Abstract
While sound is a useful cue for guiding the onshore orientation of larvae because it travels long distances underwater, it also has the potential to convey valuable information about the quality and type of the habitat at the source. Here, we provide, to our knowledge, the first evidence that settlement-stage coastal crab species can interpret and show a strong settlement and metamorphosis response to habitat-related differences in natural underwater sound. Laboratory- and field-based experiments demonstrated that time to metamorphosis in the settlement-stage larvae of common coastal crab species varied in response to different underwater sound signatures produced by different habitat types. The megalopae of five species of both temperate and tropical crabs showed a significant decrease in time to metamorphosis, when exposed to sound from their optimal settlement habitat type compared with other habitat types. These results indicate that sounds emanating from specific underwater habitats may play a major role in determining spatial patterns of recruitment in coastal crab species.
Keywords: crab, megalopae, settlement and metamorphosis cue, underwater sound, habitat type
1. Introduction
In most marine ecosystems, many organisms (such as crustaceans, fishes and molluscs) have a complex life cycle, which includes a pelagic larval phase capable of dispersal over great distances. The settlement period at the end of the larval phase, when the organism selects and settles in a benthic habitat, is a critical period in the life cycle [1]. Many post-settlement individuals are often found in greater numbers in structurally complex habitats that provide refuge against predation (e.g. fishes and crustaceans in macroalgae, coral heads or rubble) [2,3]. Although settlement habitat choice is a well-described phenomenon, the present study aims to test for the presence of a novel habitat-specific settlement cue—underwater sound.
As many reef-dwelling larvae are known to be capable of preferentially arriving at settlement habitats, a number of studies have investigated what cues are used by larvae to locate these preferred settlement habitats [4]. Many potential orientation cues exist, such as the direction of oceanic forces [5,6], tidal currents [7], magnetic and celestial [8,9], visual and polarized light [10,11], chemical [12], electric fields [13] and underwater sound [14]. However, to date, there is only clear empirical evidence to support the use of underwater sound [15], oceanic forces and, to a certain extent, visual and chemical orientation cues [12]. Many reef-dwelling larvae appear to use an arsenal of cues when attempting to find a suitable settlement habitat. However, there can be very different spatial scales over which each is effective and they are often used in a hierarchical order [16]. The effective use of multiple cues at varying special scales is common in many animals that are attempting to get from one place to another [12,16], and this is no exception with decapod crustaceans [17–19].
Ambient underwater sound has long been regarded as one of the most robust candidates for guiding onshore orientation by pelagic larvae. It can be conducted over large distances, is directional, can carry significant biological information about the habitat of origin and is independent of water currents. In a recent study, it was found that within a relatively small section of a coast (less than 20 km), it is possible to find in spectral and temporal composition of ambient underwater sound, marked differences that are associated with different types of coastal habitat [20]. Also, a number of studies have demonstrated that ambient underwater sound emanating from coastal habitats attracts the settlement stages of a broad range of families of reef fishes, crustaceans and coral [15,21–25]. Furthermore, there is initial evidence that a wide range of species are using underwater acoustic cues to locate habitats [26,27]. Settlement-stage fishes of some families have been shown to be attracted to different frequency components of underwater coral reef sound, and this was thought to be possibly associated with differences in sounds produced by various habitats, but this was not directly tested [28]. For settlement-stage larvae, what remains unclear is whether they have the ability to discriminate between different habitats based on differences in the underwater sound generated by these different habitats.
A wide range of settlement and metamorphosis cues have been identified that can be involved in the selection of suitable settlement habitat by settlement-stage brachyuran crab larvae. These cues include salinity, depth, substrate rugosity, as well as a range of chemical cues associated with conspecifics, settlement substrates, aquatic vegetation and potential prey [29]. During a more recent study, it was observed that settlement and subsequent metamorphosis was advanced in the megalopae of several species of coastal brachyuran crab when exposed to ambient levels of underwater reef sound [30]. Therefore, the aim of this present research was to determine the potential for the megalopae of coastal brachyuran crab species to discriminate among different settlement habitats based on habitat-specific differences in underwater sound. Experiments were conducted on the megalopae of coastal crab species in temperate and tropical waters to determine whether the results were consistent over a wide range of marine environments.
2. Methods
The study was undertaken during November 2009 to April 2010 in temperate waters near the Leigh Marine Laboratory in northeastern New Zealand, and also in tropical waters near the Lizard Island Research Station on the Great Barrier Reef (GBR) in northeastern Australia.
Light traps were used to capture pelagic megalopae for behavioural assays [30,31]. Up to eight light traps were deployed at night within 500 m of the shoreline and recovered the following morning. The megalopae were counted, sorted into settlement stage and identified to lowest taxonomic level possible [32,33]. Only intermoult pre-settlement megalopae of similar size and age were selected for use in the assays. The megalopae were held in a flowing filtered seawater system with natural light period and ambient water temperature until experiments began the following evening. Five species of brachyuran megalopae were used. The two temperate species, Hemigrapsus sexdentatus and Cyclograpsus lavauxi, both from the family Grapsidae, are commonly known to co-occur in nearshore subtidal and intertidal habitats, and are commonly found living under boulders, among macroalgae, and on rocky reefs and shores in New Zealand. The three tropical species, Cymo andreossyi, Schizophrys aspera and Grapsus tenuicrustatus, are members of the Xanthidae, Majidae and Grapsidae families, respectively, and are known to be associated with hard coral and coral shore habitats on many parts of the GBR.
(a). Laboratory-based behaviour assays
Each laboratory-based experiment consisted of four sound treatments (three distinct habitat sound types and one silent (control)), and within each treatment, there were three replicate water baths used to maintain a constant water temperature for megalopae throughout the experiment. The silent treatment was used as a baseline to show the potential maximum delay in metamorphosis when no auditory cues are present, that can then be compared with the other distinct habitat sound treatments. The silent treatment also acts as a control for the experimental apparatus. The baths were acoustically isolated using foam rubber mats to prevent any transfer of acoustic energy from the surrounding environment into the experimental treatment. The absence of any significant acoustic signal in the silent treatment tanks was confirmed by recording with a calibrated hydrophone (HTI 96-MIN) and digital recorder (Roland Edirol R09HR).
Each replicate water bath contained 5–10 tightly sealed plastic vials (250 ml; number determined by light trap catch rates) housing a single randomly selected megalopa in filtered (1 µm) and ultraviolet–light-sterilized seawater. It is not possible to reliably remove all possible chemical compositional cues from seawater; however, for consistency, each experiment used the same seawater for all treatments. The vials had a roughened base acting as a chemically inert settlement surface for settling megalopae. All replicates for both the sound and silent treatments had a weighted loudspeaker inside a watertight plastic bag that was submerged in the water bath. For the sound replicates only, a Sony CD Walkman D—EJ815 was connected to the speaker and used to continually play a 4 min loop of recorded ambient underwater reef sound into the water bath and through the near to acoustically transparent plastic vials holding the crabs. All megalopae in each treatment were kept under natural light period and ambient water temperature, dependent on local ambient temperature (18–21° for temperate, 29–31°C for tropical), for the duration of the experiment. It was not logistically possible to provide separate sound systems for individual megalopae in this experiment; so they were completely independent replicates. Our use of three replicate water baths, each containing replicate megalopae, represented a practical compromise for the experiment. The megalopae within the water baths were each contained in tightly sealed vials, thereby preventing any interactive effects among individuals, such as from the release of conspecific chemical settlement cues. The vials were spaced at least 500 mm apart within the water bath, and it is well known that crustacean larvae lack the visual acuity that would be required for them to be able to observe the behaviour of individuals in adjacent replicate vials. Megalopae were not fed during the experiments.
The megalopae were added to the experiment at 17.00 h on the day of their capture and the CD Walkman was switched on so as to initiate sound in the sound treatments. Every 6 h an observation period occurred, with counts made of the number of individual megalopae that had settled onto the base of the vials and metamorphosed into the first instar benthic juvenile stage. The time between the experiment commencing and the observation of a metamorphosed first instar benthic juvenile was termed time to metamorphosis (TTM). The period of observation on each occasion lasted no more than 40 min for all treatments. When the observational period occurred at night, red light was used to observe metamorphosis [34]. The experiment was terminated when all experimental megalopae in all treatments had metamorphosed.
(b). Habitat sound recordings for laboratory-based experiments
Recordings of typical ambient underwater sound were made at the three different habitats selected for the laboratory-based sound treatments. For temperate waters, the natural underwater sound samples used for replaying in the experimental sound treatments were recorded from north-eastern New Zealand during the summer at dusk on a new moon; North Reef (36°15′58″ S, 174°47′37″ E), a macroalgae-dominated rocky reef habitat; Mahurangi Harbour (36°20′51″ S, 174°45′54″ E), an extensive sandy/broken shell seafloor habitat; and Pakiri Beach (36°13′31″ S, 174°42′34″ E), an open sandy beach habitat (see the electronic supplementary material, figure S1). For tropical waters, the natural underwater sound samples used for replaying in the experimental sound treatments were recorded from waters near the Lizard Island Research Station, north-eastern Australia on the GBR during the summer at dusk on a new moon; Coconut Reef (14°40′51″ S, 145°28′17″ E), a continuous frontal fringing coral reef habitat; Horseshoe Reef (14°41′13″ S, 145°26′37″ E), an isolated coral back reef habitat interrupted with areas of sand and coral rubble; and Lagoon (14°41′26″ S, 145°27′28″ E), a lagoon habitat with extensive sandy seafloor in the centre of the Lizard Island Group, distant (approx. 400 m) from fringing reefs (see the electronic supplementary material, figure S1). These sites at both the temperate and tropical locations were selected as the most favourable, intermediate and least favourable in terms of the preferred benthic habitat for settlement in these species. The habitats associated with large rocky or coral reef are the most favourable, and harbour, lagoon or sandy-bottomed habitats being the least favourable habitats for these species.
In situ habitat sounds were recorded using a remote recording system that consisted of a calibrated HTI 96-MIN hydrophone connected to an automated recording system and a digital recorder Roland Edirol R09HR, contained in an underwater housing. The recorder was in approximately 15 m of water for the temperate treatments, excluding the Mahurangi Harbour, which was in 6 m water depth. For the tropical treatments, the recorder was at approximately 7 m of water depth, except for the Lagoon for which the recorder was in 12 m depth. The hydrophone was calibrated by recording a NetMark 1000 acoustic pinger (specifications: source level 130 dB re 1 µPa at 1 m, 10 kHz signal, 300 ms pulse length, 4 s repetition rate). Digital recordings were transferred to a PC, and the spectral composition was analysed using Matlab software. Ten typical 4 min sequences from each habitat recording were selected, and from these, three sequences were randomly selected and each transferred to a CD and used for playback in one of the three replicates for each sound treatment in the laboratory-based experiments. The three different 4 min sequences were used to avoid pseudoreplication by using the same habitat recording for each replicate within the treatment [35].
A calibrated hydrophone and recorder was used to adjust the sound level produced by the speakers in each experimental sound treatment tank to 100 dB, 80 dB and 70 dB re 1 µPa at 1 m for North Reef, Pakiri Beach and Mahurangi Harbour, respectively (see the electronic supplementary material, figure S1a–c), and to 90 dB, 75 dB and 60 dB re 1 µPa at 1 m for Coconut Reef, Horseshoe Reef and Lagoon, respectively (see the electronic supplementary material, figure S1d–f). These levels were within the typical range of ambient underwater sound levels for evening chorus at such habitats in New Zealand's coastal waters [36] and at similar habitats on the GBR in Australia [37]. To ensure that the sounds replayed in the experimental tanks were as consistent with the natural habitat sound as possible, the played back habitat sound was recorded from within the tank and analysed so that their spectral composition could be compared with the source signals recorded from the natural habitats.
(c). Field-based behaviour assays
For the two temperate species, field-based experiments consisted of three distinct habitat sites (treatments): Waterfall Reef (36°16′05″ S, 174°48′06″ E), a macroalgae-dominated rocky reef; Whangateau Harbour (36°18′55″ S, 174°45′24″ E), a harbour with an extensive area of sandy/broken shell seafloor; and Pakiri Beach (36°13′31″ S, 174°42′34″ E), an open sandy beach (see the electronic supplementary material, figure S2). For the three tropical species, field-based experiments consisted of two habitat sites (treatments): Horseshoe Reef (14°41′13″ S, 145°26′37″ E), an isolated coral back reef habitat interrupted with areas of sand and coral rubble; and Loomis Beach Lagoon (14°40′59″ S, 145°27′13″ E), which has an extensive area of sandy seafloor that is approximately 400 m from fringing reefs (see the electronic supplementary material, figure S2). Horseshoe Reef was used as a field experimental site rather than Coconut Reef (as used in the laboratory experiment), owing to diver and boating safety regulations at the field research station; however, both reefs are suitable settlement habitats for the crab species used for this study. Each habitat site consisted of three replicates, each replicate with 5–10 individually housed megalopae in a 250 ml plastic vial containing filtered (1 µm) and ultraviolet-treated seawater with a tightly sealed lid, identical to those used in laboratory-based experiments. Vials were held in a vertical position approximately 40 cm from the sea floor and were spaced at least 100 mm apart in a positively buoyant frame. The replicates were 1 m apart, tethered to the seafloor on sand 2 m from the reef at reef sites in 5–8 m of water depending on the habitat.
Megalopae were added to the habitat sites by divers at approximately 17.00 h on the day of their capture. At dawn (8.00 h) and dusk (17.00 h), divers visited the habitat sites and counts were made of the number of individuals that had settled to the base of the vials and metamorphosed into the first instar benthic juvenile stage. The experiment was terminated when all experimental megalopae in all treatments had metamorphosed or when poor weather conditions no longer permitted safe diving at the habitat sites.
Recordings of the ambient underwater sound in the vicinity of the experimental habitat sites were collected during the experiment using the remote recording system previously described (see the electronic supplementary material, figure S2). The recorder was installed in approximately 7 m of water. Recordings at noon and dusk were taken and the spectral composition analysed using Matlab software to confirm the habitats were acoustically distinct.
(d). Data analyses
For both laboratory- and field-based experiments for each crab species, non-parametric statistical methods were used to test for differences in median TTM within each sound treatment as the data were not continuous, with a non-normal distribution and unequal variance among treatments [38]. Kruskal–Wallis analysis of variance by ranks or Mann–Whitney U-tests were used to test for a difference in the distribution of median TTMs among the replicates within the same treatment (i.e. each treatment analysed separately). If this test found no difference among the three replicate water baths, the data from the three replicates were pooled for an experiment-wide analysis. To help us to ensure that the replication of megalopae within water baths was not specifically constraining their response, the total variance was decomposed to within and among components; so they could be compared. If the replicate water baths were constraining the behavioural responses of the megalopae they contained, it could be expected that variance among the water baths would be inflated relative to the variance within the water baths. The Kruskal–Wallis test was then used to compare the distribution of median TTMs for megalopae among the treatments using the data pooled from the three treatments. For all statistical tests, p-values ≤ 0.05 were considered to be significant. To isolate difference among individual treatments, a Dunn's pairwise multiple comparison of ranks procedure was used [38]. A metamorphosis rate for each treatment within each species was also calculated with a Sen's slope analysis for the data points between the last sampling event prior to the first megalopa metamorphosing and the sampling event when the last megalopa metamorphosed. This facilitated a comparison of metamorphosis rate for a cohort of settling crab megalopae among treatments. All analyses were performed using the software Sigma Stat v. 4.0 and Minitab v. 16.1.0.
3. Results
(a). Laboratory-based experiments
(i). Sound analyses
In the laboratory experiments for both temperate and tropical crab species, the played back sound within the experimental tanks had a similar overall spectral composition and sound level to the source signals recorded from the natural habitats in situ (see the electronic supplementary material for details, figure S1a–f). The treatment had no sound transfer from any other external sources. The flat response at approximately 35 dB is at the lower recording limit of the recording equipment, indicating very quiet acoustic conditions (see the electronic supplementary material, figure S1g).
The spectra for the different habitats tested in both temperate and tropical locations were different in overall appearance, indicating that the acoustic characteristics varied markedly among the habitats (see the electronic supplementary material, figure S1a–f). For detailed acoustic descriptions of individual habitat sites, see the electronic supplementary material, appendix S1.
(b). Laboratory-based behaviour assays
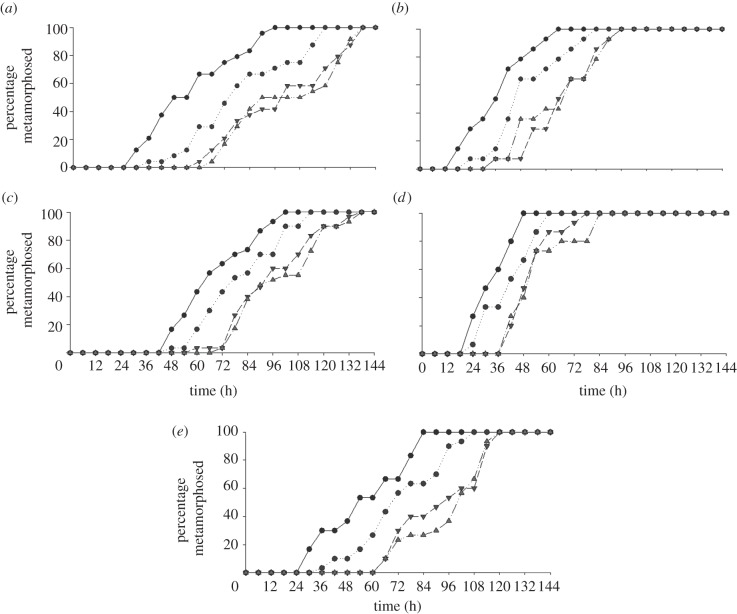
For all five crab species, there was no significant difference in the median TTM among the replicates within each of the four treatments, with a p-value > 0.1 for each. Therefore, for each crab species the TTM data for the replicates within each treatment were pooled to test for an overall treatment effect. For all pooled treatment data, the variance among the replicate water baths were consistently small relative to the variance within water baths, indicating that the replication within water baths was not constraining the behavioural responses of the megalopae they contained. Median TTM differed significantly among treatments for the megalopae of all crab species tested (Kruskal–Wallis test; table 1 and figure 1). Both temperate species, H. sexdentatus and Cyclograpsus lavauxi, had significantly different median TTM between all paired combinations of treatments (Dunn's test p < 0.05) except between Pakiri Beach and silent (p > 0.05). North Reef had the smallest median TTM (i.e. fastest settlement) in both H. sexdentatus and Cyclograpsus lavauxi of 58 h and 39 h, respectively, followed by Mahurangi Harbour of 74 h and 48 h, respectively, then Pakiri Beach of 105 h and 69 h, respectively, and finally silent of 105 h and 72 h. Hemigrapsus sexdentatus had the largest difference in median TTM between North Reef (58 h) and silent (150 h) treatments for the temperate species, a difference of 47 h (p < 0.05).
Table 1.
Statistical comparisons among median TTMs and metamorphosis rates for each treatment in laboratory-based experiments for five crab species. (***Asterisks indicate a significant difference in TTMs between treatments (p < 0.05, Kruskal–Wallis test).)
| species | total no. individuals (n) | treatment (habitat) | variance within replicates | variance among replicates | median TTM (hour) | H-statistic | p-value | metamorphosis rate |
|---|---|---|---|---|---|---|---|---|
| H. sexdentatus | 24 | North Reef | 462.6 | 57.9 | 58 | 34.0 | <0.001*** | 8.1 |
| 24 | Mahurangi Harbour | 639.2 | 37.7 | 74 | 6.8 | |||
| 24 | Pakiri Beach | 735.2 | 31.9 | 105 | 6.7 | |||
| 24 | silent | 760.5 | 2.7 | 105 | 6.8 | |||
| Cyclograpsus lavauxi | 24 | North Reef | 182.4 | 112.0 | 39 | 13.6 | 0.004*** | 11.4 |
| 24 | Mahurangi Harbour | 291.0 | 63.4 | 48 | 9.5 | |||
| 24 | Pakiri Beach | 336 | 13.0 | 69 | 9.9 | |||
| 24 | silent | 304 | 183 | 72 | 9.1 | |||
| Cymo andreossyi | 30 | Coconut Reef | 336.1 | 0.4 | 66 | 35.5 | <0.001*** | 9.4 |
| 30 | Horseshoe Reef | 370.1 | 14.9 | 78 | 8.9 | |||
| 30 | Lagoon | 405.5 | 0.4 | 96 | 7.9 | |||
| 30 | silent | 437.5 | 26.8 | 99 | 8.5 | |||
| S. aspera | 15 | Coconut Reef | 415.9 | 13.4 | 36 | 25.4 | <0.001*** | 20 |
| 15 | Horseshoe Reef | 426.9 | 9.5 | 42 | 14.7 | |||
| 15 | Lagoon | 436.7 | 1.4 | 54 | 13.8 | |||
| 15 | silent | 306 | 32.2 | 54 | 10.2 | |||
| G. tenuicrustatus | 10 | Coconut Reef | 415.9 | 13.4 | 54 | 47.9 | <0.001*** | 8.9 |
| 10 | Horseshoe Reef | 432.3 | 88.7 | 72 | 8.3 | |||
| 10 | Lagoon | 543.3 | 20.6 | 96 | 8.7 | |||
| 10 | silent | 306 | 32.2 | 102 | 9.6 |
Figure 1.
Percentage of total number of megalopae that metamorphosed over time (hour) in laboratory-based experiments: (a) Hemigrapsus sexdentatus; (b) Cyclograpsus lavauxi; (c) Cymo andreossyi; (d) Schizophrys aspera and (e) Grapsus tenuicrustatus. Key for (a,b): circles with solid line, North Reef; circles with dotted line, Mahurangi Harbour; inverted triangles with dashed line, Pakiri Beach; triangles with dot dashed line, silent. Key for (c,d,e): circles with solid line, Coconut Reef; circles with dotted line, Horseshoe Reef; inverted triangles with dashed line, Lagoon; triangles with dot dashed line, silent.
In the tropical crab species Cymo andreossyi and G. tenuicrustatus, the median TTMs between all paired combinations of treatments were significantly different (Dunn's test p < 0.05), except between the Lagoon and silent treatments. Schizophrys aspera had significantly different median TTMs between Coconut Reef versus silent, Horseshoe Reef versus silent, Coconut Reef versus Lagoon and Horseshoe Reef versus Lagoon (p < 0.05) treatments, but similar median TTM between Coconut Reef versus Horseshoe Reef, and silent versus Lagoon (p > 0.05) treatments. Coconut Reef had the shortest median TTM in all Cymo andreossyi, G. tenuicrustatus and S. aspera experiments with 66 h, 36 h and 54 h, respectively, followed by Horseshoe Reef with 78 h, 43 h and 72 h, respectively, then Lagoon with 96 h, 54 h and 96 h, and lastly silent with 99 h, 54 h and 102 h. Grapsus tenuicrustatus had the largest difference in median TTM between Coconut Reef (54 h) and silent (102 h) for the tropical species, a difference of 48 h (p < 0.05).
The difference in time from the outset of the experiments to the first megalopae to complete metamorphosis varied substantially among treatments for each species, with both H. sexdentatus and G. tenuicrustatus exhibiting the greatest difference of 36 h (figure 1). Grapsus tenuicrustatus had the greatest difference of 47 h for all megalopae to complete metamorphosis among treatments (figure 1).
Both species of temperate crabs H. sexdentatus and Cyclograpsus lavauxi had a faster metamorphosis rate in the North Reef treatment than in the silent treatment (1.2 times faster) (table 1). In the tropical species, both Cymo andreossyi and S. aspera had faster metamorphosis rates in the Coconut Reef treatment than the silent treatment, 1.1 times and 1.8 times faster, respectively (table 1). However, G. tenuicrustatus had a faster metamorphosis rate in the Silent treatment when compared with the Coconut Reef treatment (1.1 times faster).
(c). Field-based experiments
(i). Habitat site sound recording
The spectra from the underwater sound recorded from the habitat sites for both the temperate and tropical experiments showed differences in spectral composition, temporal variation and overall level among sites (see the electronic supplementary material, figure S2a–e). For detailed acoustic descriptions of individual habitat sites, see the electronic supplementary material, appendix S2.
(d). Field-based behaviour assays
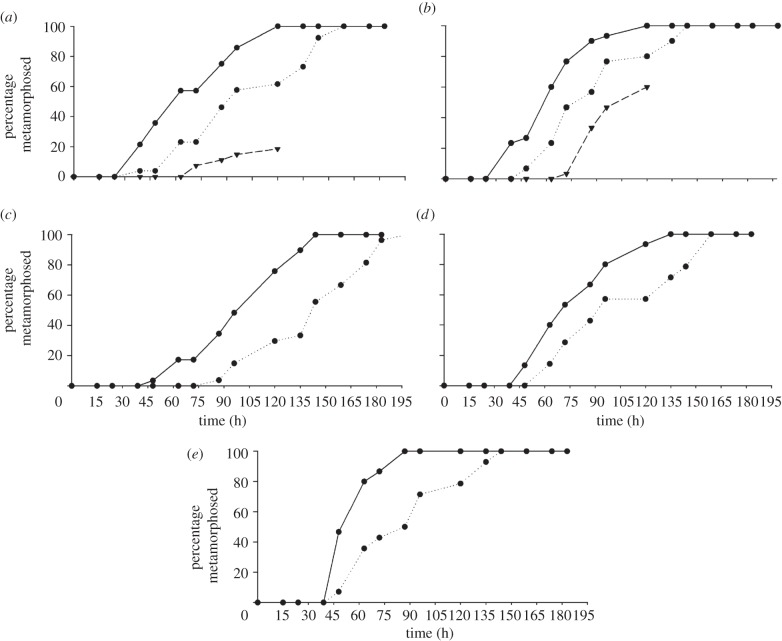
For all five crab species in the field-based experiments, there were no significant differences in the median TTM among the replicates for all of the habitat treatments (p > 0.05). Therefore, for each crab species, the TTM data for the replicates within each habitat treatment were pooled to test for an overall treatment effect. Median TTMs differed significantly among treatments for the megalopae of all crab species (table 2 and figure 2; p < 0.05). In the temperate crab species, the megalopae at the Waterfall Reef habitat had a significantly shorter median TTM than those at the Whangateau Harbour habitat. Cyclograpsus lavauxi had a median TTM of 63 h and 96 h (33 h difference), and H. sexdentatus had a median TTM of 63 h and 87 h (24 h difference), respectively (table 2; p < 0.001). Owing to poor weather, the Pakiri Beach habitat could not be sampled past 120 h for both H. sexdentatus and Cyclograpsus lavauxi and therefore could not be included in analysis. However, in the first 120 h, there was a visible lag in the TTM at the Pakiri Beach habitat when compared with the other two habitats (figure 2a,b).
Table 2.
Statistical comparisons among median TTMs and metamorphosis rates for each habitat site in field-based experiments for five crab species. (***Asterisks indicate a significant difference in TTMs between habitat sites (p < 0.05, Mann–Whitney U-test).)
| species | total no. individuals (n) | treatment (habitat) | median TTM (hour) | U-statistic | p-value | metamorphosis rate |
|---|---|---|---|---|---|---|
| H. sexdentatus | 30 | Waterfall Reef | 63 | 34.0 | <0.001*** | 15 |
| 30 | Whangateau Harbour | 87 | 13 | |||
| Cyclograpsus lavauxi | 28 | Waterfall Reef | 63 | 13.6 | <0.001*** | 14 |
| 26 | Whangateau Harbour | 96 | 11 | |||
| Cymo andressyo | 29 | Horseshoe Reef | 114 | 35.5 | 0.045*** | 13.4 |
| 27 | Loomis Beach Lagoon | 144 | 12.2 | |||
| S. asperas | 15 | Horseshoe Reef | 72 | 25.4 | <0.001*** | 15 |
| 15 | Loomis Beach Lagoon | 96 | 12 | |||
| G. tenuicurstatus | 15 | Horseshoe Reef | 63 | 47.9 | 0.002*** | 24 |
| 15 | Loomis Beach Lagoon | 92 | 13 |
Figure 2.
Percentage of total number of megalopae metamorphosed over time (h) in the field-based experiments: (a) H. sexdentatus; (b) Cyclograpsus lavauxi; (c) Cymo andreossyi; (d) S. aspera and (e) G. tenuicrustatus. Key for (a,b): circles with solid line, Waterfall Reef; circles with dotted line, Whangateau Harbour; inverted triangle with dashed line, Pakiri Beach. Key for (c,d,e): circles with solid line, Horseshoe Reef; circles with dotted line, Loomis Beach Lagoon.
The tropical species Cymo andreossyi had the largest difference in median TTMs between the two habitats, with a median TTM of 114 h at the Horseshoe Reef habitat and 144 h at the Loomis Beach Lagoon habitat, a difference of 30 h (table 2; p < 0.05). This was followed by G. tenuicrustatus with a median TTM of 63 h at the Horseshoe Reef habitat and 92 h at the Loomis Beach Lagoon habitat, a 29 h difference (table 2; p < 0.005). Schizophrys aspera had a 29 h difference, with a median TTM of 72 h and 96 h at the Horseshoe Reef and Loomis Beach lagoon habitats, respectively (table 2; p < 0.001).
The difference in time from the outset of the experiment to the first megalopae to complete metamorphosis varied among the treatments (figure 2). The tropical species Cymo andreossyi exhibited the greatest difference between the two treatments, with the first megalopae in the Horseshoe Reef treatment starting metamorphosis 39 h ahead of the first megalopae at Loomis Beach Lagoon. The tropical species G. tenuicrustatus had the greatest difference of 57 h for all megalopae to complete metamorphosis among treatments (figure 2).
All species, both temperate and tropical, had faster metamorphosis rates at Waterfall Reef and Horseshoe Reef habitats, respectively, compared with Whangateau Estuary, Pakiri Beach or Loomis Beach Lagoon habitats (table 2). The tropical species G. tenuicrustatus had the largest difference in metamorphosis rates among sites with megalopae at Horseshoe Reef being 1.8 times faster than those at the Loomis Beach Lagoon (24 h and 13 h, respectively; figure 2e), followed by S. aspera, which had a metamorphosis rate that was 1.3 times faster at Horseshoe Reef. Both temperate species, H. sexdentatus and Cyclograpsus lavauxi, had a metamorphosis rate that was 1.2 times faster at Waterfall Reef than at Whangatean Estuary. The tropical species Cymo andreossyi had the smallest difference in metamorphosis rate among sites, with megalopae at Horseshoe Reef having 1.1 times faster rates than those at Loomis Beach Lagoon (figure 2c). The metamorphosis rate at Pakiri Beach started with a similar overall rate to both the other two temperate habitat sites for Cyclograpsus lavauxi; however, in H. sexdentatus the rate was much lower (4.4 compared with 14 and 11).
4. Discussion
This study is the first, to our knowledge, to demonstrate the ability of settling stages of coastal decapod crustaceans to discriminate among suitable settlement habitats on the basis of differences in natural ambient underwater sound. The phenomenon appears to be common because it was present in all five species tested from several families of coastal brachyuran crabs, and it was present both in tropical and in temperate waters. In all species of crab tested in both laboratory- and field-based experiments, the settlement and metamorphosis responses were significantly faster in habitats that matched the preferred benthic habitat for settlement in these species, i.e. habitats associated with rocky or coral reefs. By contrast, in unfavourable settlement habitats (i.e. harbour, lagoon or sandy bottomed beach habitats), the time to settlement and metamorphosis was significantly delayed. For example, when comparing most favourable to least favourable settlement habitats, TTM was shortened by 33–47% in the laboratory-based experiments, and by 21–34% in the field-based experiments.
It could be questioned whether it is the overall sound level rather than the composition of the sound that is serving as a cue for metamorphosis; however, results from a recent study clearly shows that sound level alone does not explain the differences in metamorphosis response observed in the brachyuran megalopae when exposed to underwater sound taken from preferred settlement habitat [39]. Megalopae of H. sexdentatus and Leptograpsus variegatus previously showed no significant metamorphosis response when exposed to varying overall intensities of sound from an open sandy beach, an unfavourable habitat type, even when the sound level was at a similar level (±1 dB) to the ambient sound at their preferred settlement habitat, rocky reef [39]. This previous study also found there was no significant reduction in TTM in the megalopae for either of the two crab species tested in the treatments broadcasting Pakiri Beach recordings at 90 dB, 103 dB, 125 dB re 1 µPa, or a silent treatment, while there was a significant reduction in TTM in the ambient reef sound treatment. These results demonstrated that it is the frequency and temporal composition of underwater sound rather than the overall sound level per se that is the important characteristic of sound that mediates settlement and metamorphosis in these crab megalopae [39].
The results of the present study also reconfirm the preferential metamorphosis response to an ambient reef sound treatment over a silent treatment in settlement-stage crab larvae but with an increased range of crab species and higher experimental replication [30]. The consistency of the results between parallel laboratory and field experiments in this current study also indicates that there do not appear to be any artefacts created as a result of using a laboratory experimental setting as was used in previous studies [30], or from the need to contain replicate experimental megalopae within individual water baths in the current study.
The metamorphosis responses exhibited by the megalopae to acoustic cues are entirely consistent with what might be expected, given the differences in the distribution of juvenile and adult crabs among these habitats. Both the temperate species H. sexdentatus and Cyclograpsus lavauxi showed the strongest metamorphosis response to the macroalgae-dominated rocky reef habitat (North Reef). Both of these species are associated with this type of intertidal and subtidal habitat as settled juveniles and adults [32]. The habitat to elicit the next strongest response in these two crab species was from the Mahurangi Harbour with a sand and broken shell seafloor, perhaps because both the sound recording used in the laboratory-based experiment and the habitat used in the field-based experiments displayed some degree of acoustic similarities to the macroalgae-dominated rocky reef treatments. Although the sound level was lower in these experiments, there were similar biotic signals in the 2500–5000 Hz frequency band, indicating the presence of snapping shrimp [36,40]. All three of the tropical crab species tested, Cymo andreossyi, S. aspera and G. tenuicrustatus, showed the strongest metamorphosis response to the coral reef habitat type (Coconut Reef and Horseshoe Reef) as opposed to the lagoon habitat (Lagoon and Loomis Beach Lagoon). This could be expected, as adults of these three species are known to be strongly associated with coral reefs and use the physical complexity of this reef habitat for protection and prey capture, and they are also often found living in symbiosis with hard corals [33].
As larvae are competent to actively select a suitable habitat in which to settle, live and survive in, there is a range of potential sensory information they may draw on to guide them to their preferred settlement habitats. Many reef-dwelling larvae appear to use visual and chemical cues when settling. Conspecific odour has been found to reduce time to metamorphosis in the crab species Chasmagnathus granulate, Ulga pugilator, Panopeus herbstii and Rhithropanopeus harrisii [29]. However, both chemical and visual cues have the potential to become effective sensory cues over relatively small spatial scales compared with those over which sound cues can operate [12,41]. Chemical stimuli can be effective either downstream of the source or at small spatial scales before they become substantially diluted [42]. Visual cues allow fine-scale habitat selection; however, anything other than perfect water clarity could be expected to obstruct view to distant habitats [12]. However, compared with other potential cues, acoustic cues are able to be conducted over large distances in water, are independent of water currents and can carry information on habitat direction and quality. The different spatial ranges of various potential settlement cues, such as acoustic, chemical and visual cues, offer the potential for settling larvae to use a suite of sequential cues to improve the localization of preferred settlement habitats [12].
The results from the present study provide evidence that acoustic cues originating from coastal habitats are also important metamorphosis cues for reliably identifying a suitable habitat in which to settle. It has been observed in both the present and previous studies [29,41] that when megalopae are approaching metamorphosis their behaviour changes noticeably with reduced swimming activity in the water column, more downward swimming to the substrate, followed by increased exploratory crawling on the substrate. The replayed acoustic cue not only influences the behaviour of the megalopae, but also appears to mediate an endogenous physiological developmental process that expedites metamorphosis, which has been termed as a morphogenetic response [43]. The larvae of different species appear to differ in the extent of the dependence and specificity to the acoustic inducers that trigger settlement and metamorphosis [44]. This dependence and specificity would be associated with recognition of the inducer. It is this recognition that activates the genetically scheduled sequence of behavioural, anatomical and physiological processes that determine settlement and metamorphosis [43].
There have been numerous studies in the last decade investigating physical settlement cues and habitat selection methods in many species of pelagic larvae. In many of these studies, ambient underwater sound may have also been involved in the reported settlement habitat selection process. Many habitats that are known to attract high numbers of larval and juvenile marine organisms have similar characteristics, they provide shelter from predators with complex physical structure (such as rocks and algae or coral heads and rubble [45,46]) and are rich in food sources [46]. These habitats are often biologically complex and can emit sounds within specific frequency bands that are associated with the variety of sound-producing animal residents that characterize the habitat, such as snapping shrimp, sea urchins and fishes [40,47,48]. These optimal settlement habitats for many species of pelagic larvae are therefore potentially emitting valuable acoustic cues that can be detected, and used in conjunction with other available sources of information, as a reliable indicator of a habitat type.
There are some previous studies which provide evidence that the larval stages of reef organisms are not only able to detect and use reef sound to orientate [26] but can also discriminate and respond to differences in underwater sounds from different biological sources [28,49]. For example, the settlement-stage larvae of the reef fish Chromis atripectoralis adjusts swimming speed and direction when presented with either broadcast natural reef sound or artificial pure tones [49], suggesting that they may be capable of discriminating acoustic differences that indicate potential differences in a habitat. Certainly, a wide range of juvenile and adult reef fish species appear to be able to distinguish and respond to differences in underwater sound emanating from different habitats [28,50]. In the present study, the preferential habitat type of the settling-stage larvae of five species of crabs were clearly associated with a reduction in time to settlement and metamorphosis, i.e. the macroalgae-dominated rocky reef in the temperate species, and the dense coral reef in the tropical species. Both these habitats had the highest levels of higher frequency sound (800–15 000 Hz) compared with the other habitats, which is predominantly owing to the presence of resident soniferous marine organisms, such as sea urchins and snapping shrimp [20]. A possible explanation for this preferential response by the crabs to the greater higher frequency component of these preferred settlement habitat sounds is that there is a higher abundance and diversity of soniferous invertebrate fauna inhabiting complex rocky reef, coral reef or rocky shore structures. These habitats are also essential for the species tested in this study as it is their primary adult habitat [32,33]; therefore, the presence and abundance of other invertebrate species provides a reliable indication of habitat type and quality. Also, fish vocalizations are known to be in the lower frequencies and fishes pose a direct predatory threat to crab megalopae and therefore may not provide a reliable indicator of the suitability of the habitat [51,52].
The results of the present study provide evidence that crab megalopae can discriminate habitats by the acoustic underwater sound signature they emanate and actively select to settle and metamorphose at the preferred habitat. The ability to remotely identify a suitable habitat in which to settle by using readily available acoustic cues would be a major advantage for settlement-stage reef organisms at both close range and at some distance offshore. Collectively, the existing evidence suggests that ambient underwater sound effects the distribution and orientation of a number of important settlement-stage reef organisms, such as fishes and crabs [14,15,26,53]. Therefore, if settling stages of reef fishes and crabs are adjusting their settlement responses to varying spectral and temporal characteristic of underwater sound that can differentiate among habitats, then underwater sound is of major importance in structuring the spatial settlement and subsequent recruitment in reef organisms.
Acknowledgments
The work was conducted under University of Auckland Animal Ethics Committee approval no. R701.
We thank staff at the Leigh Marine Laboratory and Lizard Island Research Station for their assistance with logistics for this research. This research was supported by the New Zealand Marine Sciences Society Student research grant and the Glenn Family Foundation.
References
- 1.Werner E. E. 1988. Size, scaling and the evolution of complex lifecycles. In Size-structured populations (eds Ebenman B., Perssons L.), pp. 61–81 Berlin, Germany: Springer [Google Scholar]
- 2.Shulman M. J. 1984. Resource limitation and recruitment patterns in a coral-reef fish assemblage. J. Exp. Mar. Biol. Ecol. 74, 85–109 10.1016/0022-0981(84)90039-X (doi:10.1016/0022-0981(84)90039-X) [DOI] [Google Scholar]
- 3.Carr M. H. 1991. Habitat selection and recruitment of an assemblage of temperate zone reef fishes. J. Exp. Mar. Biol. Ecol. 146, 113–137 10.1016/0022-0981(91)90257-W (doi:10.1016/0022-0981(91)90257-W) [DOI] [Google Scholar]
- 4.Jeffs A. G., Montgomery J. C., Tindle C. T. 2005. How do spiny lobster post-larvae find the coast? NZ J. Mar. Freshwater Res. 39, 605–617 10.1080/00288330.2005.9517339 (doi:10.1080/00288330.2005.9517339) [DOI] [Google Scholar]
- 5.Cowen R. K., Lwiza K. M. M., Sponaugle S., Paris C. B., Olson D. B. 2000. Connectivity of marine populations: open or closed? Science 287, 857–859 10.1126/science.287.5454.857 (doi:10.1126/science.287.5454.857) [DOI] [PubMed] [Google Scholar]
- 6.Shanks A. L. 1995. Mechanisms of cross-shelf dispersal of larval invertebrates and fish. In Ecology of marine invertebrate larvae (ed. McEdward L.), pp. 323–367 Boca Raton, FL: CRC Press [Google Scholar]
- 7.Forward R. B., Jr, Tankersley R. A. 2001. Selective tidal-stream transport of marine animals. Oceanogr. Mar. Biol. 39, 305–353 [Google Scholar]
- 8.Boles L. C., Lohmann K. J. 2003. True navigation and magnetic maps in spiny lobsters. Nature 421, 60–63 10.1038/nature01226 (doi:10.1038/nature01226) [DOI] [PubMed] [Google Scholar]
- 9.Smith R. J. F., Smith M. J. 1998. Rapid acquisition of directional preferences by migratory juveniles of two amphidromous Hawaiian gobies, Awaous guamensis and Sicyopterus stimpsoni. Environ. Biol. Fishes 53, 275–282 10.1023/A:1007449021543 (doi:10.1023/A:1007449021543) [DOI] [Google Scholar]
- 10.Leis J. M., Carson-Ewart B. M. 1999. In situ swimming and settlement behaviour of larvae of an Indo-Pacific coral-reef fish, the coral trout Plectropomus leopardus (Pisces: Serranidae). Mar. Biol. 134, 51–64 10.1007/s002270050524 (doi:10.1007/s002270050524) [DOI] [Google Scholar]
- 11.Kobayashi D. R. 1989. Fine-scale distribution of larval fishes: patterns and processes adjacent to coral reefs in Kaneohe Bay, Hawaii. Mar. Biol. 100, 285–293 10.1007/BF00391141 (doi:10.1007/BF00391141) [DOI] [Google Scholar]
- 12.Kingsford M. J., Leis J. M., Shanks A., Lindeman K. C., Morgan S. G., Pineda J. 2002. Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. 70, 309–340 [Google Scholar]
- 13.Metcalfe J. D., Holford B. H., Arnold G. P. 1993. Orientation of plaice (Pleuronectes platessa) in the open sea: evidence for the use of external directional clues. Mar. Biol. 117, 559–566 10.1007/BF00349766 (doi:10.1007/BF00349766) [DOI] [Google Scholar]
- 14.Montgomery J. C., Jeffs A., Simpson S. D., Meekan M., Tindle C. 2006. Sound as an orientation cue for the pelagic larvae of reef fishes and decapod crustaceans. Adv. Mar. Biol. 51, 143–196 10.1016/S0065-2881(06)51003-X (doi:10.1016/S0065-2881(06)51003-X) [DOI] [PubMed] [Google Scholar]
- 15.Simpson S. D., Meekan M. G., Montgomery J. C., McCauley R. D., Jeffs A. 2005. Homeward sound. Science 308, 221. 10.1126/science.1107406 (doi:10.1126/science.1107406) [DOI] [PubMed] [Google Scholar]
- 16.Able K. P. 1991. Common themes and variations in animal orientation systems. Am. Zool. 31, 157–167 10.1093/icb/31.1.157 (doi:10.1093/icb/31.1.157) [DOI] [Google Scholar]
- 17.Rebach S. 1981. Use of multiple cues in short-range migrations of crustacea. Am. Midland Nat. 105, 168–180 10.2307/2425022 (doi:10.2307/2425022) [DOI] [Google Scholar]
- 18.Welch J. M., Forward R. B., Jr, Howd P. A. 1999. Behavioral responses of blue crab Callinectes sapidus postlarvae to turbulence: implications for selective tidal stream transport. Mar. Ecol. Prog. Ser. 179, 135–143 10.3354/meps179135 (doi:10.3354/meps179135) [DOI] [Google Scholar]
- 19.Tankersley R. A., McKelvey L. M., Forward R. B. 1995. Responses of estuarine crab megalopae to pressure, salinity and light: implication for flood tide transport. Mar. Biol. 122, 391–400 10.1007/BF00350871 (doi:10.1007/BF00350871) [DOI] [Google Scholar]
- 20.Radford C. A., Stanley J. A., Tindle C. T., Montgomery J. C., Jeffs A. G. 2010. Localised coastal habitats have distinct underwater sound signatures. Mar. Ecol. Prog. Ser. 401, 21–29 10.3354/meps08451 (doi:10.3354/meps08451) [DOI] [Google Scholar]
- 21.Stobutzki I. C., Bellwood D. R. 1998. Nocturnal orientation to reefs by late pelagic stage coral reef fishes. Coral Reefs 17, 103–110 10.1007/s003380050103 (doi:10.1007/s003380050103) [DOI] [Google Scholar]
- 22.Jeffs A., Tolimieri N., Montgomery J. C. 2003. Crabs on cue for the coast: the use of underwater sound for orientation by pelagic crab stages. Mar. Freshwater Res. 54, 841–845 10.1071/MF03007 (doi:10.1071/MF03007) [DOI] [Google Scholar]
- 23.Leis J. M., Lockett M. M. 2005. Localization of reef sounds by settlement-stage larvae of coral-reef fishes (Pomacentridae). Bull. Mar. Sci. 76, 715–724 [Google Scholar]
- 24.Vermeij M. J. A., Marhaver K. L., Huijbers C. M., Nagelkerken I., Simpson S. D. 2010. Coral larvae move toward reef sounds. PLoS ONE 5, e10660. 10.1371/journal.pone.0010660 (doi:10.1371/journal.pone.0010660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolimieri N., Haine O., Montgomery J., Jeffs A. 2002. Ambient sound as a navigational cue for larval reef fish. Bioacoustics 12, 214–217 10.1080/09524622.2002.9753700 (doi:10.1080/09524622.2002.9753700) [DOI] [Google Scholar]
- 26.Radford C. A., Jeffs A. G., Montgomery J. C. 2007. Directional swimming behavior by five species of crab postlarvae in response to reef sound. Bull. Mar. Sci. 80, 369–378 [Google Scholar]
- 27.Simpson S. D., Radford A. N., Tickle E. J., Meekan M. G., Jeffs A. G. 2011. Adaptive avoidance of reef noise. PLoS ONE 6, e16625. 10.1371/journal.pone.0016625 (doi:10.1371/journal.pone.0016625) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson S. D., Meekan M. G., Jeffs A., Montgomery J. C., McCauley R. D. 2008. Settlement-stage coral reef fish prefer the higher-frequency invertebrate-generated audible component of reef noise. Anim. Behav. 75, 1861–1868 10.1016/j.anbehav.2007.11.004 (doi:10.1016/j.anbehav.2007.11.004) [DOI] [Google Scholar]
- 29.Forward R. B., Jr, Tankersley R. A., Rittschof D. 2001. Cues for metamorphosis of brachyuran crabs: an overview. Am. Zool. 41, 1108–1122 10.1668/0003-1569(2001)041[1108:CFMOBC]2.0.CO;2 (doi:10.1668/0003-1569(2001)041[1108:CFMOBC]2.0.CO;2) [DOI] [Google Scholar]
- 30.Stanley J. A., Radford C. A., Jeffs A. G. 2010. Induction of settlement in crab megalopae by ambient underwater reef sound. Behav. Ecol. 21, 113–120 10.1093/beheco/arp159 (doi:10.1093/beheco/arp159) [DOI] [Google Scholar]
- 31.Meekan M. G., Wilson S. G., Halford A., Retzel A. 2001. A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar. Biol. 139, 373–381 10.1007/s002270100577 (doi:10.1007/s002270100577) [DOI] [Google Scholar]
- 32.McLay C. L. 1988. Crabs of New Zealand. Auckland, New Zealand: University of Auckland Bindery [Google Scholar]
- 33.Jones D. S., Morgan G. J. 2002. A field guide to Crustaceans of Australian waters. Sydney, Australia: New Holland Publishers (Australia) Pty Ltd. [Google Scholar]
- 34.Cronin T. W. 1986. Photoreception in marine-invertebrates. Am. Zool. 26, 403–415 [Google Scholar]
- 35.Kroodsma D. E., Byers B. E., Goodale E., Johnson S., Liu W. C. 2001. Pseudoreplication in playback experiments, revisited a decade later. Anim. Behav. 61, 1029–1033 10.1006/anbe.2000.1676 (doi:10.1006/anbe.2000.1676) [DOI] [Google Scholar]
- 36.Radford C. A., Jeffs A. G., Tindle C. T., Montgomery J. C. 2008. Temporal patterns in ambient noise of biological origin from a shallow water temperate reef. Oecologia 156, 921–929 10.1007/s00442-008-1041-y (doi:10.1007/s00442-008-1041-y) [DOI] [PubMed] [Google Scholar]
- 37.Cato D. H., McCauley R. D. 2002. Australian research into ambient sea noise. Acoust. Aust. 30, 13–20 [Google Scholar]
- 38.Zar H. J. 1999. Biostatistical analysis, 4th edn Englewood Cliffs, NJ: Prentice-Hall Inc [Google Scholar]
- 39.Stanley J. A., Radford C. A., Jeffs A. 2011. Behavioural response thresholds in New Zealand crab megalopae to ambient underwater sound. PLoS ONE 6, e28572. 10.1371/journal.pone.0028572 (doi:10.1371/journal.pone.0028572) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cato D. H. 1992. The biological contribution to the ambient noise in waters near Australia. Acoust. Aust. 20, 76–80 [Google Scholar]
- 41.Forward R. B., Jr, DeVries M. C., Rittschof D., Frankel D. A. Z., Bischoff J. P., Fisher C. M., Welch J. M. 1996. Effects of environmental cues on metamorphosis of the blue crab Callinectes sapidus. Mar. Ecol. Prog. Ser. 131, 165–177 10.3354/meps131165 (doi:10.3354/meps131165) [DOI] [Google Scholar]
- 42.Montgomery J. C., Tolimieri N., Haine O. S. 2001. Active habitat selection by pre-settlement reef fishes. Fish Fish. 2, 261–277 10.1046/j.1467-2960.2001.00053.x (doi:10.1046/j.1467-2960.2001.00053.x) [DOI] [Google Scholar]
- 43.Rodriguez S. R., Ojeda F. P., Inestrosa N. C. 1993. Settlement of benthic marine invertebrates. Mar. Ecol. Prog. Ser. 97, 193–207 10.3354/meps097193 (doi:10.3354/meps097193) [DOI] [Google Scholar]
- 44.Morse D. E. 1990. Recent progress in larval settlement and metamorphosis: closing the gaps between molecular biology and ecology. Bull. Mar. Sci. 46, 465–483 [Google Scholar]
- 45.Sale P. F. 1968. Influence of cover availability on depth preference of the juvenile manini, Acanthurus triostegus sandvicensis. Copeia 1968, 802–807 10.2307/1441847 (doi:10.2307/1441847) [DOI] [Google Scholar]
- 46.Levin P. S. 1994. Small-scale recruitment variation in a temperate fish: the roles of macrophytes and food-supply. Environ. Biol. Fishes 40, 271–281 10.1007/BF00002517 (doi:10.1007/BF00002517) [DOI] [Google Scholar]
- 47.Cato D. H. 1976. Ambient sea noise in waters near Australia. J. Acoust. Soc. Am. 60, 320–328 10.1121/1.381109 (doi:10.1121/1.381109) [DOI] [Google Scholar]
- 48.Au W. W. L., Banks K. 1998. The acoustics of the snapping shrimp Synalpheus parneomeris in Kaneohe Bay. J. Acoust. Soc. Am. 103, 41–47 10.1121/1.423234 (doi:10.1121/1.423234) [DOI] [Google Scholar]
- 49.Leis J. M., Carson-Ewart B. M., Cato D. H. 2002. Sound detection in situ by the larvae of a coral-reef damselfish (Pomacentridae). Mar. Ecol. Prog. Ser. 232, 259–268 10.3354/meps232259 (doi:10.3354/meps232259) [DOI] [Google Scholar]
- 50.Radford C., Stanley J., Simpson S., Jeffs A. 2011. Juvenile coral reef fish use sound to locate habitats. Coral Reefs 30, 295–305 10.1007/s00338-010-0710-6 (doi:10.1007/s00338-010-0710-6) [DOI] [Google Scholar]
- 51.Onuki A., Somiya H. 2004. Two types of sound and additional spinal nerve innervation to the sonic muscles in John dory, Zeus faber (Zeiformes: Teleostei). J. Mar. Biol. Assoc. UK 84, 843–850 10.1017/S0025315404010045h (doi:10.1017/S0025315404010045h) [DOI] [Google Scholar]
- 52.Connaughton M. A. 2004. Sound generation in the searobin (Prionotus carolinus), a fish with alternate sonic muscle contraction. J. Exp. Biol. 207, 1643–1654 10.1242/jeb.00928 (doi:10.1242/jeb.00928) [DOI] [PubMed] [Google Scholar]
- 53.Simpson S. D., Meekan M. G., McCauley R. D., Jeffs A. 2004. Attraction of settlement-stage coral reef fishes to reef noise. Mar. Ecol. Prog. Ser. 276, 263–268 10.3354/meps276263 (doi:10.3354/meps276263) [DOI] [Google Scholar]