Abstract
Climatic and geological changes across time are presumed to have shaped the rich biodiversity of tropical regions. However, the impact climatic drying and subsequent tropical rainforest contraction had on speciation has been controversial because of inconsistent palaeoecological and genetic data. Despite the strong interest in examining the role of climatic change on speciation in the Neotropics there has been few comparative studies, particularly, those that include non-rainforest taxa. We used bird species that inhabit humid or dry habitats that dispersed across the Panamanian Isthmus to characterize temporal and spatial patterns of speciation across this barrier. Here, we show that these two assemblages of birds exhibit temporally different speciation time patterns that supports multiple cycles of speciation. Evidence for these cycles is further corroborated by the finding that both assemblages consist of ‘young’ and ‘old’ species, despite dry habitat species pairs being geographically more distant than pairs of humid habitat species. The matrix of humid and dry habitats in the tropics not only allows for the maintenance of high species richness, but additionally this study suggests that these environments may have promoted speciation. We conclude that differentially expanding and contracting distributions of dry and humid habitats was probably an important contributor to speciation in the tropics.
Keywords: speciation, divergence times, species diversity, neotropics, Isthmus of Panama, refuge model
1. Introduction
The origins and causes of the extraordinary species diversity in the tropics is a contentious issue among biologists [1–5]. One of the most vigorously debated models to explain high Neotropical diversity is that rainforest contraction initiated speciation in small, isolated forest refuges when drier more open habitats expanded during Pleistocene (2.6–0.01 Myr ago) glacial cycles [1,6]. A lack of support for this rainforest refuge hypothesis has come from both genetic [2,6] and palaeo-climatic data [7]. Molecular phylogenetic studies have indicated that most speciation events are much older than the last glacial cycle [6,8]. Palaeo-climatic data suggest Amazonia remained mostly forested during the Pleistocene instead of being replaced by open savannah habitats [9,10].
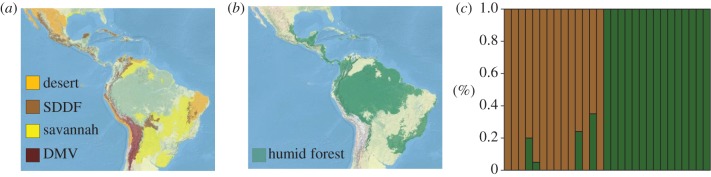
Collectively, these findings suggest that speciation events were not concentrated during the last glacial cycle and that savannah did not extensively replace rainforest as was once envisioned. This evidence, however, does not indicate that rainforests remained stable over time, nor that habitat changes were unimportant for speciation across a broader time frame. A less extreme alternative to the rainforest/savannah dichotomy is the possibility that seasonally deciduous dry forest expanded during arid periods [11]. The expansion of seasonally deciduous dry forest is consistent with the view that Neotropical forests were extensive throughout Pleistocene glacial cycles [11]. However, the expansion of more arid biomes consisting of dry forests, edge habitat and scrub (hereafter dry habitat) would have generated ecological barriers for many species that inhabit rainforest environments (hereafter humid habitat) [12,13]. Under current conditions, dry biomes are highly fragmented (figure 1a) and separated by the more-or-less continuously distributed rainforests known as humid forests (figure 1b).
Figure 1.
Neotropical biomes and climatic assemblage assignment test. Geographical distribution of (a) dry and (b) humid biomes in the Neotropics. (a) Seasonally deciduous dry forest (SDDF; brown), savannah (yellow), dry montane valley (DMV; maroon), and desert and xeric shrubland (orange). (b) Humid forest (green). Results from climatic assemblage assignment test are shown in (c). Each column represents a species pair (in alphabetical order; see the electronic supplementary material) and the colour indicates the proportion of climatic data (y-axis) from each pair (x-axis) that is assigned to the dry (brown) and humid (green) groups.
Today, many dry habitat sister taxa are geographically distant from one another, suggesting that these species have either undergone long-distance dispersal across large tracts of humid forests, or that arid regions were once more connected [14–17]. In some cases, closely related dry habitat lineages exhibit puzzling biogeographic distributions that have trans-continental gaps separated by thousands of kilometres. These distributions have been attributed to ancient dispersal events across the Isthmus of Panama during alternating periods of suitable environmental conditions [18–20]. Studies based on the mammalian fossil record have concluded that dry habitat species were able to disperse through a trans-American dry habitat corridor during periods of rainforest contraction [18,19]. This model of alternating episodes of suitable climate corridors [18,19] predicts that under wet conditions, humid habitat species will be able to disperse through habitat corridors and dry habitat species will be isolated, whereas under drier conditions, dispersal corridors for dry habitat species are formed and humid habitat populations became isolated. Surprisingly, given the intensive interest in understanding the role of climate change on speciation in the tropics, there have been few studies on the diversification of dry habitat taxa and even less on comparative studies between humid and dry habitat taxa [20].
Understanding speciation time patterns between humid and dry habitat assemblages across the Isthmus of Panama will provide insight into the role of climate change in generating species in the Neotropics. However, climatic and environmental changes probably occur at a scale that is much finer than can be inferred from molecular speciation times, given that credible intervals around some time estimates may encompass millions of years. Thus, directly attempting to correlate speciation cycles to the absolute timing of climatic changes is challenging. For example, the glacial–interglacial cycles of the Pleistocene operated on 40 000 and 100 000 year cycles with interglacial periods being much shorter than glacial periods. Earlier climatic changes in the Pliocene (5.3–2.6 Myr ago), also, probably impacted the dispersal and speciation of taxa, but the temporal scale of these changes are less well understood. Therefore, given the uncertainty around the timing of climatic cycles and speciation times examining relative speciation patterns may be most appropriate.
In this study, we used a diverse assemblage of bird species known to have dispersed across the Panamanian Isthmus to characterize temporal and spatial patterns of speciation across this barrier, and to evaluate the role of climatic changes on speciation. We selected a suite of species that have previously been identified to exhibit a genetic break across the Isthmus of Panama [21–24] and are known to occur in an array of habitats from humid evergreen forests to seasonally deciduous dry forests. Using bioclimatic data, we performed an assignment test to delimit species into one of two coarse-scale climatic assemblages or separate species into humid or dry habitat groups. We estimated speciation times across the Isthmus of Panama using a multilocus coalescent approach and examined the similarity in time estimates by summarizing the amount of overlap across species pairs. We used these summaries as proxies for different models of speciation that represented speciation of humid and dry habitat taxa during the same time periods to a model representing distinct periods of speciation between humid and dry habitat taxa. Finally, we incorporated speciation times, climatic data and current-day distributions to propose a model of how palaeo-environments shaped the assembly of humid and dry forest regional communities on each side of the Panamanian land bridge.
2. Methods
(a). Taxa and genetic data
Taxon list and laboratory protocol is in the electronic supplementary material.
(b). Speciation times
Given the high level of genetic diversification within certain pairs, our dataset was not well-suited for existing comparative phylogeographic software [25], nor were our data suitable for a gene-flow with divergence model, such as IMA [26–28] because these programs assume that sister lineages are relatively panmictic on each side of a barrier. Therefore, we used a species tree approach and estimated speciation times in the program *BEAST [29]. We used a relaxed uncorrelated rate for the ND2 gene to account for the variance observed across mtDNA rates. ([21]; lognormal distribution with mean = 0.0125; s.d. = 0.1) and a strict rate for autosomal and sex-linked rates ([30]; autosomal rate: lognormal distribution with mean = 0.0018, s.d. = 0.45; sex-linked rate: lognormal distribution with mean = 0.00195, s.d. = 0.45). We ran the analysis for 50 000 000 generations sampling every 1000 generations. All analyses implemented a Yule process on the species tree prior and lognormal distributions on sequence model prior distributions. We assessed Markov chain Monte Carlo convergence and determined the burn-in by examining effective sampling size (ESS) values and likelihood plots in Tracer v. 1.5 [31].
(c). Climatic assemblage assignment test
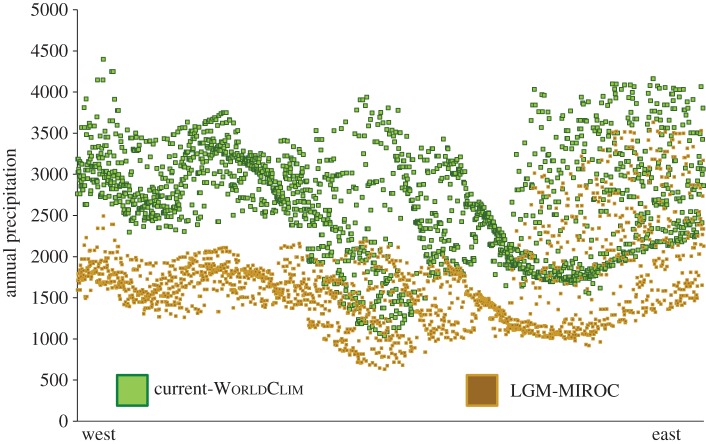
To assign species into either humid or dry climatic groups, we performed a two-step cluster analysis using climatic data extracted from locality records in the program PASW Statistics v. 18. We used variables (WorldClim dataset v. 1.4, with a resolution of 2.5 min) associated with precipitation because the climatic differences between arid and humid biomes of the Neotropics are largely defined by the amount of rainfall an area receives [32]. A full description of the methodology is given in the electronic supplementary material. The cluster analysis was fixed to estimate two clusters, which allowed us to assign species pairs to one of the climatic groups based on the proportion of each pair's climatic data in each cluster. This approach makes no assumption about niche conservatism or divergence; rather, we assumed that the climatic space of a sister pair is a conservative estimate of climatic preferences. We estimated the Euclidean geographical distance between sister lineages using digital range maps [33]. Finally, we compared average annual precipitation values across the Isthmus of Panama using current-day conditions and glacial conditions to look at how climate regimes may have changed between glacial-interglacial cycles. Using the program DIVA-GIS (www.diva-gis.org), we extracted precipitation values from randomly generated points less than or equal to 1500 m from a present-day climatic layer (WorldClim dataset v. 1.4) and a palaeo-layer for the last glacial maximum (LGM) [a model for interdisciplinary research on climate (MIROC)].
(d). Speciation time patterns
We assessed if there were temporal differences in the *BEAST speciation times of dry and humid forest species by sequentially estimating the probability that species assemblages diverged in either the same or in different time periods. We divided the last six million years into one-million-year intervals and calculated the probability that a species diverged in each interval. One-million-year intervals provided a time frame comparable to the speciation times, which often had 95% credible intervals ranging over millions of years. Given that we were interested in comparing patterns between humid and dry pairs and not directly estimating the number of vicariance events, we a priori estimated the probability of speciation in three intervals per group. This allowed us to test a model of completely alternating cycles of speciation from 0 to 6 Myr ago. Specifically, we calculated the joint probability that the sister pairs which inhabit dry habitat diverged in three intervals (Id), and the sister pairs that inhabit humid habitat diverged in three intervals (Ih):
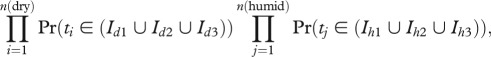 |
where n(dry) and n(humid) represents the total numbers of dry and humid pairs, respectively, and interval (I) is one million years in length. The parameter t comes from the posterior distribution of the *BEAST speciation time across the Isthmus of Panama. We calculated probabilities for different temporal scenarios by allowing the intervals (I) to shift from 0.0 to 6.0 million years at units of 0.5 million years. Each interval permutation was used to evaluate the probability of alternative temporal models.
3. Results
(a). Species pairs and climatic assemblage assignment
We included 29 sister pairs with representatives from 13 avian families (see the electronic supplementary material). The climatic assemblage assignment test provided robust results in designating pairs to the humid or dry forest group (figure 1c) by assigning 15 pairs to the former group and 14 to the latter group. All 15 humid habitat pairs were 100 per cent assigned to one cluster (see the electronic supplementary material). We observed more variance in the dry habitat pairs probably because dry habitat species can use edge habitat and gallery forests in wetter regions [34], and some pairs exhibited wider climatic tolerances. Two species in our dataset, Granatellus pelzelni and Periporphyrus erythromelas, are found in wetter regions than their cross-Isthmus counterparts. The clustering analysis assigned each pair to the dry habitat group because the climatic data for these species indicated that they were found in habitats on the drier end of humid forests.
(b). Speciation times and patterns
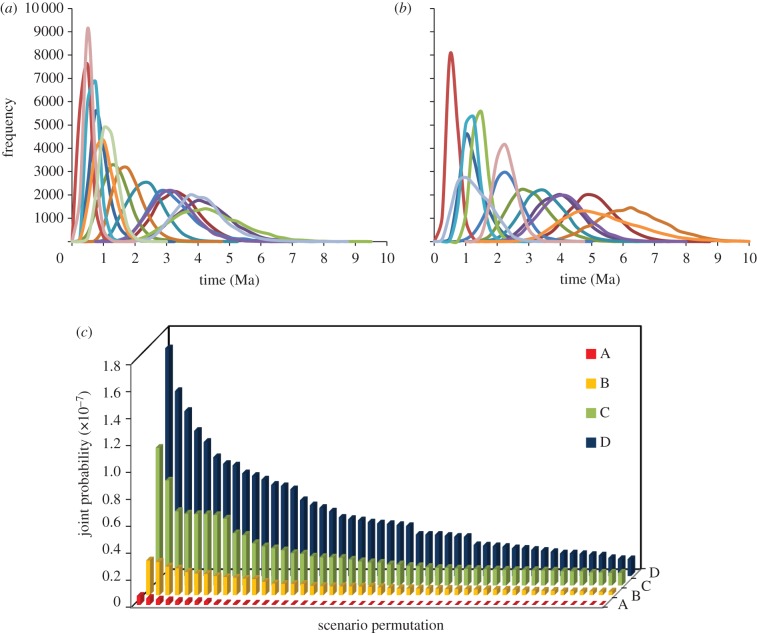
All parameters in *BEAST analyses had ESS values of more than 200. Mean speciation times ranged from 0.31 to 4.18 Myr ago for humid habitat pairs (see figure 2a and the electronic supplementary material) and 0.47 to 6.13 Myr ago for dry habitat pairs (see figure 2b and the electronic supplementary material). We separated speciation scenarios into four categories based on the number of unique intervals shared between them. The scenarios defined represent a continuum from completely overlapping speciation intervals (scenario A) to temporally unique intervals (scenario D). We reported the 50 most probable permutations for each scenario (figure 2c). To make the joint probability of each model more interpretable, we calculated a weighted probability for each model based on the approach used in information theory [35]. Weighted probabilities (electronic supplementary material, table S1) indicate that the probability of a scenario increased with the number of unique intervals (scenario A wk = 0.04; scenario B wk = 0.19; scenario C wk = 0.32; scenario D wk = 0.45). Scenario D (temporally unique bouts of speciation between humid and dry habitat pairs) was over 11 times more probable than scenario A (no discernible differences in speciation times between groups). The permutation with the highest probability (scenario D; log of the joint probability = −15.61) had the intervals 0–1.0, 1.0–2.0 and 3.0–4.0 Myr ago for the humid pairs and 0.5–1.5, 2.0–3.0 and 4.0–5.0 Myr ago for the dry pairs (electronic supplementary material). The most probable overlapping speciation permutation (scenario A; log of the joint probability = −19.12) had the intervals 0.5–1.5, 2.0–3.0 and 3.5–4.5 Myr ago.
Figure 2.
Speciation times and speciation scenario probabilities. Posterior distributions of speciation times across the Isthmus of Panama in (a) humid and (b) dry pairs. (c) Rank of differing speciation scenarios. The coloured models (c) represent four different scenarios that differ by the number of unique intervals. The scenarios represent a continuum of completely overlapping intervals or time periods (scenario A; red) to temporally unique intervals among habitat groups (scenario D; blue). On the x-axis are the top 50 permutations and on the y-axis is the joint probability of each permutation.
(c). Spatial and climatic speciation patterns
There was a positive linear relationship between speciation time and geographical distance (r = 0.41; p = 0.03), indicating that geographical distance between sister lineages generally increases with time since speciation. Additionally, there was a stronger negative linear relationship between geographical distance and the average annual precipitation of the pair's habitat (r = −0.66; p < 0.00). We found that sister lineage pairs found in dry habitats have a larger between-lineage geographical distance than humid habitat pairs (student's t-test: t = −4.26; p-value < 0.00). Current-day precipitation conditions are overall wetter than conditions from a palaeo-climate simulation of the LGM (21 000 years ago; figure 3).
Figure 3.
Present-day and palaeo precipitation transect across the Isthmus of Panama. West to east transects across the Isthmus of Panama that shows average annual precipitation under current conditions (green) and glacial conditions (brown).
4. Discussion
(a). Speciation patterns and contracting rainforests
Here, we present comparative findings of speciation times between humid and dry habitat taxa across a prominent biogeographic barrier in the Neotropics. These data suggest overall that dry and humid taxa exhibit temporally different patterns of speciation. Contrary to the idea that rainforests were stable during the Pleistocene, speciation across the Isthmus of Panama occurred frequently over the last two million years in humid habitat taxa. Speciation in dry habitat taxa occurred earlier and slowed down before the onset of the major glacial cycles. While it is tempting to correlate these speciation times to specific Earth history events, the differences in scale between climatic and genetic data make such comparisons problematic. A comparison of the relative difference in speciation times along with the geographical distances between sister pairs favours a model of multiples episodes of expansion, contraction, and speciation.
Our estimate of speciation times indicates that humid taxa began diverging around 3–4 Myr ago and continued throughout the Pleistocene (figure 2). Moreover, these sister taxa are often in close geographical proximity to one another, and some lineages may have come back into secondary contact. The bird assemblages found in these humid forests are composed of taxa that have dispersed across the Isthmus during different times, suggesting that these communities are assembling over a long period of time instead of simultaneously. The high proportion of sister pairs that diverged during the Pleistocene suggest that humid forests probably expanded and contracted multiple times over the last two million years. This finding is further supported by our comparison of precipitation values between current-day and palaeo conditions that indicate the Isthmus of Panama may have been drier in the past, which suggests that the current-day homogeneous rainforest across the area may have been a more heterogeneous environment during glacial periods (figure 3).
Speciation times in dry habitat birds began earlier and occurred across a longer time frame than humid habitat birds (figure 2). This pattern in dry habitat birds is consistent with the mammalian fossil record that demonstrates a pre-Pleistocene pulse in dispersal for large grazing herbivores that inhabited non-rainforest environments [36]. Divergence time estimates between isolated areas in other Neotropical dry habitat taxa, such as in plants and frogs, have generally been older than the Pleistocene [37,38]. In our dataset, we inferred only a single mid-Pleistocene divergence event in the dry habitat species (figure 2). It is unclear why dry habitat taxa stopped dispersing across the land bridge over the last 500 000 years. One possible explanation is that arid biome source faunas may have become too geographically distant from the Isthmus, with some currently more than 1000 km from the Isthmus and several 1000 km from each other.
(b). Molecular dating
We were unable to define speciation times in discrete alternating episodes as envisioned by palaeontologists who studied the mammalian fossil record. However, we did find that there was a greater probability that humid and dry birds diverged during different times than during the same time periods (figure 2). Future work may further clarify the role of climate change on speciation in the tropics with the usage of next-generation sequencing that will allow researchers to generate genomic scale datasets [39]. Larger genetic datasets should greatly reduce the uncertainty around divergence times, perhaps allowing speciation and climatic events to be compared at similar scales.
(c). Climatic change and high tropical species diversity
The matrix of forest types in the Neotropics represents one of the most heterogeneous environments on Earth, allowing these areas to maintain extraordinary species diversity. We posit that this habitat heterogeneity has also promoted speciation by a mechanism similar to Haffer's refuge model [40]. However, our findings highlight the importance of non-rainforest species in the speciation model. Given the array of available habitats in the Neotropics and high levels of species diversity, we can predict that under different climatic scenarios, some species will expand or contract their distributions, depending on specific habitat requirements. Under such a model, range expansion and fragmentation would be constant and dynamic owing to shifts in the prevailing climatic conditions. Diversification rate analysis on a widely distributed and speciose clade of birds [41] supports the view that diversification has been a constant process in the Neotropics. By contrast, more homogeneous environments found in the temperate region appear to synchronously respond to climatic changes, such as the repeated contraction of eastern North American forests into glacial refugia [42]. The interplay between the constant expansion and contraction of different habitats across the Neotropics may help us explain the high species diversity of the tropics, as well, as the present-day distributional patterns of humid and dry biome taxa.
5. Conclusion
The role of climate change in initiating speciation in the tropics has been an intensely debated topic; however, most studies have focused on speciation patterns in rainforest taxa at the exclusion of taxa that occur in drier habitats. In this study, we examined temporal patterns of speciation across the Isthmus of Panama in an ecologically diverse suite of birds and found that humid and dry habitat taxa exhibit temporal differences in speciation times. In addition, each climatic assemblage of birds consists of both ‘old’ and ‘young’ lineages, indicating that community assembly has been a protracted process instead of a simultaneous event. Under current wet conditions, divergent sister lineages have come in close or secondary contact in the now largely continuous rainforests, whereas dry habitat taxa have been isolated in arid fragments scattered across the Neotropics. We suggest that the cyclical contraction and expansion of rainforests was an important process in generating the observed high species diversity of the Neotropics, not only for rainforest taxa but also for dry habitat species.
Acknowledgments
The following individuals and institutions provided samples for the study: R. Brumfield, D. Dittman (LSUMZ), M. Miller (STRI), P. Escalante (CNAV), B. Hernández-Baños, A. G. Navarro-Sigüenza (MZFC), P. Sweet and G. Barrowclough (AMNH), S. Rohwer and S. Birks (UWBM), M. Robbins (KU), D. Willard and S. Hackett (FMNH), J. Fjeldså and J.B. Kristensen (MZUC), and J. Pérez-Eman and A. Rodríguez-Ferraro. We thank two anonymous reviewers and the associate editor for providing comments on an earlier draft of this manuscript. We thank J.P.E., R.W.B., M.G., C.D., R.T., A.C., M.H., G.S., D.F., A.R.-F., T.J. and D.C. This work was partially funded by a UNLV International Studies Scholarship, a UNLV GPSA grant and an AMNH Chapman Grant.
References
- 1.Haffer J. 1969. Speciation in Amazonian forest birds. Science 165, 131–137 10.1126/science.165.3889.131 (doi:10.1126/science.165.3889.131) [DOI] [PubMed] [Google Scholar]
- 2.Hoorn C., et al. 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330, 927–931 10.1126/science.1194585 (doi:10.1126/science.1194585) [DOI] [PubMed] [Google Scholar]
- 3.Claramunt S., Derryberry E. P., Remsen J. V., Jr, Brumfield R. T. 2011. High dispersal ability inhibits speciation in a continental radiation of passerine birds. Proc. R. Soc. B 279, 1567–1574 10.1098/rspb.2011.1922 (doi:10.1098/rspb.2011.1922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rull V. 2011. Origins of biodiversity. Science 33, 398–399 10.1126/science.331.6016.398-c (doi:10.1126/science.331.6016.398-c) [DOI] [PubMed] [Google Scholar]
- 5.Wiens J. J., Pyron R. A., Moen D. C. 2011. Phylogenetic origins of local-scale diversity patterns and causes of Amazonian megadiversity. Ecol. Lett. 14, 643–652 10.1111/j.1461-0248.2011.01625.x (doi:10.1111/j.1461-0248.2011.01625.x) [DOI] [PubMed] [Google Scholar]
- 6.Moritz C., Patton C. J., Schneider C. J., Smith T. B. 2000. Diversification of rainforest faunas: an integrated molecular approach. Annu. Rev. Ecol. Syst. 31, 533–563 10.1146/annurev.ecolsys.31.1.533 (doi:10.1146/annurev.ecolsys.31.1.533) [DOI] [Google Scholar]
- 7.Colinvaux P. A., de Oliveira P. E., Bush M. B. 2000. Amazonian and neotropical plant communities on glacial timescales: the failure of the aridity and refuge hypotheses. Quat. Sci. Rev. 19, 141–169 10.1016/S0277-3791(99)00059-1 (doi:10.1016/S0277-3791(99)00059-1) [DOI] [Google Scholar]
- 8.Rull V. 2008. Speciation timing and Neotropical biodiversity: the Tertiary–Quaternary debate in the light of molecular phylogenetic evidence. Mol. Ecol. 17, 2722–2729 10.1111/j.1365-294X.2008.03789.x (doi:10.1111/j.1365-294X.2008.03789.x) [DOI] [PubMed] [Google Scholar]
- 9.Colinvaux P., De Oliveira P., Moreno J., Miller M., Bush M. 1996. A long pollen record from lowland Amazonia: forest and cooling in glacial times. Science 274, 85–88 10.1126/science.274.5284.85 (doi:10.1126/science.274.5284.85) [DOI] [Google Scholar]
- 10.Mayle F. E., Beerling D. J., Gosling W. D., Bush M. B. 2004. Responses of Amazonian ecosystems to climatic and atmospheric carbon dioxide changes since the last glacial maximum. Proc. R. Soc. Lond. B 359, 499–514 10.1098/rstb.2003.1434 (doi:10.1098/rstb.2003.1434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennington R. T., Lavin M., Prado D. E., Pendry C. A., Pell S. K., Butterworth C. A. 2004. Historical climate change and speciation: Neotropical seasonally dry forest plants show patterns of both Tertiary and Quaternary diversification. Proc. R. Soc. Lond. B 359, 515–538 10.1098/rstb.2003.1435 (doi:10.1098/rstb.2003.1435) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson W. D., Brawn J. D., Robinson S. K. 2000. Forest bird community structure in central Panama: influence of spatial scale and biogeography. Ecol. Monogr. 70, 209–235 10.1890/0012-9615(2000)070[0209:FBCSIC]2.0.CO;2 (doi:10.1890/0012-9615(2000)070[0209:FBCSIC]2.0.CO;2) [DOI] [Google Scholar]
- 13.Graham C. H., Blake J. G. 2001. Influence of patch- and landscape-level factors on bird assemblages in a fragmented tropical landscape. Ecol. Appl. 11, 1709–1721 10.1890/1051-0761(2001)011[1709:IOPALL]2.0.CO;2 (doi:10.1890/1051-0761(2001)011[1709:IOPALL]2.0.CO;2) [DOI] [Google Scholar]
- 14.Prado D. E., Gibbs P. E. 1993. Patterns of species distributions in the dry seasonal forests of South America. Ann. Miss. Bot. Gard. 80, 902–927 10.2307/2399937 (doi:10.2307/2399937) [DOI] [Google Scholar]
- 15.Pennington R. T., Prado D. E., Pendry C. A. 2000. Neotropical seasonally dry forests and Quaternary vegetation changes. J. Biogeogr. 27, 261–273 10.1046/j.1365-2699.2000.00397.x (doi:10.1046/j.1365-2699.2000.00397.x) [DOI] [Google Scholar]
- 16.Caetano S., Prado D., Pennington R. T., Beck S., Oliveira-Filho A., Spichiger R., Naciri Y. 2008. The history of seasonally dry tropical forests in eastern South America: inferences from the genetic structure of the tree Astronium urundeuva (Anacardiaceae). Mol. Ecol. 17, 3147–3159 10.1111/j.1365-294X.2008.03817.x (doi:10.1111/j.1365-294X.2008.03817.x) [DOI] [PubMed] [Google Scholar]
- 17.Wuster W., Ferguson J. E., Quijada-Mascarenas A., Pook C. E. 2005. Tracing an invasion: landbridges, refugia, and the phylogeography of the Neotropical rattlesnake (Serpentes: Viperidae: Crotalus durissus). Mol. Ecol. 14, 1095–1108 10.1111/j.1365-294X.2005.02471.x (doi:10.1111/j.1365-294X.2005.02471.x) [DOI] [PubMed] [Google Scholar]
- 18.Webb S. D. 1991. Ecogeography and the Great American Interchange. Paleobiology 17, 266–280 [Google Scholar]
- 19.Vrba E. S. 1992. Mammals as a key to evolutionary theory. J. Mammal. 73, 1–28 10.2307/1381862 (doi:10.2307/1381862) [DOI] [Google Scholar]
- 20.Ribas C. C., Maldonado M. C., Smith B. T., Cabanne G. S., D'Horta F. M., Naka L. N. In press Towards an integrated historical biogeography of the Neotropical lowland avifauna: combining diversification analysis and landscape evolution. Ornitología Neotropical. [Google Scholar]
- 21.Smith B. T., Klicka J. 2010. The profound influence of the Late Pliocene Panamanian uplift on the exchange, diversification, and distribution of New World birds . Ecography 33, 333–342 10.1111/j.1600-0587.2009.06335.X (doi:10.1111/j.1600-0587.2009.06335.X) [DOI] [Google Scholar]
- 22.Barker F. K. 2007. Avifaunal interchange across the Panamanian Isthmus: insights from Campylorhynchus wrens. Biol. J. Linn. Soc. 90, 687–702 10.1111/j.1095-8312.2007.00758.x (doi:10.1111/j.1095-8312.2007.00758.x) [DOI] [Google Scholar]
- 23.DaCosta J. M., Klicka J. 2008. the Great American Interchange in birds: a phylogenetic perspective with the genus Trogon. Mol. Ecol. 17, 1328–1343 10.1111/j.1365-294X.2007.03647.x (doi:10.1111/j.1365-294X.2007.03647.x) [DOI] [PubMed] [Google Scholar]
- 24.Weir J. T., Bermingham E., Schluter D. 2009. The Great American biotic Interchange in birds. Proc. Natl Acad. Sci. USA 106, 21 737–21 742 10.1073/pnas.0903811106 (doi:10.1073/pnas.0903811106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickerson M. J., Stahl E., Takebayashi N. 2007. MSBayes: a flexible pipeline for comparative phylogeographic inference using approximate Bayesian computation (ABC). BMC Bioinformatics 8, 268. 10.1186/1471-2105-8-268 (doi:10.1186/1471-2105-8-268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hey J., Nielsen R. 2007. Integration within the Felsenstein equation for improved Markov chain Monte Carlo methods in population genetics. Proc. Natl Acad. Sci. USA 104, 2785–2790 10.1073/pnas.0611164104 (doi:10.1073/pnas.0611164104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becquest C., Przeworksi M. 2009. Learning about modes of speciation by computational approaches. Evolution 63, 2547–2562 10.1111/j.1558-5646.2009.00662.x (doi:10.1111/j.1558-5646.2009.00662.x) [DOI] [PubMed] [Google Scholar]
- 28.Strasburg J. L., Riesenberg L. H. 2010. How robust are ‘Isolation with Migration’ analyses to violations of the IM model? A simulation study. Mol. Biol. Evol. 27, 297–310 10.1093/molbev/msp233 (doi:10.1093/molbev/msp233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heled J., Drummond A. J. 2010. Bayesian inference of species trees from multilocus data. Mol. Biol. Evol. 27, 570–580 10.1093/molbev/msp274 (doi:10.1093/molbev/msp274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Axelson E., Smith N. G. C., Sundstrom H., Berlin S., Ellegren H. 2004. Male biased mutation rate and divergence in autosomal, Z-linked and W-linked introns of chicken and turkey. Mol. Biol. Evol. 21, 1538–1547 10.1093/molbev/msh157 (doi:10.1093/molbev/msh157) [DOI] [PubMed] [Google Scholar]
- 31.Rambaut A., Drummond A. J. 2010. Tracer v. 1.5. Oxford, UK: Oxford University Press [Google Scholar]
- 32.Gentry A. H. 1995. Diversity and floristic composition of Neotropical dry forests. In Seasonally dry tropical forests (eds Bullock S. H., Mooney H. A., Medina E.), pp. 146–194 Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Ridgely R. S., Allnutt T. F., Brooks T., McNicol D. K., Mehlman D. W., Young B. E., Zook J. R. 2007. Digital distribution maps of the birds of the Western Hemisphere, v. 3.0 Arlington, VA: NatureServe [Google Scholar]
- 34.Stotz D. F., Fitzpatrick J. W., Parker T. A., III, Moskovits D. K. 1996. Neotropical birds: ecology and conservation. Chicago, IL: University of Chicago Press [Google Scholar]
- 35.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference. New York, NY: Springer [Google Scholar]
- 36.Webb S. D., Rancy A. 1996. Late Cenozoic evolution of the Neotropical mammal fauna. In Evolution and environment in tropical America (eds Jackson J., Budd A. F., Coates A. G.), pp. 335–358 Chicago, IL: University of Chicago Press [Google Scholar]
- 37.Pennington R. T., Lavin M., Oliveira-Filho A. 2009. Woody plant diversity, evolution, and ecology in the tropics: perspectives from seasonally dry tropical forests. Annu. Rev. Ecol. Syst. 40, 437–457 10.1146/annurev.ecolsys.110308.120327 (doi:10.1146/annurev.ecolsys.110308.120327) [DOI] [Google Scholar]
- 38.Crawford A. J., Bermingham E., Polanía C. 2007. The role of tropical dry forest as a long-term barrier to dispersal: a comparative phylogeographical analysis of dry forest tolerant and intolerant frogs. Mol. Ecol. 16, 4789–4807 10.1111/j.1365-294X.2007.03524.x (doi:10.1111/j.1365-294X.2007.03524.x) [DOI] [PubMed] [Google Scholar]
- 39.McCormack J. E., Maley J. M., Hird S. M., Derryberry E. P., Graves G., Brumfield R. T. 2012. Next-generation sequencing reveals phylogeographic structure and a species tree for recent bird divergences. Mol. Phyl. Evol. 62, 397–406 10.1016/j.ympev.2011.10.012 (doi:10.1016/j.ympev.2011.10.012) [DOI] [PubMed] [Google Scholar]
- 40.Haffer J., Prance T. 2001. Climatic forcing of evolution in Amazonia during the Cenozoic: on the refuge theory of biotic differentiation. Amazoniana 16, 579–607 [Google Scholar]
- 41.Derryberry E. P., Claramunt S., Derryberry G., Chesser R. T., Cracraft J., Aleixo A., Pérez-Emán J., Remsen J. V., Jr, Brumfield R. T. 2011. Lineage diversification and morphological evolution in a large-scale continental radiation: the Neotropical ovenbirds and woodcreepers (Aves: Furnariidae). Evolution 65, 2973–2986 10.1111/j.1558-5646.2011.01374.x (doi:10.1111/j.1558-5646.2011.01374.x) [DOI] [PubMed] [Google Scholar]
- 42.Hewitt G. 2000. The genetic legacy of the Quaternary ice ages. Nature 405, 907–913 10.1038/35016000 (doi:10.1038/35016000) [DOI] [PubMed] [Google Scholar]