Abstract
Chronic exposure to food of low quality may exert conflicting selection pressures on foraging behaviour. On the one hand, more active search behaviour may allow the animal to find patches with slightly better, or more, food; on the other hand, such active foraging is energetically costly, and thus may be opposed by selection for energetic efficiency. Here, we test these alternative hypotheses in Drosophila larvae. We show that populations which experimentally evolved improved tolerance to larval chronic malnutrition have shorter foraging path length than unselected control populations. A behavioural polymorphism in foraging path length (the rover–sitter polymorphism) exists in nature and is attributed to the foraging locus (for). We show that a sitter strain (fors2) survives better on the poor food than the rover strain (forR), confirming that the sitter foraging strategy is advantageous under malnutrition. Larvae of the selected and control populations did not differ in global for expression. However, a quantitative complementation test suggests that the for locus may have contributed to the adaptation to poor food in one of the selected populations, either through a change in for allele frequencies, or by interacting epistatically with alleles at other loci. Irrespective of its genetic basis, our results provide two independent lines of evidence that sitter-like foraging behaviour is favoured under chronic larval malnutrition.
Keywords: competition, experimental evolution, feeding, nutritional stress, PKG, rover–sitter
1. Introduction
Food that animals consume is ultimately metabolized to release energy for survival and reproduction; however, foraging for food is itself energetically expensive and risky, and the optimal balance between the benefits and costs is likely to be environment-dependent. Foraging success and efficiency may be affected by metabolic and physiological traits [1,2], but in particular depend on behaviour. One of the main aspects of foraging behaviour is whether and when to leave the currently exploited food patch and look for potentially greener pastures [3]. Here, we focus on the mobility aspect of foraging in Drosophila larvae in the context of chronic nutritional stress.
Drosophila melanogaster larvae may face different forms of nutritional stress during their development on ephemeral habitats in nature, which can be to some degree imitated under laboratory conditions. When reared at high densities, Drosophila larvae experience a temporally variable food source: as a result of intraspecific competition and accumulation of waste products, the initially rich and plentiful food deteriorates in both quality and quantity as the larvae develop. Larvae must crawl longer distances around other larvae, drowned pupae and adults to reach a nearby food patch. Additionally, high competition favours the ability to find the patches that still remain relatively underexploited or unpolluted. It is thus not surprising that Drosophila populations maintained under high larval density over multiple generations evolve increased mobility while foraging, compared with populations reared at low-densities [4]. Other feeding-associated behaviours favoured under larval crowding include an increase in larval competitive ability and a higher feeding rate [5,6].
A difference in mobility during foraging has been reported between natural allelic variants at the gene foraging (for) encoding the PKG (cGMP-dependent protein kinase) [7,8]. Larvae with the rover (forR) allele travel significantly longer distances while feeding on yeast paste and move more between food patches than larvae that are homozygous for the sitter (fors) allele. However, even though this difference in path lengths of these natural variants is consistently observed on high- and low-quality food, they do not differ in their rate of locomotion in the absence of food [7–11]. The for locus also contributes to the difference in foraging path lengths among populations experimentally evolved under high and low densities [4]. In natural populations, the polymorphism is presumably maintained by density- and frequency-dependent selection operating at the larval stage [12]. Taken together, those results consistently indicate that crowded conditions on nutritionally rich food favour greater mobility while foraging in Drosophila larvae.
In this study, we investigated whether a change in the mobility aspect of larval foraging behaviour in D. melanogaster is favoured by another form of nutritional stress: chronic malnutrition under a low larval density. In our malnutrition regime, larvae are reared on a food medium that is extremely poor in nutrition, such that larvae not adapted to those conditions survive poorly and take twice as long to develop from egg to pupa, and the resulting adults are less than half the weight of flies reared on standard food [13,14]. However, this poor food is available at a large quantity, such that the total energetic content per larva is at least 10 times the energetic content of a large pre-pupation larva [14]. This chronic malnutrition scenario is thus in several ways different from larval crowding implemented in the studies cited above: food is poor from the start and does not deteriorate further in quality as rapidly as under the larval crowding regime. Because larval density is low, the concentration of metabolic waste probably remains low compared with the crowded regimes.
We hypothesized that these poor food conditions would result in conflicting selection pressures on larval foraging mobility. On the one hand, one could expect that greater mobility would allow the larvae to better exploit heterogeneities in food quality, resulting from both the initial imperfection of food homogenization and from the foraging activity of other larvae. This, as with larval crowding, would favour rover-like phenotypes. On the other hand, locomotion in Drosophila larvae is energetically expensive [15], so selection for metabolic efficiency would favour sitter-like phenotypes, which move less while feeding, and thus conserve energy.
To test these alternative hypotheses, we first studied the foraging-related mobility (i.e. the foraging path length) in six D. melanogaster populations that, in the course of over 80 generations of experimental evolution, have become adapted to the chronic malnutrition conditions. As the control, we used six unselected populations originally derived from the same base population [13]. Because the malnutrition-tolerant populations turned out to have a shorter foraging path, we independently verified the adaptive significance of this trait on the poor food by comparing viability and developmental rate of a rover (forR) and a sitter mutant (fors2) strain on both poor and standard food. In parallel to the selected (i.e. malnutrition-tolerant) populations [13], we expected the sitter strain to show higher viability than the rover strain on the poor food, and faster development on both food types. Furthermore, increased expression of at least some for transcripts induces longer foraging path [7] and other rover-like behaviours [16,17]. Reduced for expression is thus one possible mechanism mediating the evolution of shorter foraging path in our malnutrition-tolerant selected populations. To address this possibility, we compared larval for expression between the selected and control populations using quantitative reverse-transcription PCR. Finally, we used a quantitative complementation test [18] on one selected and one control population in an attempt to verify whether the for locus contributes to the difference in malnutrition tolerance between them. A failure to complement would either indicate that the phenotypic difference between populations is at least in part owing to different allele frequency at the for locus, or that the for locus interacts epistatically with loci responsible for the difference [18].
2. Material and methods
(a). Fly stocks and maintenance
Six replicate D. melanogaster populations originating from a single base population were maintained on poor larval food for over 80 generations; six control populations derived from the same base population were concurrently maintained on standard food (15 g agar, 30 g sucrose, 60 g glucose, 12.5 g dry yeast, 50 g cornmeal, 0.5 g MgSO4, 0.5 g CaCl2, 30 ml ethanol, 6 ml propionic acid and 1 g nipagin per litre of water) [13]. The poor larval food contained 25 per cent of the amounts of sugars, yeast and cornmeal of the standard food. Adult flies from both regimes upon emergence were always maintained on standard food with supplemented yeast. Prior to the assays described below, all populations were reared for two to three generations on standard food to eliminate maternal effects.
To minimize differences in genetic background between rover and sitter strains, we used the natural rover strain (forR) isolated in Toronto and sitter mutant (fors2) strain generated on rover genetic background [7,19]; both strains were maintained on standard food. All populations were maintained and assays were performed at 25°C, 70 per cent humidity.
(b). Larval foraging assay
The length of the path traversed by larvae while feeding on yeast paste was measured for six control and six selected populations after 84 generations of selection using the method described by Sokolowski [9]. Briefly, eggs from the control and selected populations were collected and reared on standard food for 92 ± 2 h at the density of approximately 200–250 larvae per bottle containing 30 ml of standard food. The foraging larvae were collected, rinsed with water and individually placed on a thin layer of yeast paste (2 : 1 ratio by weight of water : live yeast) filled in a shallow circular well (0.5 mm depth and 85 mm diameter) and covered with Petri dish lids. Each larva was allowed to feed on the yeast paste for 5 min and the path traversed by the larvae was traced onto a transparency sheet. The traced foraging path of larvae was scanned and digitized using custom-made software to calculate the distance traversed (path length in pixels) for each larva. The experiment was split into four blocks and per block path lengths for 10 larvae per population (12 lines in total) were measured; 480 larvae were assayed in total.
To exclude any differences in larval locomotion that might confound the measurements of foraging behaviour described above, we measured the path traversed by larvae from control and selected populations in the absence of food after 86 generations of selection. Larvae were raised in the same way and of the same age as in the foraging assay. Ten foraging larvae per population were used for this assay. Each larva was placed in an individual 5 cm Petri plate lined with 2 per cent agar and its movement was traced for 2 min. The path traversed was scanned and its length was digitally measured. The foraging path length and locomotion path length data were analysed using mixed-model analysis of variance (ANOVA) in JMP v. 8. The selection regime was included as a main effect while the replicate populations were nested within the selection regime and was treated as a random effect in the model; block was treated as a random effect for the foraging data.
(c). for polymorphism and larval malnutrition
To compare the fitness of rover and sitter strains, which differ in their foraging strategies, on our standard and poor food, we used a rover strain homozygous for a natural allele forR derived from a natural population, and a sitter strain homozygous for allele fors2, a mutant derived from the forR genetic background [7,19]. Eight bottles were set up for each strain (four with 30 ml of standard food and four with equal volume of poor food), and each bottle was seeded with 200 eggs. The 16 bottles were incubated and the number of flies emerging in each bottle was recorded every 24 h. Egg-to-adult viability and the developmental rate for each strain/food vial were calculated. We compared the viability and developmental rate of forR and fors2 on both diets (standard and poor) using a two-way ANOVA.
(d). for gene expression
We used quantitative RT-PCR to compare the pooled relative expression of for transcripts in control and selected populations (after 123 generations of selection). Larvae were raised in the same way and were of the same age as in the foraging assay. Two groups of 25 larvae per population from two replicate bottles were collected and snap frozen within 1.5 ml eppendorf tubes containing approximately 15–20 glass beads. These larvae were homogenized at 4°C in a tissue homogenizer and its total RNA was extracted using RNA kit (RNeasy mini kit, Qiagen). The quality and quantity of the RNA extracted were estimated on an Agilent Bioanalyzer 2100. Reverse transcription (cDNA synthesis) was performed in a 10 µl reaction using 1 µl total RNA (0.6 ng) using Supercript II (Invitrogen). The cDNA templates were then diluted to 1/10 in water (MilliQ).
The quantitative PCR was performed using Sybr GREEN I (Invitrogen) on Applied Biosystems 7900HT SDS. The primers used (forward: 5′-GTA GAG CTG GTC CAA ACT AAT GG-3′, reverse: 5′-AAC TCT CCA TCA GCA TGT ACA GG-3′) amplify a 202 bp fragment in the catalytic domain (2L:3623601..3623803) common to all known transcripts of the for gene (Aaron Allen and Marla B. Sokolowski 2012, personal communication; www.flybase.org). We used three reference genes: (i) rp49 (forward: 5′-GAC GCT TCA AGG GAC AGT ATC TG-3′, reverse 5′-AAA CGC GGT TCT GCA TGA G -3′) [20], (ii) ef1α (forward 5′-GTG AAG CAG CTG ATC GTT GGT-3′, reverse 5′-CAT AAC GGG CCT CGC TGT AT -3′; U. Friedrich 2009, unpublished data) and (iii) actin5c (forward 5′-CAA GTG CGA GTG GTG GAA GTT-3′, reverse 5′-GCA GGT GGT TCC GCT CTT T-3′) [21]. Three technical replicates were set up on the 384-well PCR plate for each of the two biological samples and each gene.
The cycle threshold values (Ct) were analysed with qBasePLUS (Biogazelle). The primer efficiency of all primer pairs were greater than 1.98 and were hence set to a default value of 2. The normalized relative quantities (NRQs) for each biological replicate were calculated by the software using average Ct values across the technical replicates, relative to the three reference genes. One biological replicate for control population C6 was excluded from the analysis as its expression levels were 11-fold higher than all other samples. The log-transformed NRQs of the control and selected populations were compared using a mixed-model ANOVA as described for the foraging and locomotion assays above.
(e). Quantitative complementation assay
The design of the quantitative complementation followed [18]. We crossed virgin females from one control population (C1) and one selected population (S1) with males from either the rover strain forR or the sitter strain fors2, and assayed the viability of the resulting offspring on the poor food. fors2 is a recessive allele, characterized by a highly reduced PKG activity [7,16]. Therefore, a smaller phenotypic difference between C1 × forR and S1 × forR crosses than between C1 × fors2 and S1 × fors2 crosses would imply either that some alleles at the for locus contribute to the divergence between C1 and S1 populations, or that the for locus interacts epistatically with other loci responsible for that divergence [18].
The quantitative complementation assay was carried out after 96 generations of selection. In addition to the four crosses discussed above, we assayed in parallel the four parental lines (i.e. C1, S1, forR and fors2). This was performed in two blocks two generations apart; for each block, four bottles with 30 ml poor food were set up per parental strain or cross. Each bottle was seeded with 200 eggs. The number of flies emerging in each bottle was recorded every day. Egg-to-adult viability for each parental strain or cross was calculated as the proportion of eggs that survived to produce an adult. The proportions were arcsine-square-root-transformed. The complementation hypothesis was tested with an ANOVA as the interaction between the maternal strain (selected or control) and the paternal strain (sitter or rover). Additional planned comparisons were carried out as contrasts; block effect was included as a random factor.
3. Results
(a). Larval foraging assay
Larvae from the selected populations traced on average a shorter path while feeding on yeast paste than larvae from the control populations (figure 1a; F1,10 = 8.7, p = 0.015), although there was significant variation among replicate populations (F10,465 = 3.8, p < 0.0001). In particular, four of the selected populations consistently showed very short foraging path, while the means of the remaining selected populations (S2 and S3) overlapped with the range of variation of the control lines (figure 1a). In contrast, the path length traversed by larvae in the absence of food (i.e. on agar surface) did not differ between the selection regimes (figure 1b; F1,10 = 1.1, p = 0.33), although there was variation among populations (F10,108 = 2.7, p = 0.005). A significant block effect was found in the foraging assay (F3,465 = 8.1, p < 0.0001).
Figure 1.
Larval traits of the selected (dark grey bars) and control (light grey bars) populations; for each trait, data indicate (i) selection regime means ± s.e. and (ii) means ± s.e. of individual replicate populations within each selection regime. (a) Path length traversed by larvae during foraging on yeast in 5 min. (b) Path length traversed by larvae on agar in 2 min. (c) Relative expression level of for transcripts.
(b). for polymorphism and larval malnutrition
On poor food, the sitter strain fors2 had a higher egg-to-adult viability than the rover strain forR (figure 2a; F1,6 = 9.4, p = 0.02), while the reverse was true on the standard food (F1,6 = 47.0, p = 0.0005), indicating a strong genotype × environment interaction (F1,12 = 25.4, p = 0.0003). The sitter strain developed more slowly than the rover strain on both food types (figure 2b; poor food: F1,6 = 17.9, p = 0.006; standard food: F1,6 = 71.4, p < 0.0001; interaction: F1,12 = 0.032, p = 0.86).
Figure 2.

Developmental traits (mean ± s.e.) of foraging strains forR (light grey bars) and fors2 (dark grey bars) on poor and standard larval food. (a) Egg-to-adult viability. (b) Egg-to-adult developmental rate. Both strains were maintained for three generations on standard food before the assay.
(c). for gene expression
Reduction in for expression in the selected populations was one hypothetical mechanisms underlying their sitter-like foraging. We found no support for it; if anything, the expression of pooled for transcripts tended to be higher in selected than in control populations (F1,10 = 3.1, p = 0.11; figure 1c). Even though there was significant variation among populations within both regimes (F10,23 = 4.6, p = 0.009), it was not correlated across populations with the foraging path (r = −0.4, p = 0.10).
(d). Quantitative complementation assay
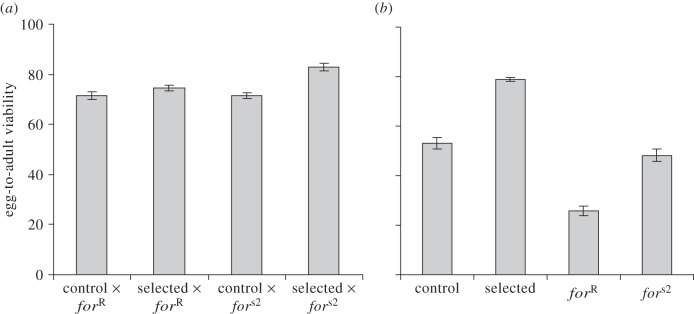
The pattern of egg-to-adult viability on poor food in the four crosses involved in the quantitative complementation test (figure 3a) qualitatively conformed to the expectation under failure to complement. There was an interaction between the maternal strain (selected versus control) and the for allele (F1,27 = 9.2, p = 0.005). F1 offspring of the selected population crossed with sitter strain fors2 had a significantly higher viability than progeny of the control population crossed with sitter strain fors2 (F1,27 = 32.7, p < 0.0001). However, no difference in viability was found between the progeny of the selected and the control population crossed with the rover strain forR (F1,27 = 2.1, p = 0.16). This pattern was observed in both blocks; the block effect was not significant (F1,27 = 0.2, p = 0.63).
Figure 3.
Quantitative complementation assays measuring egg-to-adult viability on poor larval food of (a) four crosses between one control population (C1), one selected population (S1) and foraging strains (forR and fors2), and (b) the four parental strains used for the crosses.
Nonetheless, the differences in viability among the crosses were much smaller than the difference (assayed in parallel) between the selected and the control line, or between the sitter and the rover strain (figure 3b). All crosses survived as well, or almost as well, as the selected line, and clearly better than the control line or the rover strain (figure 3a,b). Again, this was observed in both blocks of the experiment.
The egg-to-adult viability of the four parental strains (figure 3b) confirmed previous findings. The sitter strain fors2 had higher viability than the rover strain forR on poor food (F1,27 = 113, p < 0.0001) and, as expected, the selected population had higher viability than the control population (F1,27 = 158, p < 0.0001; figure 3b). The block effect was significant (F1,27 = 21.4, p < 0.0001).
4. Discussion
We demonstrate here the evolution of a shorter foraging path in four of our six selected D. melanogaster populations which had adapted to chronic larval malnutrition, paralleling the well-described sitter phenotype [16,19]. Consistent with this result, a sitter strain homozygous for the fors2 allele showed higher viability on the poor food than the rover strain forR, while the reverse was true on the standard food. Thus, two independent lines of evidence indicate that sitter-like behaviour is favoured under our poor food regime, possibly because it is more energy-efficient: locomotion is energetically costly, and less food is ingested while the larva roves. The poor food medium is initially fairly homogeneous, and even though some heterogeneity presumably arises as a result of larval feeding and excretion, it is apparently not large enough to compensate for the costs of search [15].
Our results seem to contradict an earlier study where rovers survived better under poor food conditions [16]. It is difficult to identify the reasons for this apparent discrepancy, as the composition of the media used in that study differs from that used here. In particular, our poor food contained less than half of the lowest yeast concentration used by Kaun et al. [16] (3.1 versus 7.5 g per litre). Physiological studies [16,22,23] throw some light on the complex relationship between nutritional level and the rover–sitter foraging polymorphism. On good food rovers have higher glucose absorption rate despite having lower feeding rate than the sitters. On poorer foods, both rovers and sitters increase their feeding rate to a common maximum where rovers in addition maintain their higher absorption rates [16]. However, when subjected to short bouts of starvation, rovers show a greater reduction in blood (haemolymph) sugar than sitters, and when allowed to feed again rovers had reduced feeding rate and slower recovery than the sitters [24]. Possibly, the importance of these pleiotropic effects of the rover–sitter polymorphism depends in a subtle way on the details of the food composition and other experimental conditions, tipping the balance in favour of one or another strain for reasons independent of the difference in foraging behaviour.
Enhanced for expression leads to longer foraging path [7], and natural rover genotypes show a higher for PKG activity in larval bodies [16]. Hence, a reduced expression of for might have been one candidate mechanism mediating the shorter foraging path of the selected populations. This hypothesis was not supported by the quantitative RT-PCR data; if anything, the global expression of pooled for transcripts tended to be higher in the selected populations. This does not preclude a potential role of subtle differences in the expression of specific transcripts in specific cell types, or differences at a post-transcriptional regulation. for is a complex gene with at least 11 transcripts, of which neither function nor expression pattern has been elucidated (http://flybase.org; Marla B. Sokolowski 2012, personal communication).
Lack of change in genes expression does not preclude a contribution of a locus to an evolved phenotypic change [18]. Interestingly, the quantitative complementation test offers some tentative support for the involvement of the for locus; either allelic variants at the for locus would have directly contributed to the divergence, or some other polymorphisms underlying the divergence would interact epistatically with the for locus [18]. However, the conclusion of the quantitative complementation test is weakened by the unexpected heterosis shown by the crosses. The simplest explanation for this heterosis—high degree of inbreeding of the control lines, and hence restoration of heterozygosity at many loci—seems not very likely. The viability of the control lines, on the poor food was very low from the first generation of experimental evolution, before any substantial inbreeding would have occurred [13]. Furthermore, we have not observed heterosis in crosses between replicate populations (R. K. Vijendravarma 2010, unpublished data). Rather, we suspect that this may reflect heterozygote advantage at a specific locus or complementation between some specific alleles in the genetic backgrounds of our populations and both for strains. Thus, while suggestive, the quantitative complementation does not provide compelling evidence for the involvement of the for locus in the divergence between the selected and control lines. Possibly, the evolution of a shorter foraging path in the selected lines has some other genetic basis.
The parallelism between the selected populations and the sitter strain in our study did not extend to developmental time. Rather, sitters developed more slowly than rovers on both food types, whereas the selected populations evolved faster development, possibly in response to the cut-off on time to emergence during selection [13,25]. Rovers have been reported to develop faster than sitters, particularly on poor food [16], and increased foraging path has been reported as a correlated response to selection for accelerated development [26]. However, developmental rate is a highly polygenic trait and a net outcome of the effects of many underlying traits. In particular, a major mechanism of the faster development of our selected population is their smaller critical size at which metamorphosis is initiated [25]. Thus, our finding that the selected populations develop faster and show sitter-like foraging does not necessarily contradict the existence of mechanisms that tie long foraging path with faster development, such as feeding rate [26] or the rate of food absorption in the gut [16].
Our results support the argument that low-quality food conditions at low population density favour different adaptations than crowded conditions on limited high-quality food. In contrast to results reported here, in two separate evolutionary experiments, high larval densities favoured rover-like foraging phenotype (i.e. greater foraging path length) [4,5]. Both studies also reported an increased feeding rate; in contrast, our selected populations do not differ in their feeding rates from the control populations [27]. Other differences between experimental adaptation to chronic malnutrition and adaptation to crowded conditions are discussed in Vijendravarma et al. [25,27]. Thus, although both larval crowding and larval malnutrition cause nutritional stress during development, and the plastic responses for both are similar (reduced size at emergence, slower development and low viability), the evolutionary forces acting on populations under these scenarios are different, or even opposite.
Acknowledgements
This work has been supported by the Swiss National Science Foundation. We thank M. B. Sokolowski, A. Allen and U. Friedrich for advice and primer sequences, M. Zini for maintaining the fly cultures and H. Richter for technical assistance with RT-PCR. We also thank the two anonymous reviewers, M. B. Sokolowski and B. Hollis for comments.
References
- 1.Stubbs R. J., Tolkamp B. J. 2006. Control of energy balance in relation to energy intake and energy expenditure in animals and man: an ecological perspective. Br. J. Nutr. 95, 657–676 10.1079/bjn20041361 (doi:10.1079/bjn20041361) [DOI] [PubMed] [Google Scholar]
- 2.Wang T., Hung C. C. Y., Randall D. J. 2006. The comparative physiology of food deprivation: from feast to famine. Annu. Rev. Physiol. 68, 223–251 10.1146/annurev.physiol.68.040104.105739 (doi:10.1146/annurev.physiol.68.040104.105739) [DOI] [PubMed] [Google Scholar]
- 3.Pyke G. H. 1984. Optimal foraging theory—a critical review. Annu. Rev. Ecol. Syst. 15, 523–575 10.1146/annurev.ecolsys.15.1.523 (doi:10.1146/annurev.ecolsys.15.1.523) [DOI] [Google Scholar]
- 4.Sokolowski M. B., Pereira H. S., Hughes K. 1997. Evolution of foraging behavior in Drosophila by density-dependent selection. Proc. Natl Acad. Sci. USA 94, 7373–7377 10.1073/pnas.94.14.7373 (doi:10.1073/pnas.94.14.7373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi A., Mueller L. D. 1988. Evolution of higher feeding rate in Drosophila due to density-dependent natural selection. Evolution 42, 1090–1093 10.2307/2408924 (doi:10.2307/2408924) [DOI] [PubMed] [Google Scholar]
- 6.Mueller L. D. 1988. Evolution of competitive ability in Drosophila by density-dependent natural selection. Proc. Natl Acad. Sci. USA 85, 4383–4386 10.1073/pnas.85.12.4383 (doi:10.1073/pnas.85.12.4383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne K. A., Robichon A., Burgess E., Butland S., Shaw R. A., Coulthard A., Pereira H. S., Greenspan R. J., Sokolowski M. B. 1997. Natural behavior polymorphism due to a cGMP-dependent protein kinase of Drosophila. Science 277, 834–836 10.1126/science.277.5327.834 (doi:10.1126/science.277.5327.834) [DOI] [PubMed] [Google Scholar]
- 8.de Belle J. S., Sokolowski M. B. 1987. Heridity of rover sitter: alternative foraging strategies of Drosophila melanogaster larvae. Heredity 59, 73–83 10.1038/hdy.1987.98 (doi:10.1038/hdy.1987.98) [DOI] [Google Scholar]
- 9.Sokolowski M. B. 1980. Foraging strategies of Drosophila melanogaster—a chromosomal analysis. Behav. Genet. 10, 291–302 10.1007/bf01067774 (doi:10.1007/bf01067774) [DOI] [PubMed] [Google Scholar]
- 10.Sokolowski M. B., Hansell R. I. C., Rotin D. 1983. Drosophila larval foraging behaviour. II. Selection in the sibling species Drosophila melanogaster and Drosophila simulans. Behav. Genet. 13, 169–177 10.1007/bf01065665 (doi:10.1007/bf01065665) [DOI] [PubMed] [Google Scholar]
- 11.Graf S. A., Sokolowski M. B. 1989. Rover/sitter Drosophila melanogaster larval foraging polymorphism as function of larval development, food-patch quality and starvation. J. Insect Behav. 2, 301–313 10.1007/bf01068057 (doi:10.1007/bf01068057) [DOI] [Google Scholar]
- 12.Fitzpatrick M. J., Feder E., Rowe L., Sokolowski M. B. 2007. Maintaining a behaviour polymorphism by frequency-dependent selection on a single gene. Nature 447, 210–215 10.1038/nature05764 (doi:10.1038/nature05764) [DOI] [PubMed] [Google Scholar]
- 13.Kolss M., Vijendravarma R. K., Schwaller G., Kawecki T. J. 2009. Life-history consequences of adaptation to larva nutritional stress in Drosophila. Evolution 63, 2389–2401 10.1111/j.1558-5646.2009.00718.x (doi:10.1111/j.1558-5646.2009.00718.x) [DOI] [PubMed] [Google Scholar]
- 14.Vijendravarma R. K., Narasimha S., Kawecki T. J. 2011. Plastic and evolutionary responses of cell size and number to larval malnutrition in Drosophila melanogaster. J. Evol. Biol. 24, 897–903 10.1111/j.1420-9101.2010.02225.x (doi:10.1111/j.1420-9101.2010.02225.x) [DOI] [PubMed] [Google Scholar]
- 15.Berrigan D., Pepin D. J. 1995. How maggots move—allometry and kinematics of crawling in larval diptera. J. Insect Physiol. 41, 329–337 10.1016/0022-1910(94)00113-u (doi:10.1016/0022-1910(94)00113-u) [DOI] [Google Scholar]
- 16.Kaun K. R., Riedl C. A. L., Chakaborty-Chatterjee M., Belay A. T., Douglas S. J., Gibbs A. G., Sokolowski M. B. 2007. Natural variation in food acquisition mediated via a Drosophila cGMP-dependent protein kinase. J. Exp. Biol. 210, 3547–3558 10.1242/jeb.006924 (doi:10.1242/jeb.006924) [DOI] [PubMed] [Google Scholar]
- 17.Mery F., Belay A. T., So A. K. C., Sokolowski M. B., Kawecki T. J. 2007. Natural polymorphism affecting learning and memory in Drosophila. Proc. Natl Acad. Sci. USA 104, 13 051–13 055 10.1073/pnas.0702923104 (doi:10.1073/pnas.0702923104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackay T. F. C. 2001. Quantitative trait loci in Drosophila. Nat. Rev. Genet. 2, 11–20 10.1038/35047544 (doi:10.1038/35047544) [DOI] [PubMed] [Google Scholar]
- 19.de Belle J. S., Hilliker A. J., Sokolowski M. B. 1989. Genetic localization of foraging (for)- a major gene for larval behaviour in Drosophila melanogaster. Genetics 123, 157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchon N., Broderick N. A., Poidevin M., Pradervand S., Lemaitre B. 2009. Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200–211 10.1016/j.chom.2009.01.003 (doi:10.1016/j.chom.2009.01.003) [DOI] [PubMed] [Google Scholar]
- 21.Colombani J., Bianchini L., Layalle S., Pondeville E., Dauphin-Villemant C., Antoniewski C., Carre C., Noselli S., Leopold P. 2005. Antagonistic actions of ecdysone and insulins determine final size in Drosophila. Science 310, 667–670 10.1126/science.1119432 (doi:10.1126/science.1119432) [DOI] [PubMed] [Google Scholar]
- 22.Kent C. F., Daskalchuk T., Cook L., Sokolowski M. B., Greenspan R. J. 2009. The Drosophila foraging gene mediates adult plasticity and gene–environment interactions in behaviour, metabolites, and gene expression in response to food deprivation. Plos Genet. 5, 13 10.1371/journal.pgen.1000609 (doi:10.1371/journal.pgen.1000609) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reed L. K., Williams S., Springston M., Brown J., Freeman K., DesRoches C. E., Sokolowski M. B., Gibson G. 2010. Genotype-by-diet interactions drive metabolic phenotype variation in Drosophila melanogaster. Genetics 185, 1009–1019 10.1534/genetics.109.113571 (doi:10.1534/genetics.109.113571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaun K. R., Chakaborty-Chatterjee M., Sokolowski M. B. 2008. Natural variation in plasticity of glucose homeostasis and food intake. J. Exp. Biol. 211, 3160–3166 10.1242/jeb.010124 (doi:10.1242/jeb.010124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vijendravarma R. K., Narasimha S., Kawecki T. J. 2012. Chronic malnutrition favours smaller critical size for metamorphosis initiation in Drosophila melanogaster. J. Evol. Biol 25, 288–292 10.1111/j.1420-9101.2011.02419.x (doi:10.1111/j.1420-9101.2011.02419.x) [DOI] [PubMed] [Google Scholar]
- 26.Mueller L. D., Folk D. G., Nguyen N., Nguyen P., Lam P., Rose M. R., Bradley T. 2005. Evolution of larval foraging behaviour in Drosophila and its effects on growth and metabolic rates. Physiol. Entomol. 30, 262–269 10.1111/j.1365-3032.2005.00458.x (doi:10.1111/j.1365-3032.2005.00458.x) [DOI] [Google Scholar]
- 27.Vijendravarma R. K., Narasimha S., Kawecki T. J. 2012. Adaptation to abundant low quality food improves the ability to compete for limited rich food in Drosophila melanogaster. PLoS ONE 7, e30650. [DOI] [PMC free article] [PubMed] [Google Scholar]