the no song condition in both populations, but experience of male calling songs during development had opposite effects on females from the two populations. In this analysis, female size also affected mounting latencies (table 2), with heavier females taking longer to mount males.
Table 2.
Results from a GLM of female latency to mount silent males in Kauai, comparing Ancestral Kauai with Contemporary Kauai. Significant p-values are indicated in bold.
| d.f. | F | p | |
|---|---|---|---|
| population | 1 | 10.66 | 0.002 |
| treatment | 1 | 1.32 | 0.256 |
| population × treatment | 1 | 8.02 | 0.007 |
| female mass | 1 | 8.27 | 0.006 |
| male mass | 1 | 0.65 | 0.424 |
| error | 53 |
Figure 3.
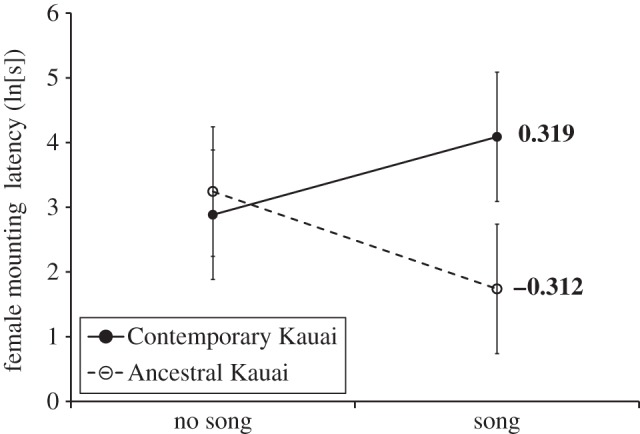
The influence of acoustic experience on female choosiness in Ancestral Kauai versus Contemporary Kauai. Choosiness is indicated on the y-axis by natural log-transformed female mounting latency, and values of Ψ estimated from regression coefficients (see text for details) are indicated on the right. Both were significantly different from zero, as indicated in bold. Error bars represent one s.e. Filled circles with continuous line, Contemporary Kauai; open circles with continuous line, Ancestral Kauai.
Overall, females that developed in a song-rich acoustic environment grew larger, attaining a higher mass at adulthood (GLM: F1,527 = 30.37, p < 0.001; figure 4). However, as with the behavioural data, a significant two-way interaction indicated that acoustic effects on mass were not equivalent across populations (GLM for mass: F4,527 = 8.59). The interaction was driven by a reversal in the effect of acoustic experience on female mass in the Mission Beach population; females from Mission Beach that were reared with song developed to be smaller (figure 4).
Figure 4.
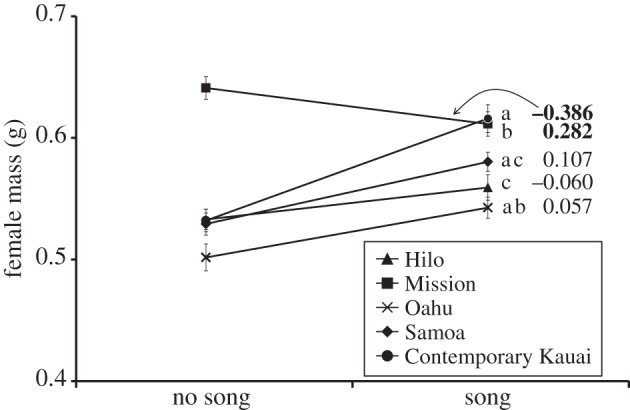
The influence of acoustic experience on female mass in contemporary T. oceanicus populations. Mass is indicated on the y-axis, and values of Ψ estimated from regression coefficients (see text for details) are indicated on the right. Error bars represent one s.e., and those populations for which Ψ differed significantly from zero are indicated in bold. Populations that differed significantly from one another in Ψ do not share one of the small letters to the right. Symbols are the same as given in figure legend 2.
4. Discussion
The evolutionary impacts of indirect genetic effects are becoming increasingly clear [33]. Quantitative genetic models incorporating IGEs have made the theoretical case that IGEs can accelerate or decelerate the evolution of interacting traits [2,6]. IGEs can also shift the direction of selection on traits expressed in a social context [34]. However, these dual influences are critically dependent on the interaction coefficient Ψ, and as such, any consequent effects on evolutionary processes such as the establishment and maintenance of reproductive isolation or population divergence, are also critically dependent on Ψ. Population subdivision plays an important role in exaggerating the evolutionary impacts of IGEs [4], and geographical and temporal variation in Ψ is therefore expected to be important for conferring different evolutionary rates and trajectories among subdivided populations. The present study provides evidence that such variation is likely to occur in nature.
Sexual selection is a logical context in which to examine IGEs and evaluate variation in Ψ, because mate choice necessarily involves social interactions, and social flexibility in female choice has been documented across numerous taxa [35,36]. Empirically, it is well established that females change their mate preferences based on prior experience [37–41], that they can become choosier when a range of male phenotypes is experienced [21,42], and that both sexes can learn about the distribution of male phenotypes in the environment based on interactions with conspecifics [26,43,44]. Taken together, these observations validate theoretical models that predict that females should flexibly modify their choosiness based on social interactions, that different populations should vary in that flexibility and that populations experiencing sexual selection should exhibit strong, positive Ψ [9,45]. Quantifying Ψ for traits involved in mate choice should yield insights about the dynamics of sexual selection in a given population or species [46].
In T. oceanicus, both the strength and direction of Ψ varied in different populations for a trait, female choosiness, which is known to impact the strength and direction of sexual selection on male traits implicated in reproductive isolation. Acoustic experience also affected female size differently in different populations, demonstrating that IGEs arising from the social environment provided by male calling songs in T. oceanicus affect not only behavioural traits, but also morphological traits. The present study cannot address the cause of population-level variation in Ψ, but there are several possible reasons for it. First, the dramatic reversal in the sign of Ψ in the Contemporary versus Ancestral Kauai population corroborates evidence of its evolvability provided by an artificial selection study in Drosophila serrata [16]. In that study, a similar reversal in Ψ was documented for social influences on the expression of a cuticular hydrocarbon, 2MeC26, after 16 generations of experimental evolution. In T. oceanicus, the Ancestral Kauai population showed a strong negative Ψ, such that acoustic experience made females less choosy. This pattern was echoed in Hilo (figures 2 and 3). However, females from Contemporary Kauai increased their choosiness of mates when reared in the presence of male calling song. The shift in Ψ had to have occurred over the course of approximately 24–32 generations, assuming between three and four generations per year since the Ancestral Kauai laboratory population was established in 2001, and this dramatic and rapid reversal in Ψ confirms the potentially important role of IGEs in the diversification of recently subdivided or isolated populations. In the wild, feedback from IGEs could accelerate the evolution of asymmetric mating isolation in subdivided populations if variation in Ψ causes heterogeneous selection on female choosiness. Asymmetrical mating isolation has in fact been documented in T. oceanicus and in other Oceanic gryllids [25,47], and future research would profit from testing causal links between variation in Ψ and patterns of mating isolation.
The direction of Ψ in Ancestral versus Contemporary Kauai could have switched for several reasons. One is that the flatwing mutation has rapidly spread through the Contemporary Kauai population, altering the acoustic environment as it propagated with each generation. This shift in the acoustic environment could have exerted selection favouring females who were able to facultatively decrease how choosy they were when song was scarce or absent, to facilitate finding a rare singing male or encountering a satellite male [22]. There are alternative explanations, however. The Ancestral Kauai population has been maintained as a laboratory colony since its inception in 2001; it was impossible to supplement because the aim was to maintain the population without introducing flatwing genotypes. Ancestral Kauai may therefore have experienced alternative selection over a longer period in the laboratory, which favoured the evolution of a negative Ψ because of the reduced importance of long-range signalling. It is intriguing, however, that the only other Hawaiian population not containing flatwing males showed a similarly negative Ψ, and that Oahu, which now contains approximately 50 per cent flatwing males, had a Ψ indistinguishable from zero. Another plausible alternative is that Ψ changes as a result of genetic drift or random sorting of ancestral variation as populations become subdivided. The role of drift could be equally important in setting up different responses to social cues in populations that have become subdivided, or which were subjected to repeated founder effects such as may have occurred as the range of T. oceanicus expanded eastward across Oceania during serial bottlenecks [25].
The geographical variation in social flexibility in female choice we have documented suggests parallels with models of speciation by sexual selection, which describe how traits under sexual selection can drift to different equilibrium points or runaway in different directions in isolated populations, thereby facilitating reproductive isolation [48]. In T. oceanicus, population-level variation in Ψ in the context of sexual selection can similarly alter the direction and strength of reproductive isolation among populations if it occurs for traits such as female preference or female choosiness, even if the composition of the social environment remains static across populations. For example, sexual selection on males may be strengthened in populations where acoustic experience increases female choosiness, such as in Oahu, while the same social experience appears to decrease female choosiness in Hilo, in turn relaxing selection on males (figure 2). While the underlying reasons for variation in Ψ in T. oceanicus female choice are not yet apparent, the fact that it does vary is sufficient to indicate an important role in driving traits implicated in precopulatory isolation towards different equilibrium values or evolutionary trajectories, above and beyond the effects of sexual selection. Variation in Ψ could thus provide an additional source of divergent selection on reproductively isolating traits, superimposed on sexual selection and evaluating spatial variation in Ψ is likely to be critical for understanding sexual selection in many systems in which social environments influence mating traits and populations are subdivided [4].
Considering IGEs can provide superior power for predicting evolutionary change in many contexts, including studies of evolutionary ecology, speciation and animal breeding [33,49]. IGEs provide a genetic framework for understanding the effects that behaviours involved in social interactions can have on the rate and direction of evolution, a topic that has attracted considerable interest in the behavioural literature but remains unresolved [1,2,50,51]. In particular, Ψ describes a specific property of those interactions that turns out to be of considerable importance. A fuller understanding of the dynamics of the interaction coefficient Ψ in a comparative context, both across and within species, is necessary to enhance the predictive capacity afforded by the IGE framework. The evidence we provide here for population-level variation in Ψ, and our corroboration that Ψ shows heritable genetic variation [16], suggest several future research priorities. First, models of IGEs would benefit from relaxing the assumption that Ψ is fixed. The effects that an evolving Ψ would have on the evolutionary dynamics imposed by IGEs are presently unclear. Second, empirical estimates of the heritability of and variation in Ψ would help to paramaterize such models. For example, Ψ may not always be linear. It could manifest as a threshold trait such that interaction with social partners does not affect a focal individual's phenotype unless a social partner's phenotype exceeds a certain value. Developing theory to address nonlinearity in Ψ and implementing it in subsequent empirical studies would provide a fruitful avenue of inquiry in the future. Social effects on male sexual trait expression [52] are also an important factor that should be incorporated in future studies. Finally, identifying the forces that cause evolutionary change in Ψ would yield insights into circumstances under which Ψ is expected to play a more important role in processes such as speciation or population divergence.
Acknowledgements
Y. Hira and B. Zelimkhanian provided much-appreciated assistance rearing crickets and performing behavioural assays, A. J. Moore and M. G. Ritchie provided helpful feedback on earlier versions of the manuscript, and two anonymous reviewers and the Associate Editor R. G. Harrison provided comments that improved the manuscript. This work was supported by a Natural Environment Research Council grant to N.W.B. (NE/G014906/1), a Pacific Rim Research Programme grant to N.W.B. and M. Z. (PRRP 05 0029) and a National Science Foundation grant to M. Z. (IOS 0641325).
References
- 1.West-Eberhard M. J. 1983. Sexual selection, social competition, and speciation. Q. Rev. Biol. 58, 155–183 10.1086/413215 (doi:10.1086/413215) [DOI] [Google Scholar]
- 2.Moore A. J., Brodie E. D., III, Wolf J. B. 1997. Interacting phenotypes and the evolutionary process. I. Direct and indirect genetic effects of social interactions. Evolution 51, 1352–1362 10.2307/2411187 (doi:10.2307/2411187) [DOI] [PubMed] [Google Scholar]
- 3.Wolf J. B., Brodie E. D., III, Moore A. J. 1999. Interacting phenotypes and the evolutionary process. II. Selection resulting from social interactions. Am. Nat. 153, 254–266 10.1086/303168 (doi:10.1086/303168) [DOI] [PubMed] [Google Scholar]
- 4.Agrawal A. F., Brodie E. D., III, Wade M. J. 2001. On indirect genetic effects in structured populations. Am. Nat. 158, 308–323 10.1086/321324 (doi:10.1086/321324) [DOI] [PubMed] [Google Scholar]
- 5.McGlothlin J. W., Moore A. J., Wolf J. B., Brodie E. D., III 2010. Interacting phenotypes and the evolutionary process. III. Social evolution. Evolution 64, 2558–2574 10.1111/j.1558-5646.2010.01012.x (doi:10.1111/j.1558-5646.2010.01012.x) [DOI] [PubMed] [Google Scholar]
- 6.Wolf J. B., Brodie E. D., III, Cheverud J. M., Moore A. J., Wade M. J. 1998. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13, 64–69 10.1016/S0169-5347(97)01233-0 (doi:10.1016/S0169-5347(97)01233-0) [DOI] [PubMed] [Google Scholar]
- 7.Moore A. J., Pizzari T. 2005. Quantitative genetic models of sexual conflict based on interacting phenotypes. Am. Nat. 165, S88–S98 10.1086/429354 (doi:10.1086/429354) [DOI] [PubMed] [Google Scholar]
- 8.Bleakley B. H., Wolf J. B., Moore A. J. 2010. The quantitative genetics of social behaviour. In Social behaviour (eds Székely T., Moore A. J., Komdeur J.), pp. 29–54 Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Bailey N. W., Moore A. J. In press Runaway sexual selection without genetic correlations: social environments and flexible mate choice initiate and enhance the Fisher process. Evolution. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Svensson E. I., Eroukhmanoff F., Karlsson K., Runemark A., Brodin A. 2010. A role for learning in population divergence of mate preferences. Evolution 64, 3101–3113 10.1111/j.1558-5646.2010.01085.x (doi:10.1111/j.1558-5646.2010.01085.x) [DOI] [PubMed] [Google Scholar]
- 11.Godin J.–G. J., Herdman E. J. E., Dugatkin L. A. 2005. Social influences on female choice in the guppy, Poecilia reticulata: generalized and repeatable trait-copying behaviour. Anim. Behav. 69, 999–1005 10.1016/j.anbehav.2004.07.016 (doi:10.1016/j.anbehav.2004.07.016) [DOI] [Google Scholar]
- 12.Fowler-Finn K. D., Rodríguez R. L. 2012. Experience-mediated plasticity in mate preferences: mating assurance in a variable environment. Evolution 66, 459–468 10.1111/j.1558-5646.2011.01446.x (doi:10.1111/j.1558-5646.2011.01446.x) [DOI] [PubMed] [Google Scholar]
- 13.ten Cate C., Vos D. 1999. Sexual imprinting and evolutionary processes in birds. Ard. Stud. Behav. 28, 1–31 10.1016/S0065-3454(08)60214-4 (doi:10.1016/S0065-3454(08)60214-4) [DOI] [Google Scholar]
- 14.Kent C., Azanchi R., Smith B., Formosa A., Levine J. D. 2008. Social context influences chemical communications in D. melanogaster males. Curr. Biol. 18, 1384–1389 10.1016/j.cub.2008.07.088 (doi:10.1016/j.cub.2008.07.088) [DOI] [PubMed] [Google Scholar]
- 15.Bleakley B. H., Brodie E. C., III 2009. Indirect genetic effects influence antipredator behavior in guppies: estimates of the coefficient of interaction psi and the inheritance of reciprocity. Evolution 63, 1796–1806 10.1111/j.1558-5646.2009.00672.x (doi:10.1111/j.1558-5646.2009.00672.x) [DOI] [PubMed] [Google Scholar]
- 16.Chenoweth S. F., Rundle H. D., Blows M. W. 2010. Experimental evidence for the evolution of indirect genetic effects: changes in the interaction coefficient, psi (Ψ), due to sexual selection. Evolution 64, 1849–1856 10.1111/j.1558-5646.2010.00952.x (doi:10.1111/j.1558-5646.2010.00952.x) [DOI] [PubMed] [Google Scholar]
- 17.Simmons L. W., Zuk M., Rotenberry J. T. 2001. Geographic variation in female preference functions and male songs of the field cricket Teleogryllus oceanicus. Evolution 55, 1386–1394 [DOI] [PubMed] [Google Scholar]
- 18.Bailey N. W. 2008. Love will tear you apart: different components of female choice exert contrasting selection pressures on male field crickets. Behav. Ecol. 19, 960–966 10.1093/beheco/arn054 (doi:10.1093/beheco/arn054) [DOI] [Google Scholar]
- 19.Zuk M., Rotenberry J. T., Tinghitella R. M. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 22, 521–524 10.1098/rsbl.2006.0539 (doi:10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinghitella R. M. 2008. Rapid evolutionary change in a sexual signal: genetic control of the mutation ‘flatwing’ that renders male field crickets (Teleogryllus oceanicus) mute. Heredity 100, 261–267 10.1038/sj.hdy.6801069 (doi:10.1038/sj.hdy.6801069) [DOI] [PubMed] [Google Scholar]
- 21.Bailey N. W., Zuk M. 2008. Acoustic experience shapes female mate choice in field crickets. Proc. R. Soc. B 275, 2645–2650 10.1098/rspb.2008.0859 (doi:10.1098/rspb.2008.0859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey N. W., Zuk M. 2009. Field crickets change mating preferences using remembered social information. Biol. Lett. 5, 449–451 10.1098/rsbl.2009.0112 (doi:10.1098/rsbl.2009.0112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebar D., Zuk M., Bailey N. W. 2011. Mating experience in field crickets modifies pre- and post-copulatory female choice in parallel. Behav. Ecol. 22, 303–309 10.1093/beheco/arq195 (doi:10.1093/beheco/arq195) [DOI] [Google Scholar]
- 24.Bailey N. W. 2011. Mate choice plasticity in the field cricket Teleogryllus oceanicus: effects of social experience in multiple modalities. Behav. Ecol. Sociobiol. 65, 2269–2278 10.1007/s00265-011-1237-8 (doi:10.1007/s00265-011-1237-8) [DOI] [Google Scholar]
- 25.Tinghitella R. M., Zuk M. 2009. Asymmetric mating preferences accommodated the rapid evolutionary loss of a sexual signal. Evolution 63, 2087–2098 10.1111/j.1558-5646.2009.00698.x (doi:10.1111/j.1558-5646.2009.00698.x) [DOI] [PubMed] [Google Scholar]
- 26.Bailey N. W., Gray B., Zuk M. 2010. Acoustic experience shapes alternative mating tactics and reproductive investment in field crickets. Curr. Biol. 20, 845–849 10.1016/j.cub.2010.02.063 (doi:10.1016/j.cub.2010.02.063) [DOI] [PubMed] [Google Scholar]
- 27.Balakrishnan R., Pollack G. S. 1997. The role of antennal sensory cues in female responses to courting males in the cricket Teleogryllus oceanicus. J. Exp. Biol. 200, 511–522 [DOI] [PubMed] [Google Scholar]
- 28.Ryan K. M., Sakaluk S. K. 2009. Dulling the senses: the role of the antennae in mate recognition, copulation and mate guarding in decorated crickets. Anim. Behav. 77, 1345–1350 10.1016/j.anbehav.2009.02.011 (doi:10.1016/j.anbehav.2009.02.011) [DOI] [Google Scholar]
- 29.Shackleton M. A., Jennions M. D., Hunt J. 2005. Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice tests. Behav. Ecol. Sociobiol. 58, 1–8 10.1016/j.anbehav.2009.02.011 (doi:10.1016/j.anbehav.2009.02.011) [DOI] [Google Scholar]
- 30.Tinghitella R. M., Zuk M., Beveridge M., Simmons L. W. 2011. Island hopping introduces Polynesian field crickets to novel environments, genetic bottlenecks and rapid evolution. J. Evol. Biol. 24, 1199–1211 10.1111/j.1420-9101.2011.02255.x (doi:10.1111/j.1420-9101.2011.02255.x) [DOI] [PubMed] [Google Scholar]
- 31.Zuk M., Simmons L. W., Cupp L. 1993. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav. Ecol. Sociobiol. 33, 339–343 [Google Scholar]
- 32.Simmons L. W. 2004. Genotypic variation in calling song and female preferences of the field cricket Teleogryllus oceanicus. Anim. Behav. 68, 313–322 10.1016/j.anbehav.2003.12.004 (doi:10.1016/j.anbehav.2003.12.004) [DOI] [Google Scholar]
- 33.Bijma P. 2010. Estimating indirect genetic effects: precision of estimates and optimum designs. Genetics 186, 1013–1028 10.1534/genetics.110.120493 (doi:10.1534/genetics.110.120493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bijma P., Wade M. J. 2008. The joint effects of kin, multilevel selection and indirect genetic effects on response to genetic selection. J. Evol. Biol. 21, 1175–1188 10.1111/j.1420-9101.2008.01550.x (doi:10.1111/j.1420-9101.2008.01550.x) [DOI] [PubMed] [Google Scholar]
- 35.Jennions M. D., Petrie M. 1997. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. Camb. Phil. Soc. 72, 283–327 10.1017/S0006323196005014 (doi:10.1017/S0006323196005014) [DOI] [PubMed] [Google Scholar]
- 36.Widemo F., Sæther S. A. 1999. Beauty is in the eye of the beholder: causes and consequences of variation in mating preferences. Trends Ecol. Evol. 14, 26–31 10.1016/S0169-5347(98)01531-6 (doi:10.1016/S0169-5347(98)01531-6) [DOI] [PubMed] [Google Scholar]
- 37.Collins S. A. 1995. The effect of recent experience on female choice in zebra finches. Anim. Behav. 49, 479–486 10.1006/anbe.1995.0062 (doi:10.1006/anbe.1995.0062) [DOI] [Google Scholar]
- 38.Hebets E. A. 2003. Subadult experience influences adult mate choice in an arthropod: exposed female wolf spiders prefer males of a familiar phenotype. Proc. Natl Acad. Sci. USA 100, 13 390–13 395 10.1073/pnas.2333262100 (doi:10.1073/pnas.2333262100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King A. P., West M. J., White D. J. 2003. Female cowbird song perception: evidence for plasticity of preference. Ethology 109, 1–13 10.1046/j.1439-0310.2003.00837.x (doi:10.1046/j.1439-0310.2003.00837.x) [DOI] [Google Scholar]
- 40.Welch A. M. 2003. Genetic benefits of a female mating preference in gray tree frogs are context-dependent. Evolution 57, 883–893 [DOI] [PubMed] [Google Scholar]
- 41.Kozak G. M., Boughman J. W. 2009. Learned conspecific mate preference in a species pair of sticklebacks. Behav. Ecol. 20, 1282–1288 10.1093/beheco/arp134 (doi:10.1093/beheco/arp134) [DOI] [Google Scholar]
- 42.Lehmann G. U. C. 2007. Density-dependent plasticity of sequential mate choice in a bushcricket (Orthoptera: Tettigoniidae). Aust. J. Zool. 55, 123–130 (doi:10.1071/ZO06105) [Google Scholar]
- 43.Dukas R. 2005. Learning affects mate choice in female fruit flies. Behav. Ecol. 16, 800–804 10.1093/beheco/ari057 (doi:10.1093/beheco/ari057) [DOI] [Google Scholar]
- 44.Kasumovic M. M., Hall M. D., Try H., Brooks R. C. 2011. The importance of listening: juvenile allocation shifts in response to acoustic cues of the social environment. J. Evol. Biol. 24, 1325–1334 10.1111/j.1420-9101.2011.02267.x (doi:10.1111/j.1420-9101.2011.02267.x) [DOI] [PubMed] [Google Scholar]
- 45.Kokko H., Mappes J. 2005. Sexual selection when fertilization is not guaranteed. Evolution 59, 1876–1885 [PubMed] [Google Scholar]
- 46.Petfield D., Chenoweth S. F., Rundle H. D., Blows M. W. 2005. Genetic variance in female condition predicts indirect genetic variance in male sexual display traits. Proc. Natl Acad. Sci. USA 102, 6045–6050 10.1073/pnas.0409378102 (doi:10.1073/pnas.0409378102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shaw K. L., Lugo E. 2001. Mating asymmetry and the direction of evolution in the Hawaiian cricket genus Laupala. Mol. Ecol. 10, 751–759 10.1046/j.1365-294x.2001.01219.x (doi:10.1046/j.1365-294x.2001.01219.x) [DOI] [PubMed] [Google Scholar]
- 48.Lande R. 1981. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA 78, 3721–3725 10.1073/pnas.78.6.3721 (doi:10.1073/pnas.78.6.3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wade M. J., Bijma P., Ellen E. D., Muir W. 2010. Group selection and social evolution in domesticated animals. Evol. Appl. 3, 453–465 10.1111/j.1752-4571.2010.00147.x (doi:10.1111/j.1752-4571.2010.00147.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West-Eberhard M. J. 1989. Developmental plasticity and evolution. Oxford, UK: Oxford University Press [Google Scholar]
- 51.Wcislo W. T. 1989. Behavioral environments and evolutionary change. Annu. Rev. Ecol. Syst. 20, 137–169 10.1146/annurev.es.20.110189.001033 (doi:10.1146/annurev.es.20.110189.001033) [DOI] [Google Scholar]
- 52.Meffert L. M. 1995. Bottleneck effects on genetic variance for courtship repertoire. Genetics 139, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]


