Abstract
All animals are under the constant threat of pathogenic infection. However, little is known regarding the influence of acute infection on sperm viability, particularly in female insects. This information is crucial for our understanding of mating and immune system coevolution, considering that females store sperm and serve as the site of sperm competition. Using the fruitfly, Drosophila melanogaster, we examined the influence of infection on sperm viability and storage. Twenty-four hours after haemocoel inoculation with a pathogen mimic (peptidoglycan, PGN) both sexes exhibited reduced sperm viability, indicating that systemic immune activation played a significant role in gamete survival. Surprisingly, sperm death did not appear to result from a reproductive-immune system trade-off, considering that sperm survived 24 h in vitro once removed from their somatic resources. Instead, our results are most consistent with death owing to immune effector collateral damage. We also examined the potential for sexually transmitted pathogens to influence sperm storage. Females mated with ‘infected’ males (created by dipping genitalia into a PGN solution) exhibited a higher proportion of empty sperm stores 48 h after mating compared to their controls. Remarkably, these data indicate that females may increase their fitness by removing ‘infected’ ejaculates from storage over time.
Keywords: sperm viability, sperm storage, infection, sexually transmitted pathogen, reproductive and immune system trade-off, collateral damage
1. Introduction
Across insect species, the viability of sperm is positively associated with the degree of sperm competition [1]. Within species, sperm viability may also predict the outcome of sperm competition and male fitness [2]. Thus, variation in viability appears to be shaped by selection and probably plays a key role in how insect mating systems evolve. Intraspecific variation in sperm viability may be the result of male physiological condition [3,4], male genotype [5], meiotic drive [2], or female mating history [6]. Recent work in vertebrates also suggests that variation may be influenced by a male's current pathogen load [7,8]. However, robust information regarding the role of acute infection on sperm viability in insects is surprisingly lacking, despite the recent interest among evolutionary biologists in understanding the selective pressures that shape mating and immune system coevolution in animals [9–11].
Pathogenic infection can influence sperm quality through either direct or indirect interactions. With regard to direct interactions, recent vertebrate studies suggest that sperm motility and viability decrease when sperm and bacteria are co-incubated in vitro [7,12,13]. Such an interaction should create intense selection against mating with infected partners owing to reduced fertility [14,15], as well as selection for a strong immune response to mating if infections are common. However, pathogenic infection can still reduce sperm quality in the absence of direct pathogen–gamete contact. Again, evidence for such an influence comes from a variety of vertebrate taxa (including mammals, birds and fishes), where bacterial and viral systemic infections in males were associated with reduced sperm number, motility and viability [16–19]. Unfortunately, the underlying mechanism(s) influencing sperm viability in these studies is (are) largely unknown, but may be owing to: (i) the migration of toxins from a replicating pathogen in the somatic tissue to the reproductive tract, (ii) a resource trade-off between the immune response and sperm maintenance, and (iii) unintended collateral damage caused by immune effectors aimed at curtailing a pathogenic insult.
Until this date, very little data existed regarding the influence of acute infection on sperm viability in male insects. Moreover, we know nothing about how infection influences sperm viability within the reproductive tract of females. This is crucial to our understanding of mating and immune system coevolution, considering the prevalence of female sperm storage among insects, as well as the fact that the female reproductive tract serves as the arena for sperm competition. In order to obtain a robust understanding of the role infection plays in sperm viability, we addressed the following questions in the fruitfly, Drosophila melanogaseter. First, does systemic pathogenic infection indirectly affect sperm viability in both sexes? Second, is a viability effect the result of: (i) the replicating pathogen (e.g. migrating exotoxins), (ii) a resource trade-off between immune and reproductive systems, or (iii) collateral damage of immune effectors? Third, can a direct reproductive tract infection via sexually transmitted pathogens also influence sperm viability and sperm storage? If the latter is true, then sexually transmitted pathogens may play a key role in shaping the evolution of the mating system.
2. Methods
(a). Stock maintenance
Flies used in the study were derived from wild caught females collected in Macon, GA in 2005 (courtesy of D. Promislow). Individuals were maintained as a moderately outbred population (approx. 200 flies per generation) in 30 ml plastic vials containing a cornmeal–sugar medium supplemented with live yeast. Vials were housed in incubators (Percival Model I36V) at 24°C on a 12 L : 12 D photoperiod. Experimental flies were separated under light CO2 anaesthesia by sex upon adult eclosion and held at a moderate density (20 per vial) as virgins. All flies were between 4 and 5 days old upon beginning each experiment.
(b). Sperm viability assay
Sperm viability was quantified using a live/dead sperm viability kit (Molecular Probes no. L-7011) that uses SYBR-14 and propidium iodide to differentially stain live (green fluorescence) and dead (red fluorescence) cells, respectively. Individuals were CO2 anaesthetized, and the sperm storage organs (either male seminal vesicles or female seminal receptacles) were removed from the reproductive tract and placed onto a clean microscope slide containing 20 μl of Drosophila Ringer's solution. It was washed once in this solution and then transferred to a new slide that contained 8 μl of Drosophila Ringer's solution. In males, the seminal vesicle was gently punctured to release the stored sperm. In females, sperm were gently pulled out of the seminal receptacle using fine forceps (sperm emerge in a rope-like structure).
Once the sperm were free of the storage organ, 1 μl of diluted SYBR-14 (1.6 μl of SYBR-14 in 10 μl of dimethyl sulphoxide) was added, and the sample was mixed gently using a fine needle and incubated for 3 min, in a dark, humid chamber. Subsequently, 1 μl of diluted propidium iodide (1.6 μl in 18 μl of Drosophila Ringer's solution) was added and the sample was mixed gently again and incubated in the dark for 1 min, in a humid chamber. Sperm were then viewed at 400× using a UMNG2 filter (Olympus, Japan) for viewing dead sperm (propidium iodide) and a U-MNBA2 filter (Olympus, Japan) for viewing live sperm (SYBR-14). The slide was then photographed using a Zeiss camera (AxioCam MRc5) attached to a Leica fluorescence microscope (Olympus, Japan) and the numbers of dead (red) and alive (green) sperm counted. Any sperm fluorescing both red and green were considered moribund and marked as dead. All sperm pictures and sperm counts were taken blind by coding each of the treatments in order to avoid bias. To avoid artefactual sperm death owing to prolonged exposure to the stains, all samples were processed and photographed within approximately 6 min of dissection (see [20] for the effects of dissection time on viability).
(c). Systemic infection
All individuals were mated 24 h prior to infection. Males and females were then randomly assigned to either a bacterial (Pseudomonas aeruginosa) or a control infection group. Flies were lightly CO2 anaesthetized and their thorax pierced with a fine-tipped needle dipped into either a diluted bacterial culture or sterile Luria broth (LB) (methods modified from Fedorka et al. [21]). The bacterial culture concentration was created by diluting log-phase bacteria in LB broth to an ocular density of 0.20, established using a microplate reader at 490 nm (Bio Rad model 680). From this solution, the bacteria were further diluted to a 1 × 10–3 concentration that corresponds to a sublethal dose in this D. melanogaster strain. Twenty-four hours after inoculation, individuals were assayed for their sperm viability as described above. The haemocoel inoculation allowed us to assess the ‘indirect’ influence of infection on sperm viability, considering that a chitin barrier between the reproductive and somatic tissue prohibits micro-organism migration.
To determine whether systemic immune activation alone could influence sperm viability (either through resource trade-offs or collateral damage of immune effectors), we inoculated individuals with peptidoglycan (PGN) derived from the cell walls of Escherichia coli (Invivogen no. K12-TLR2). In this capacity, PGN serves to activate the immune system without introducing a replicating, exotoxin-producing pathogen [22]. As with the bacterial infections, the thorax of lightly CO2 anaesthetized flies was pierced with a fine-tipped needle dipped in a PGN solution. The PGN solution was prepared by dissolving 0.001 g of PGN in 1 ml of sterile water and sonicating this solution for two to three minutes until the solution was completely clear and free of debris. Control flies were CO2 anaesthetized and pierced with a needle dipped in sterile water.
(d). Resource trade-off
To determine whether a resource trade-off between the reproductive and immune systems could influence sperm viability, we removed either a male's seminal vesicle or a female's seminal receptacle and placed them onto a microscope slide containing 200 μl of Drosophila Ringer's solution, thereby cutting off somatic resources. We rested the slide on top of a small 3.5 cm Petri dish that itself was placed into a larger 9 cm Petri dish filled with water. This prevented the sample from desiccating as well as kept the slide from touching the water. After 24 h at room temperature, we pipetted the sperm storage organ, along with 20 μl of Ringer's solution onto a new slide and assayed sperm viability. We used freshly dissected seminal vesicle and seminal receptacle as our controls (i.e. these were dissected moments before the sperm assay).
(e). Sexually transmitted infection
In order to determine whether reproductive tract infections influenced sperm viability and storage within the female, we allowed males to sexually transmit PGN during mating. To this end, virgin males were lightly CO2 anaesthetized until major motion stopped, whereupon they had their genitalia dipped in either a PGN solution (0.001 g PGN/1 ml sterile water), or sterile water. This was accomplished by touching a 200 μl pipette-tip containing 10 μl of solution to the tip of the abdomen for 20 s that deposited a small droplet covering the whole genital plate [22]. Males were then immediately placed into a vial containing a virgin female where they awoke and were allowed to mate (females were placed into their mating vials under CO2 anaesthesia 24 h prior to mating). Previous work in D. melanogaster has shown that this method is an effective means of sexual pathogen transmission [23]. Twenty-four hours after mating, females were dissected and their sperm viability assayed.
(f). Statistical analysis
All sperm viability data were analysed via ANCOVA, using arcsine square root transformed sperm viability as the dependent variable, treatment (e.g. infected versus control) as the categorical independent variable, sperm number as the covariate and the interaction between treatment and sperm number. A total of two outliers were identified via a Dixon test and removed, including one male control observation in the PGN inoculation experiment and one control observation in the sexually transmitted infection experiment [24]. Their removal did not impact the experimental conclusions. The statistical results are presented for the arcsine square root transformed data. However, all figures are presented with the non-transformed least square means. All analyses were conducted in JMP (v. 9.0).
3. Results
(a). Systemic infection
In all, 50 males and 50 females were assessed for sperm viability 24 h after inoculation with either a sublethal dose of P. aeruginosa or a sterile broth control. We found that females inoculated with bacteria did not exhibit a decline in sperm viability compared with the controls (table 1a and figure 1a). By contrast, males exhibited a sharp decline in viability when exposed to the pathogen (table 1a and figure 1b).
Table 1.
Systemic infection and in vitro assay. (Statistically significant values highlighted in bold.)
| source | d.f. | F-statistic | p-value |
|---|---|---|---|
| (a) live pathogen infection | |||
| female | |||
| treatment | 1.48 | 0.19 | 0.6623 |
| sperm number | 1.48 | 1.21 | 0.2762 |
| treat × number | 1.48 | 1.07 | 0.3072 |
| male | |||
| treatment | 1.48 | 21.57 | 0.0001 |
| sperm number | 1.48 | 3.19 | 0.0806 |
| treat× number | 1.48 | 2.53 | 0.1184 |
| (b) PGN inoculation | |||
| female | |||
| treatment | 1.29 | 7.04 | 0.0132 |
| sperm number | 1.29 | 0.07 | 0.7879 |
| treat× number | 1.29 | 0.01 | 0.9191 |
| male | |||
| treatment | 1.18 | 11.32 | 0.0039 |
| sperm number | 1.18 | 0.33 | 0.5726 |
| treat× number | 1.18 | 0.33 | 0.5726 |
| (c) in vitro assay | |||
| female | |||
| treatment | 1.11 | 0.13 | 0.7278 |
| sperm number | 1.11 | 0.80 | 0.3956 |
| treat× number | 1.11 | 0.31 | 0.5915 |
| male | |||
| treatment | 1.11 | 0.10 | 0.7571 |
| sperm number | 1.11 | 0.01 | 0.9096 |
| treat× number | 1.11 | 0.03 | 0.8581 |
Figure 1.
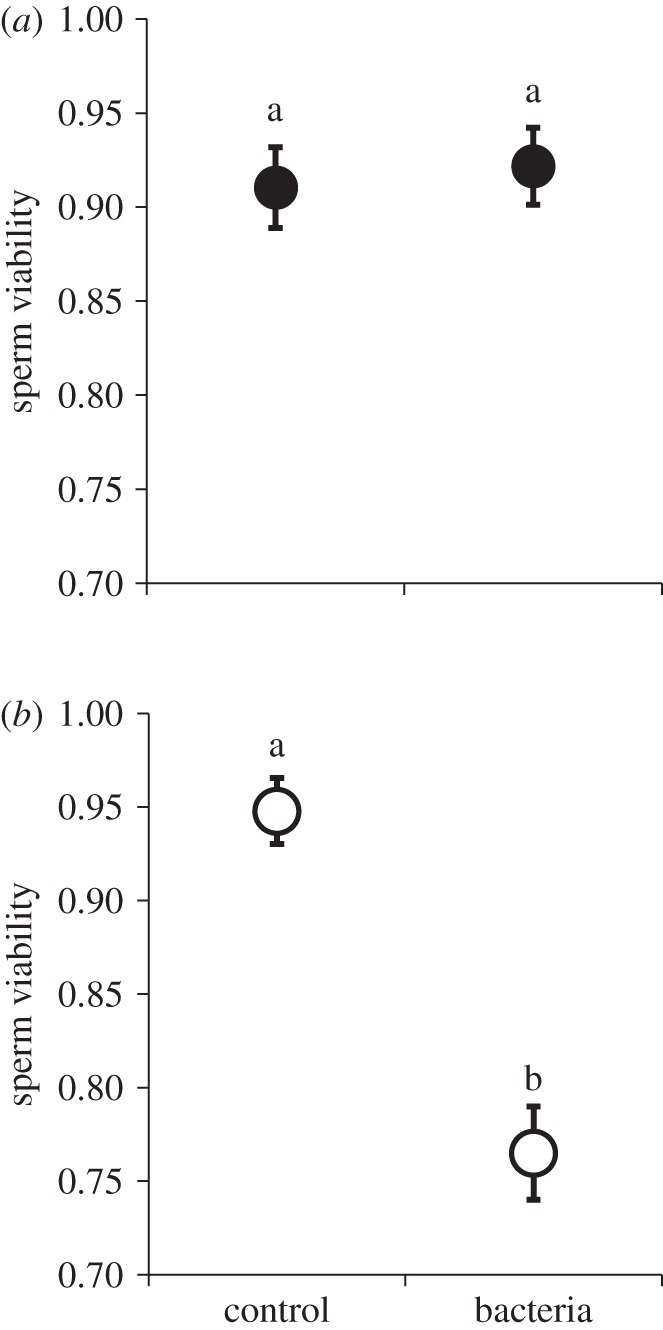
Systemic infection with live pathogen and sperm viability: (a) females, (b) males. Haemocoel inoculation with Pseudomonas aeruginosa had no influence on sperm viability in the female reproductive tract 24 h after infection. However, males inoculated with bacteria exhibited a significant decrease in sperm viability. Data points represent means and standard errors. Means connected by the same letter are not statistically different (α = 0.05).
To determine whether systemic immune activation alone could influence viability, we next inoculated both sexes with isolated PGN (n = 20 males and 31 females). In contrast to their bacterial pattern, PGN females exhibited a significant reduction in sperm viability (table 1b and figure 2a). PGN males likewise exhibited a similar magnitude of decline in the proportion of viable sperm (table 1b and figure 2b). These data suggest that immune system activation via haemocoel injections had a deleterious influence on sperm survival within both the male and female sperm storage organs.
Figure 2.
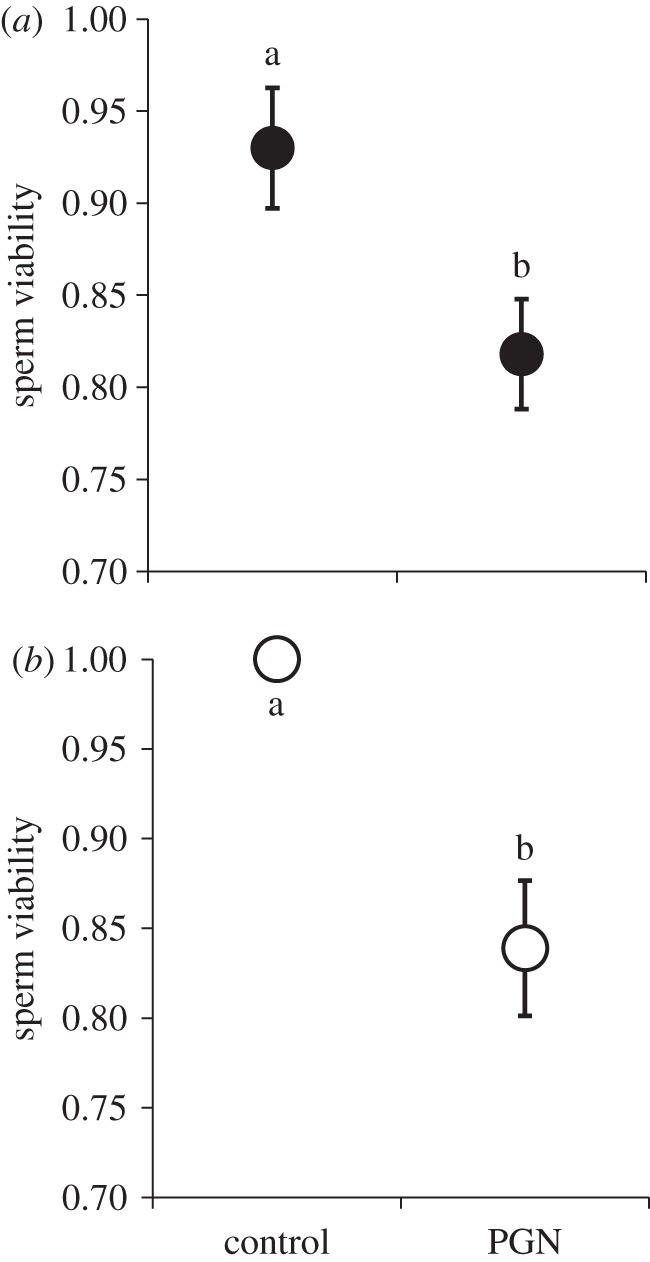
Systemic infection with peptidoglycan (PGN) and sperm viability: (a) females, (b) males. Haemocoel inoculations with PGN (a non-replicating immune activator) derived from bacterial cell walls had a significant effect on both male and female sperm viability. Means connected by the same letter are not statistically different (α = 0.05).
(b). Resource trade-off
To determine whether a resource trade-off between the reproductive and immune system could underlie the earlier-mentioned pattern, we placed both male and female sperm storage organs in Ringer's solution for 24 h and then assayed viability (sperm from organs dissected moments prior to the assayed served as controls). We found no difference in sperm viability between our 24 h treatment and their temporal controls in either sex (table 1c and figure 3). These data suggest that the resource trade-off is unlikely, considering that the 24 h treatment exhibited no significant sperm death when cut-off from somatic resources.
Figure 3.
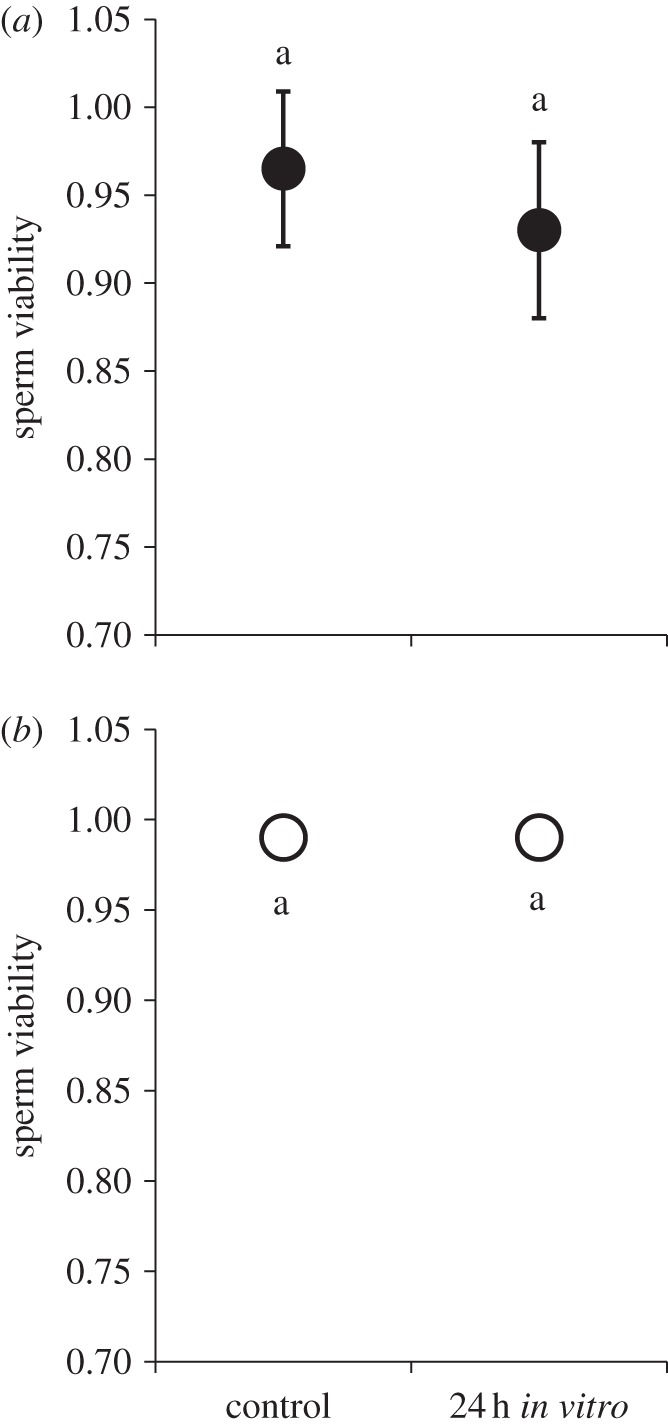
Sperm viability 24 h post dissection: (a) females, (b) males. After being cut-off from somatic resources via dissection, sperm remained viable in the male seminal vesicle and female seminal receptacle for 24 h. These data suggest that any reduction in sperm viability 24 h after infection was not the consequence of a resource trade-off between reproductive and immune systems. Means connected by the same letter are not statistically different.
(c). Sexually transmitted infection
In all, 30 females were assessed for sperm viability in their seminal receptacle 24 h after mating with PGN infected or control males (15 females in each treatment). We found no effect of sexually transmitted PGN on female sperm viability (all p > 0.68). However, we did find that an overwhelming proportion of females in the PGN treatment contained no sperm in the seminal receptacle compared with the controls (80% versus 26%, respectively; χ2 = 9.05, p = 0.0026). This pattern led us to hypothesize that pathogen-contaminated ejaculates were displaced soon after mating.
To test this hypothesis, we exposed females (n = 123) to either PGN or control-dipped males in the same manner as above and assessed sperm viability and storage in the seminal receptacle 1.25, 24 and 48 h after mating. The 1.25 h treatment also had the bursa copulatrix examined (site of sperm deposition within the female prior to storage in the seminal receptacle). One and a quarter hours after mating, both treatments contained a similar number of sperm in their reproductive tract (control: 318 ± 47.2 versus infected: 268 ± 36.8, F = 0.68, p = 0.4146) and all females possessed sperm (figure 4). With regard to sperm viability, females mated with PGN males exhibited a slight but significant reduction in sperm viability in the bursa copulatrix prior to sperm storage (0.93 ± 0.014 versus 0.99 ± 0.018, respectively; table 2a). Importantly, these observations indicate that females received relatively viable ejaculates (greater than or equal to 93% viable) from all males regardless of treatment. However, we found no difference in sperm viability within the seminal receptacle at any time point after mating (table 2b–d). With regard to sperm storage, we found that a higher proportion of PGN females lacked sperm in the seminal receptacle compared with the controls 48 h after mating (0.53 versus 0.07, respectively; χ2 = 8.89, p = 0.0029; figure 4), supporting the hypothesis that pathogen-contaminated ejaculates are removed over time.
Figure 4.
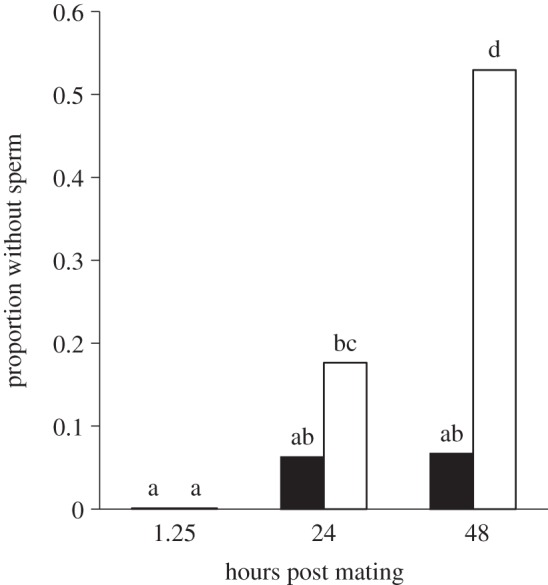
Female sperm storage after sexual transmission of peptidoglycan (PGN). Females mated with males whose genitalia were dipped in PGN exhibited in increasing lack of sperm in the seminal receptacle over time. Furthermore, a higher proportion of PGN females lacked sperm compared with the controls 48 h after mating. Means connected by the same letter are not statistically different (α = 0.05). White bars denote PGN; black bars denote control.
Table 2.
Sexually transmitted infection. (Statistically significant values highlighted in bold.)
| source | d.f. | F-statistic | p-value |
|---|---|---|---|
| (a) bursa copulatrix: 1.25 h post-mating | |||
| sperm viability | |||
| treatment | 1.25 | 4.33 | 0.0488 |
| sperm number | 1.25 | 1.06 | 0.3130 |
| treat × number | 1.25 | 1.84 | 0.1875 |
| (b) seminal receptacle: 1.25 h post-mating | |||
| sperm viability | |||
| treatment | 1.24 | 1.34 | 0.2598 |
| sperm number | 1.24 | 3.16 | 0.0892 |
| treat × number | 1.24 | 1.56 | 0.2254 |
| stored sperm | |||
| treatment | 1 | χ2 = 0.3 | 0.8613 |
| (c) seminal receptacle: 24 h post-mating | |||
| sperm viability | |||
| treatment | 1.27 | 0.85 | 0.3655 |
| sperm number | 1.27 | 0.12 | 0.7276 |
| treat × number | 1.27 | 2.19 | 0.1516 |
| stored sperm | |||
| treatment | 1 | χ2 = 0.92 | 0.3371 |
| (d) seminal receptacle: 48 h post-mating | |||
| sperm viability | |||
| treatment | 1.20 | 1.79 | 0.1967 |
| sperm number | 1.20 | 0.02 | 0.8679 |
| treat × number | 1.20 | 0.37 | 0.5493 |
| stored sperm | |||
| treatment | 1 | χ2 = 8.89 | 0.0029 |
4. Discussion
In this study, we examined the potential for systemic and reproductive tract infection to influence sperm viability and sperm storage. We found that systemic infection with a replicating pathogen (P. aeruginosa) reduced sperm viability in males but not in females. Furthermore, we found that systemic inoculation with PGN (a non-replicating component of bacterial cell walls) reduced sperm viability in both sexes. Thus, systemic immune system activation (sin pathogen) appears to have a significant and indirect influence on sperm viability.
As stated earlier, systemic infection may indirectly reduce sperm viability through three main mechanisms, including: (i) the migration of toxins from a live replicating pathogen into sperm storage, (ii) a resource trade-off between reproductive and immune systems, and (iii) collateral damage of the immune effectors. Our data suggest that the observed reduction in viability was not the consequence of migrating exotoxins or a resource trade-off, considering that no live pathogen was introduced in the PGN experiments and that sperm were able to survive for 24 h after being removed from the organism and its resources. Most likely, sperm death was caused by an unintended deleterious interaction between immune effectors and sperm, although this hypothesis remains to be tested directly. One potential group of immune effectors that could cause such an effect is reactive oxygen species (ROS). Oxidative bursts (i.e. the rapid and copious production of ROS upon infection) appear to be a highly conserved immune response against invading pathogens in both plants and animals [25]. Furthermore, sperm are exceedingly susceptible to such molecules [26–28]. Therefore, collateral damage to spermatozoa may have occurred if ROS were produced during the immune response and migrated from the haemocoel to the reproductive tract [26]. We are currently investigating the potential for ROS or other immune effectors to influence the viability of stored sperm.
With regard to the reproductive tract infections, we found that females mated with PGN-dipped males exhibited a small reduction in sperm viability (approx. 6%) shortly after mating. This reduction may have been caused by the direct influence of PGN, considering previous studies indicate that human sperm viability is reduced in the presence of lipopolysaccharides derived from Chlamydia trachomatis cell walls [29,30]. A direct and detrimental influence of bacteria (and their cell wall components) on sperm may help to explain why male ejaculate fluid in insects has evolved antibacterial activity [31,32] and why males and females increase antimicrobial activity in their reproductive tract shortly after mating [33,34]. Alternatively, the reduction in viability may be been caused by a strong female immune response to PGN-laced genitalia; especially if such a response was composed in part of ROS (see earlier text). Although the reproductive tracts of both sexes produce antioxidants to minimize ROS damage [27], these molecules may have been simply overwhelmed.
Perhaps the most interesting aspect of our sexual transmission data is that females mated with PGN males exhibited relatively empty sperm stores 48 h after mating. This is a fascinating observation which suggests that contaminated ejaculates are purged to: (i) remove dead/dying sperm, and/or (ii) avoid infection. Regardless of why sperm is lost, our results clearly show sperm removal is the action of the female. All females were single-mated and received a full complement of sperm regardless of their mate's infection status (table 2 and figure 4). However, only the females mated with infected males lost sperm over time. This is not too surprising, considering that dumping of resident sperm appears to be an adaptive behaviour under female control in D. melanogaster [35,36]. Although studies with Drosophila simulans and Mediterranean flour moths (Ephestia kuehniella) have shown that males infected with the endosymbiotic bacterium Wolbachia pipientis transfer fewer fertile sperm at mating than uninfected males [37,38], none have established a post-mating effect of infection on sperm storage within females, as we show here.
Although we provide evidence for a deleterious interaction between infection and sperm viability/storage, some aspects of our results appear inconsistent with this interpretation. First, females infected with P. aeruginosa did not exhibit a reduction in viability compared with females infected with isolated E. coli PGN. This discrepancy may owing to a greater amount of PGN having been transferred in the isolated PGN experiment, which would increase the strength of the female immune response. Alternatively, the discrepancy may be the result of a fundamental difference in how the PGN of the two different bacterial species elicit an immune response, or in how the female immune system responds to whole bacteria versus freely injected PGN. Second, males exhibited a response to the live pathogen inoculation while females did not. This pattern may be owing to the fact that females are larger than males, again creating a dose effect between the sexes. Alternatively, males may be more susceptible to Gram-negative pathogenic infection, which may help to explain why males tend to exhibit higher standing levels of Gram-negative immune gene expression [34]. Third, we found no reduction in sperm viability within the seminal receptacle when PGN was sexually transmitted to the female. As noted earlier, sperm viability within the bursa appears to have been slightly reduced. If dead sperm tend not to be moved from the bursa into storage (which seems reasonable), then we would expect little effect of sexually transmitted PGN on the viability of sperm within the seminal receptacle.
The results presented here have several important implications for mating and immune system coevolution. First, we support a fundamental prediction in several parasite-mediated sexual selection models, which expect a negative relationship between male ejaculate quality and immune activity [15,39]. Such a trade-off may cause males to: (i) honestly signal their ejaculate quality under the assumption that immune activation also reduces the sexual signal, and (ii) be at a disadvantage during sperm competition. Second, our data suggest that female immunological condition may also play a role in sperm competition, considering that a reduction in resident sperm viability owing to immune activation would put subsequent sperm donations at a competitive advantage. Third, our data suggest that females have the capacity to remove ejaculates that reduce their fitness (i.e. contain dead sperm or pose a health risk), which should have a profound impact on mating and sperm competition in the wild where sexually transmitted pathogens are of environmental origin [40].
Acknowledgements
We thank Julia Garnett for assisting with the sperm viability assays and fly stock maintenance. This work was supported by a National Science Foundation grant to K.M.F. (IOS-0722123).
References
- 1.Hunter F. M., Birkhead T. R. 2002. Sperm viability and sperm competition in insects. Curr. Biol. 12, 121–123 10.1016/S0960-9822(01)00647-9 (doi:10.1016/S0960-9822(01)00647-9) [DOI] [PubMed] [Google Scholar]
- 2.Fry C. L., Wilkinson G. S. 2004. Sperm survival in female stalk-eyed flies depends on seminal fluid and meiotic drive. Evolution 58, 1622–1626 [DOI] [PubMed] [Google Scholar]
- 3.Urbach D., Bittner D., Lenz T. L., Bernet D., Wahli T., Wedekind C. 2007. Sperm velocity in an Alpine whitefish: effects of age, size, condition, fluctuating asymmetry and gonad abnormalities. J. Fish Biol. 71, 672–683 10.1111/j.1095-8649.2007.01537.x (doi:10.1111/j.1095-8649.2007.01537.x) [DOI] [Google Scholar]
- 4.Rudolfsen G., Muller R., Urbach D., Wedekind C. 2008. Predicting the mating system from phenotypic correlations between life-history and sperm quality traits in the Alpine whitefish Coregonus zugensis. Behav. Ecol. Sociobiol. 62, 561–567 10.1007/s00265-007-0480-5 (doi:10.1007/s00265-007-0480-5) [DOI] [Google Scholar]
- 5.Civetta A., Rosing K. R., Fisher J. H. 2008. Differences in sperm competition and sperm competition avoidance in Drosophila melanogaster. Anim. Behav. 75, 1739–1746 10.1016/j.anbehav.2007.10.031 (doi:10.1016/j.anbehav.2007.10.031) [DOI] [Google Scholar]
- 6.Thomas M. L., Simmons L. W. 2007. Male crickets adjust the viability of their sperm in response to female mating status. Am. Nat. 170, 190–195 10.1086/519404 (doi:10.1086/519404) [DOI] [PubMed] [Google Scholar]
- 7.Hosseinzadeh S., Brewis I. A., Eley A., Pacey A. A. 2001. Co-incubation of human spermatozoa with Chlamydia trachomatis serovar E causes premature sperm death. Hum. Reprod. 16, 293–299 10.1093/humrep/16.2.293 (doi:10.1093/humrep/16.2.293) [DOI] [PubMed] [Google Scholar]
- 8.Ochsendorf F. R. 2008. Sexually transmitted infections: impact on male fertility. Andrologia 40, 72–75 10.1111/j.1439-0272.2007.00825.x (doi:10.1111/j.1439-0272.2007.00825.x) [DOI] [PubMed] [Google Scholar]
- 9.Forbes M. R. L. 1993. Parasitism and host reproductive effort. Oikos 67, 444–450 10.2307/3545356 (doi:10.2307/3545356) [DOI] [Google Scholar]
- 10.Sheldon B. C., Verhulst S. 1996. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321 10.1016/0169-5347(96)10039-2 (doi:10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 11.Rolff J., Siva-Jothy M. T. 2002. Copulation corrupts immunity: a mechanism for a cost of mating in insects. Proc. Natl Acad. Sci. USA 99, 9916–9918 10.1073/pnas.152271999 (doi:10.1073/pnas.152271999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zan Bar T., Yehuda R., Hacham T., Krupnik S., Bartoov B. 2008. Influence of Campylobacter fetus subsp. fetus on ram sperm cell quality. J. Med. Microbiol. 57, 1405–1410 10.1099/jmm.0.2008/001057-0 (doi:10.1099/jmm.0.2008/001057-0) [DOI] [PubMed] [Google Scholar]
- 13.Yaniz J. L., Marco-Aguado M. A., Mateos J. A., Santolaria P. 2010. Bacterial contamination of ram semen, antibiotic sensitivities, and effects on sperm quality during storage at 15°C. Anim. Reprod. Sci. 122, 142–149 10.1016/j.anireprosci.2010.08.006 (doi:10.1016/j.anireprosci.2010.08.006) [DOI] [PubMed] [Google Scholar]
- 14.Hamilton W. D., Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites. Science 218, 384–387 10.1126/science.7123238 (doi:10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 15.Folstad I., Skarstein F. 1997. Is male germ line control creating avenues for female choice? Behav. Ecol. 8, 109–112 10.1093/beheco/8.1.109 (doi:10.1093/beheco/8.1.109) [DOI] [Google Scholar]
- 16.Liljedal S., Folstad I., Skarstein F. 1999. Secondary sex traits, parasites, immunity and ejaculate quality in the Arctic charr. Proc. R. Soc. Lond. B 266, 1893–1898 10.1098/rspb.1999.0863 (doi:10.1098/rspb.1999.0863) [DOI] [Google Scholar]
- 17.Al-Qarawi A. A., Omar H. M., Abdel-Rahman H. A., El-Mougy S. A., El-Belely M. S. 2004. Trypanosomiasis-induced infertility in dromedary (Camelus dromedarius) bulls: changes in plasma steroids concentration and semen characteristics. Anim. Reprod. Sci. 84, 73–82 10.1016/j.anireprosci.2003.10.013 (doi:10.1016/j.anireprosci.2003.10.013) [DOI] [PubMed] [Google Scholar]
- 18.Boltz C. R., Boltz D. A., Bunick D., Scherba G., Bahr J. M. 2007. Vaccination against the avian infectious bronchitis virus affects sperm concentration, sperm quality and blood testosterone concentrations in cockerels. Br. Poultry Sci. 48, 617–624 10.1080/00071660701592375 (doi:10.1080/00071660701592375) [DOI] [PubMed] [Google Scholar]
- 19.Lorusso F., Palmisano M., Chironna M., Vacca M., Masciandaro P., Bassi E., Luigi L. S., Depalo R. 2010. Impact of chronic viral diseases on semen parameters. Andrologia 42, 121–126 10.1111/j.1439-0272.2009.00970.x (doi:10.1111/j.1439-0272.2009.00970.x) [DOI] [PubMed] [Google Scholar]
- 20.Radhakrishnan P., Fedorka K. M. 2011. Influence of female age, sperm senescence and multiple mating on sperm viability in female Drosophila melanogaster. J. Physiol. 57, 778–783 10.1016/j.jinsphys.2011.02.017 (doi:10.1016/j.jinsphys.2011.02.017) [DOI] [PubMed] [Google Scholar]
- 21.Fedorka K. M., Linder J. E., Winterhalter W., Promislow D. 2007. Post-mating disparity between potential and realized immune response in Drosophila melanogaster. Proc. R. Soc. B 274, 1211–1217 10.1098/rspb.2006.0394 (doi:10.1098/rspb.2006.0394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gendrin M., Welchman D. P., Poidevin M., Herve M., Lemaitre B. 2009. Long-range activation of systemic immunity through peptidoglycan diffusion in Drosophila. PLoS Pathogens 5, e1000694 10.1371/journal.ppat.1000694 (doi:10.1371/journal.ppat.1000694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miest T. S., Bloch-Qazi M. C. 2008. Sick of mating: sexual transmission of a pathogenic bacterium in Drosophila melanogaster. Fly 2, 215–219 [DOI] [PubMed] [Google Scholar]
- 24.Sokal R. R., Rohlf F. J. 1995. Biometry. San Francisco, CA: Freeman Press [Google Scholar]
- 25.Nappi A. J., Ottaviani E. 2000. Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays 22, 469–480 (doi:10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 26.Dowling D. K., Simmons L. W. 2009. Reactive oxygen species as universal constraints in life-history evolution. Proc. R. Soc. B 276, 1737–1745 10.1098/rspb.2008.1791 (doi:10.1098/rspb.2008.1791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heifetz Y., Rivlin P. K. 2010. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology 73, 723–739 10.1016/j.theriogenology.2009.11.001 (doi:10.1016/j.theriogenology.2009.11.001) [DOI] [PubMed] [Google Scholar]
- 28.Ribou A. C., Reinhardt K. 2012. Reduced metabolic rate and oxygen radicals production in stored insect sperm. Proc. R. Soc. B 279, 2196–2203 10.1098/rspb.2011.2422 (doi:10.1098/rspb.2011.2422) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eley A., Hosseinzadeh S., Hakimi H., Geary I., Pacey A. A. 2005. Apoptosis of ejaculated human sperm is induced by co-incubation with Chlamydia trachomatis lipopolysaccharide. Hum. Reprod. 20, 2601–2607 10.1093/humrep/dei082 (doi:10.1093/humrep/dei082) [DOI] [PubMed] [Google Scholar]
- 30.Hakimi H., Geary I., Pacey A., Eley A. 2006. Spermicidal activity of bacterial lipopolysaccharide is only partly due to lipid A. J. Androl. 27, 774–779 10.2164/jandrol.106.000083 (doi:10.2164/jandrol.106.000083) [DOI] [PubMed] [Google Scholar]
- 31.Lung O., Kuo L., Wolfner M. F. 2001. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 47, 617–622 10.1016/S0022-1910(00)00151-7 (doi:10.1016/S0022-1910(00)00151-7) [DOI] [PubMed] [Google Scholar]
- 32.Otti O., Naylor R. A., Siva-Jothy M. T., Reinhardt K. 2009. Bacteriolytic activity in the ejaculate of an insect. Am. Nat. 174, 292–295 10.1086/600099 (doi:10.1086/600099) [DOI] [PubMed] [Google Scholar]
- 33.McGraw L. A., Gibson G., Clark A. G., Wolfner M. F. 2004. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr. Biol. 14, 1509–1514 10.1016/j.cub.2004.08.028 (doi:10.1016/j.cub.2004.08.028) [DOI] [PubMed] [Google Scholar]
- 34.Winterhalter W. E., Fedorka K. M. 2009. Sex-specific variation in the emphasis, inducibility and timing of the post-mating immune response in Drosophila melanogaster. Proc. R. Soc. B 276, 1109–1117 10.1098/rspb.2008.1559 (doi:10.1098/rspb.2008.1559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snook R. R., Hosken D. J. 2004. Sperm death and dumping in Drosophila. Nature 428, 939–941 10.1038/nature02455 (doi:10.1038/nature02455) [DOI] [PubMed] [Google Scholar]
- 36.Manier M. K., Belote J. M., Berben K. S., Novikov D., Stuart W. T., Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science 328, 354–357 10.1126/science.1187096 (doi:10.1126/science.1187096) [DOI] [PubMed] [Google Scholar]
- 37.Champion de Crespigny F. E., Wedell N. 2006. Wolbachia infection reduces sperm competitive ability in an insect. Proc. R. Soc. B 273, 1455–1458 10.1098/rspb.2006.3478 (doi:10.1098/rspb.2006.3478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewis Z., de Crespigny F. E. C., Sait S. M., Tregenza T., Wedell N. 2011. Wolbachia infection lowers fertile sperm transfer in a moth. Biol. Lett. 7, 187–189 10.1098/rsbl.2010.0605 (doi:10.1098/rsbl.2010.0605) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillgarth N., Ramenofsky M., Wingfield J. 1997. Testosterone and sexual selection. Behav. Ecol. 8, 108–109 10.1093/beheco/8.1.108 (doi:10.1093/beheco/8.1.108) [DOI] [Google Scholar]
- 40.Knell R. J., Webberley K. M. 2004. Sexually transmitted diseases of insects: distribution, evolution, ecology and host behaviour. Biol. Rev. 79, 557–581 10.1017/S1464793103006365 (doi:10.1017/S1464793103006365) [DOI] [PubMed] [Google Scholar]


