Abstract
Social groups face a fundamental problem of overcoming selfish individuals capable of destroying cooperation. In the social amoeba Dictyostelium discoideum, there is evidence that some clones (‘cheaters’) contribute disproportionately to the viable spores in a fruiting body while avoiding the dead stalk cell fate. It remains unclear, however, whether this cheating is actually the product of selection. Here, I report the results of an experimental evolution study designed to test whether clones of D. discoideum will evolve resistance to cheating in the laboratory with genetic variation created only through spontaneous mutation. Two strains, one green fluorescent protein (GFP)-labelled and one wild-type, were allowed to grow and develop together before the wild-type strain was removed and replaced with a naïve strain evolving in parallel. Over the course of 10 social generations, the GFP-labelled strain reliably increased its representation in the spores relative to control populations that had never experienced the competitor. This competitive advantage extended to the non-social, vegetative growth portion of the life cycle, but not to pairwise competition with two other strains. These results indicate strong antagonism between strains, mediated by ample mutational variation for cheating and also suggest that arms races between strains in the wild may be common.
Keywords: antagonistic coevolution, social amoeba, Dictyostelium discoideum, altruism, social conflict, social evolution
1. Introduction
The persistence of cooperation in social groups faces a constant threat from selfish individuals that benefit from the altruistic actions of others but fail to reciprocate. Dictyostelium discoideum, a social amoeba, has emerged as a model system for studying this tension between cooperation and conflict. These unicellular amoebae live in forest soil, consuming bacteria and dividing asexually, until food becomes scarce. Thousands of amoebae then come together to form a motile slug that eventually develops into a fruiting body (figure 1). This fruiting body contains viable spore cells held aloft by dead stalk cells, the product of altruistic self-sacrifice.
Figure 1.
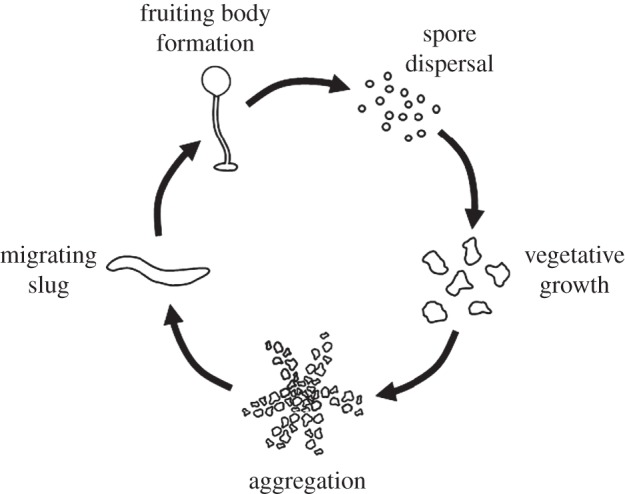
The life cycle of Dictyostelium discoideum. Unicellular amoebae feed and divide until food is scarce, at which point tens of thousands of cells aggregate to form a motile slug. After migration, the slug develops into a fruiting body in which 20% of amoebae die to form a stalk that holds the remaining 80% aloft as spores awaiting dispersal.
Unlike the genetically identical soma of many multicellular organisms that originate from one cell, aggregates can and often do contain amoebae of different genotypes that readily form chimeric fruiting bodies [1,2]. Differences in the genetic composition of aggregates should lead to conflict and, potentially, the breakdown of cooperation through selection favouring cheaters that contribute disproportionately to the viable spores. Indeed, cheaters have been found in dictyostelids in nature [3,4] and generated in the laboratory via mutagenesis [5,6]. There appear to be multiple, independent pathways to cheating [6], some of which do not carry any obvious cost to cheaters developing alone. Some genotypes also appear to discriminate against competitors that are genetically different [7] or collected from the same geographical area [8]. These findings suggest that coevolutionary dynamics may be important in natural D. discoideum populations and the cause of cheating phenotypes recovered in wild isolates, but this has never been experimentally demonstrated. A simple alternative explanation remains plausible: cheating in nature may represent a by-product of divergence between strains through either drift or adaptation to another aspect of the environment.
There are several reasons to suspect that identified cheaters [4] might not have evolved through social competition. First, relatedness in fruiting bodies in the wild appears to be high [2], suggesting that the chimeras generated in laboratories might be anomalous in nature. If most aggregates originate as single cells colonizing a novel area, then development in Dictyostelium would be as conflict-free as in most multicellular animals. Considering realistic estimates of the selective advantage of cheaters in mixed groups and the rate of mutation to cheating phenotypes, it is clear that even intermittent single-cell bottlenecks can keep the frequency of obligate cheaters in check [9].
Furthermore, cheater mutants that have been generated in the laboratory through mutagenesis or deletions [5,6,10,11] appear to cheat by failures in signalling or cell–cell interaction (e.g. by failing to recognize signals to accept a prestalk fate in an aggregate, or failures in cell–cell adhesion). Ending up with a large share of the spores, then, is a side effect of this failure and does not result from manipulative interactions moulded by selection. Additionally, many of these cheaters result from deletions or null alleles created by restriction enzyme-mediated integration and are therefore not representative of natural variation. The mutants often demonstrate aberrant morphology and fail to develop when grown clonally [5,10,11], limiting the possibility of success in the wild. Although there appear to be more than 100 genes that generate cheating phenotypes when knocked out [6], the coevolution of antagonistic phenotypes from natural mutational variation, expected to be more subtle in effect but pervasive in scope, has not been experimentally demonstrated.
Here, I used experimental evolution to ask whether cheating or defence against cheating can evolve in a population of D. discoideum with only natural mutational variation and a focal competitor prevented from coevolving. After only 10 social generations, defence against cheating in the social stage had evolved repeatedly along with differences in competitive growth rate prior to the social stage. I then tested the strains with alternative competitors in order to determine whether the observed competitive advantages over control strains were general or instead represented targeted antagonism. The experimentally evolved strains showed no advantage over their ancestor in this challenge, suggesting the adaptation that occurred in this experiment was specific to the opposition encountered in the strains' evolutionary history.
2. Material and methods
(a). Dictyostelium clones and maintenance
The wild-type D. discoideum strain (NC4) and a fluorescently labelled axenic strain (AX2-green fluorescent protein (GFP)) were used in the evolution experiments. Another axenic strain (AX4) and a wild isolate (NC75.2) were used in subsequent tests of the generality of evolved antagonism. All cells were grown throughout the experiment on Petri dishes with a diluted Sussman's medium (peptone 1 g, yeast 0.1 g, glucose 1 g, KH2PO4 1.9 g, K2HPO4 0.6 g, MgSO4 0.1 g, agar 20 g, H2O 1 l) and Escherichia coli B/r as prey. For social fitness measurements (see later text), cells were placed on starving media (KH2PO4 2.25 g, K2HPO4 0.67 g, agar 20 g, H2O 1 l). At the outset of the experiment, the wild-type D. discoideum strain NC4 was a strong cheater of fluorescently labelled AX2-GFP (comprising 51% of total aggregates and 83% of spores, measured as outlined below).
(b). Experimental evolution
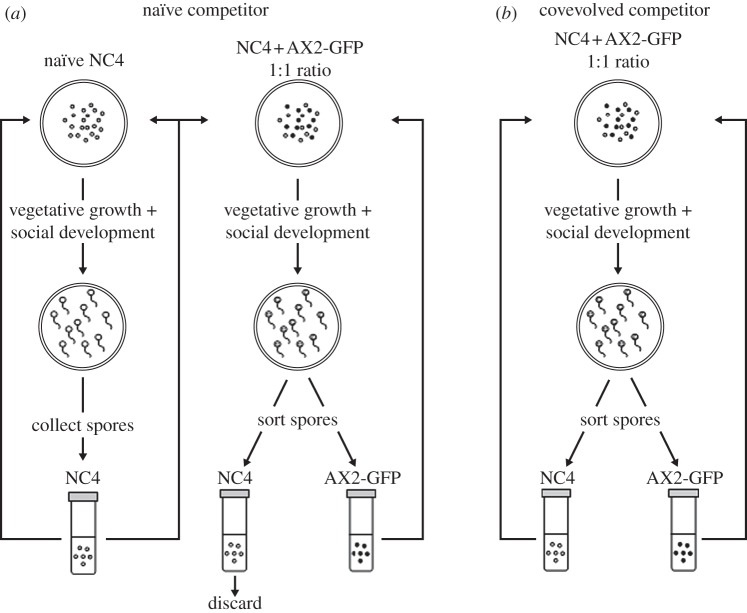
Mixtures of NC4 and AX2-GFP were initiated from 2 × 105 spores in a 1 : 1 ratio. In the ‘naïve’ NC4 competitor regime (figure 2a), the two strains were allowed to grow vegetatively and develop together, in competition, for one social generation. In order to allow AX2-GFP to adapt to the presence of a non-responding NC4 population, spores were collected from Petri dishes after 3–5 days and the NC4 spores were filtered out. This was accomplished by separating spores based on their fluorescence with a cell sorter. In order to minimize the number of spores that were sorted incorrectly, two steps were taken. First, conservative gates were used in order to discard all spores that did not clearly fall into one class (i.e. those that were probably GFP + but on the lower-fluorescence tail of the population's distribution). This generally ensured high purity of the collections (95–100% pure AX2-GFP or NC4), but a second enrichment sort was performed when post-sort checks revealed lower purity. The removed NC4 spores were then discarded and replaced with a naïve (non-coevolving) NC4 strain to begin the next cycle.
Figure 2.
Experimental coevolution design. Populations were initiated at a 1 : 1 ratio of NC4 and AX2-GFP strains of Dictyostelium discoideum and allowed to grow vegetatively and develop into fruiting bodies together. In the ‘naïve competitor’ selection regime (a), NC4 spores were filtered out each social generation and replaced with a non-responding NC4 population. In the ‘coevolved competitor’ selection regime (b), the NC4 spores were retained for the next cycle. A third ‘control competitor’ regime, where both NC4 and AX2-GFP spores were reared separately throughout the experiment, is not shown.
In the ‘coevolving’ NC4 competitor regime (figure 2b), amoebae were reared in the same way, but the NC4 spores from a round of competitive vegetative growth and development were retained after sorting and used for propagation of the next generation. In the ‘control’ regime, the two strains were maintained separately throughout the experiment, without any manipulations, and never interacted prior to measurements of fitness. For each of these three selection regimes, there were three replicates, each consisting of a unique AX2-GFP and NC4 population that were genetically isolated from one another owing to entirely asexual reproduction.
(c). Measuring competitive vegetative growth rate
After 10 social generations of experimental evolution, NC4 and AX2-GFP spores from all replicates were grown separately for one social generation prior to testing components of fitness. In order to measure competitive vegetative growth rate, the NC4 and AX2-GFP spores from each population were then plated at a 1 : 1 ratio (2 × 105 total spores, three to five replicate plates per population) and allowed to grow for 42 h. Plates were then harvested prior to the onset of development by flooding with approximately 3 ml cold KK2 buffer (KH2PO4 2.25 g, K2HPO4 0.67 g, H2O 1 l) and scraping the surface of the medium, then collecting the suspension into centrifuge tubes containing 40 ml cold KK2 buffer. Amoebae were separated from bacteria by three rounds of centrifugation (4 min at 1500 r.p.m) and the frequency of each type determined with a haemocytometer and fluorescent microscope.
(d). Measuring social fitness
In order to measure the competitive ability of NC4 and AX2-GFP cells from each replicate during the social phase, both genotypes from each population were grown in isolation and then harvested, separated from bacteria by sequential centrifugation and counted on a haemocytometer as described earlier. Mixing experiments were then performed by combining the NC4 and AX2 amoebae in a 1 : 1 ratio (approx. 107 cells per plate, three to five plates per population) in a thin line along one side of a starving media plate devoid of bacteria. The plates were then wrapped in aluminium foil and a pin-sized hole punched out on the opposite end of the dish from where the amoebae were deposited. This allowed light to enter from only one direction, ensuring that after aggregation slugs would migrate across the dish towards the light source. After 40 h, the foil was removed from each plate and half of the slugs that had migrated approximately halfway across the plate were collected and mechanically disaggregated. The proportion of each type in this slug collection was then determined with a haemocytometer and fluorescent microscope. Because the remaining slugs were now exposed to light from all directions instead of only one direction, they immediately initiated culmination without further migration. On the next morning, the spores from all of these fruiting bodies were collected, and the proportion of each type determined for the sorus collection with a haemocytometer and fluorescent microscope. Examination of fruiting bodies and the surrounding area on plates revealed no differential sloughing of cells between the times of these two measurements. The same protocol was followed for mixing evolved AX2-GFP cells with two alternative competitors, AX4 and NC75.2, which are also GFP- and easily distinguished.
The ratio of the proportion of AX2-GFP cells in the sorus collection to the proportion of AX2-GFP cells in the slug collection provides a measure of social fitness, where a value of one indicates equal representation in the slug and sorus, values less than one indicate that the AX2-GFP cells are being cheated and values greater than one indicate that the AX2-GFP cells are cheaters. Note that this method of evaluating cheating in mixtures of D. discoideum is slightly different than what has been employed in past work [4] because we begin with mixtures at a 1 : 1 ratio and quantify the proportion of each strain in the entire slug (as opposed to prespore and prestalk investment measured independently by dividing the slug) immediately before fruiting body formation. This can then be compared with the proportions of each type in the sorus, providing an accurate estimate of the level of exploitation.
(e). Analysis
Data were analysed with generalized linear mixed models. When significant effects were found, contrasts were used to determine which of the three selection treatments differed from one another. For the vegetative growth data, the number of AX2-GFP cells out of the total counted for each plate was the binomial response variable with a fixed effect of selection regime (naïve competitor, coevolved competitor or control competitor) and a random replicate effect nested within regime. A significant selection regime effect indicates evolved differences between selection treatments in competitive vegetative growth rate.
For the social fitness data, the response variable was the ratio of the proportion of AX2-GFP cells in the sorus to the proportion of AX2-GFP cells in the slug, giving one value for each plate, with the same fixed (selection regime) and random (replicate population) effects. A selection regime effect here indicates evolved differences between selection treatments in the overall degree of cheating. The social fitness data were also decomposed, so that the sorus and slug contributions could be analysed independently. Here, the number of AX2-GFP cells (or spores) in the slug (or sorus) out of the total number was modelled with the same effects. Both analyses are reported below. Analysis of the generality of evolved antagonism between strains, where mixes were performed with alternative competitors, was performed in the same fashion but with only two levels for selection regime (naïve competitor or control competitor).
3. Results
(a). Competitive vegetative growth rate
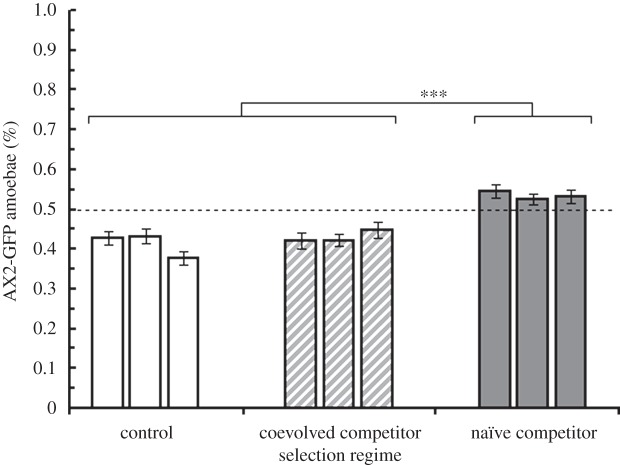
After 10 social generations, the three populations of AX2-GFP with an evolutionary history of naïve competitors exhibited the highest competitive growth rate during the vegetative growth portion of the D. discoideum life cycle (figure 3). The significant overall effect of selection regime (F2,6 = 34.63, p < 0.001) was driven by this enhanced competitive ability in the naïve competitor regime (naïve competitor–coevolved competitor contrast: t6 = 6.42, p < 0.001; naïve competitor–control competitor contrast: t6 = 7.58, p < 0.001), and these were the only three populations to outperform their NC4 competitor in the assay. There was no difference between coevolved competitor and control competitor populations (coevolved competitor–control competitor contrast: t6 = 1.04, p = 0.340).
Figure 3.
Competitive vegetative growth of experimentally evolved strains. After 10 social generations, AX2-GFP strains from the naïve competitor selection regime (filled bars) were superior competitors versus their NC4 competitor than AX2-GFP strains from either the control competitor regime (white bars) or coevolved competitor regime (hatched bars). ***p < 0.01.
(b). Social fitness
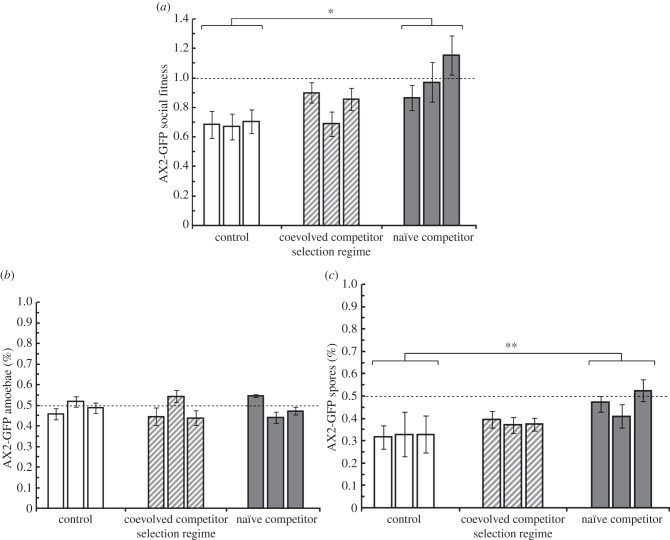
In addition to the evolved difference in competitive vegetative growth rate, AX2-GFP populations in the naïve competitor regime evolved significantly greater resistance to cheating by NC4 during social development than did AX2-GFP populations in the control competitor regime (figure 4a; overall selection regime effect: F2,6 = 6.75, p = .029, naïve competitor–control competitor contrast: t6 = 3.66, p = 0.011). Indeed, one of the three replicate populations from the naïve competitor regime actually became a weak cheater of NC4. The coevolved competitor treatment exhibited intermediate exploitation levels and was not significantly different from either control or naïve competitor regimes (coevolved competitor–control competitor contrast: t6 = 1.62, p = 0.156; coevolved competitor–naïve competitor contrast: t6 = −2.08, p = 0.0831). The control competitor regime populations look similar to the base strains measured at the outset of the experiment in that NC4 is still a weak cheater of AX2-GFP, although this effect is not as pronounced.
Figure 4.
Social fitness for experimentally evolved strains against NC4 competitors. (a) After 10 social generations, AX2-GFP strains from the naïve competitor selection regime (filled bars) had evolved superior social fitness relative to those from the control competitor regime (white bars). AX2-GFP strains from the coevolved competitor regime (hatched bars) exhibited intermediate social fitness and were not significantly different from either naïve competitor or control competitor regimes. (b) Representation of AX2-GFP cells in migrated slugs was not significantly different between selection regimes. (c) Representation of AX2-GFP spores in the sorus of fruiting bodies was significantly greater in the naïve competitor regime than in the control competitor regime, driving the effect present in (a). *p < 0.05; **p < 0.01.
Breaking down net exploitation into its components, slugs were composed of on average 48 per cent AX2-GFP (figure 4b) across regimes, and there was no difference between selection regimes in slug composition (F2,6 = 0.06, p = 0.945). Representation in the spores (figure 4c) was significantly different between selection regimes (F2,6 = 14.16, p < 0.01) and this difference is what drove the selection regime effect on overall exploitation (figure 4a).
(c). Generality of antagonism
In order to determine whether this evolved social performance difference was specific to the NC4 competitor or represented a general adaptation, we mixed naïve competitor and control competitor populations of AX2-GFP with two alternative competitors: AX4, a common laboratory strain; and NC75.2, a wild isolate that has been shown to be a cheater in at least some pairwise mixtures [4]. There was no difference detected between selection regimes in overall resistance to cheating (AX4 competitor: F1,4 = 0.45, p = 0.540, figure 5a; NC75.2 competitor: F1,4 = 0.12, p = 0.745, figure 5b). In mixes with AX4, the proportion of AX2-GFP in migrated slugs averaged 43 per cent and was not different between selection regimes (F1,4 = 0.05, p = 0.837). In mixes with NC75.2, there were fewer AX2-GFP cells in migrated slugs—on average 17 per cent—but again there was no difference between naïve competitor and control competitor regimes (F1,4 = 0.96, p = 0.383). Likewise, the selection regimes did not differ in contribution to spores with either of these competitors (AX4: F1,4 < 0.00, p = 0.967; NC75.2: F1,4 = 1.67, p = 0.266).
Figure 5.
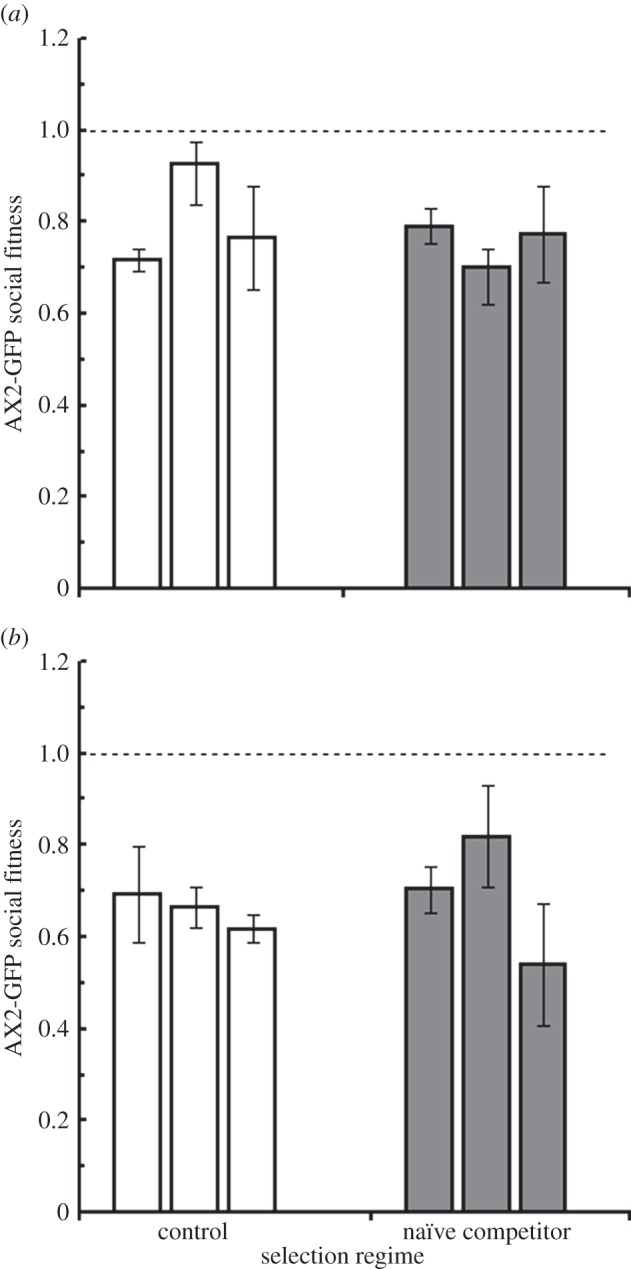
Social fitness for experimentally evolved strains against (a) AX4 and (b) NC75.2 competitors. After 10 social generations, there was no significant difference between naïve competitor and control competitor selection regimes for AX2-GFP social fitness versus either alternative competitor.
4. Discussion
Dictyostelium has become an important model system in the study of social evolution since the discovery of cheaters in nature [3,4] and the development via mutagenesis of genotypes that both cheat and resist cheating in the laboratory [5,6,12]. Surprisingly, there is still no direct evidence that the cheating detected in nature has actually been shaped by selection. Instead, the over-representation of one wild genotype in the spores of a chimeric fruiting body generated in the laboratory could represent a failure in social interactions within specific pairwise mixtures that seldom occur in natural environments. The experiment reported here addresses this concern by demonstrating that antagonistic coevolution—a putatively important sculpting force in natural populations—does take place between clones. Strains that grew and developed with a competitor that was replaced each social generation exhibited increased vegetative and social competitive ability relative to control strains. Furthermore, natural mutational input was responsible for creating the genetic variation necessary for these populations to respond to competitors on a rapid timescale. Antagonism may have also evolved to a lesser extent in strains that experienced a coevolving competitor, where social fitness measures were intermediate between naïve competitor and control regimes, but this difference was not significant.
In past work on competitive social interactions in D. discoideum, a linear dominance hierarchy has been found in wild isolates [13]. This transitivity of social dominance has also been found in the social bacterium, Myxococcus xanthus [14,15]. The adaptation present in this experiment, however, exists as a targeted response towards a competitor, similar to the cheater-resistant genotypes of D. discoideum [12]. If evolved antagonistic interactions between strains are often not transitive, then arms race dynamics that result in churning of low-frequency cheating and cheating-resistant alleles are likely in natural populations that experience some level of mixing.
Allowing strains to adapt to a stationary target in 1 : 1 mixtures undoubtedly facilitated the rapid response observed in 10 social generations. For the AX2-GFP strains evolving with naïve competitors, mixing removed any costs that might have been incurred through clonal development (development without a competitor to exploit). Because there were also no costs incurred through counter-adaptations in the opposition, the conditions were favourable for antagonism to evolve. On the other hand, experimental populations of AX2-GFP cells experienced a bottlenecked population size of 105 spores each social generation, so it is impressive that natural mutational processes could generate sufficient variation for selection to operate on in an experiment on this timescale. Furthermore, the antagonistic genetic variation arising in AX2-GFP genotypes in our naïve competitor populations was present in both the typical ‘social’ stage (fruiting body development) and also during competitive vegetative growth. This social antagonism extends beyond what is typically appreciated in D. discoideum and is consistent with work indicating competitive social interactions in the vegetative growth stage of the life cycle [16]. If genetic variation for antagonism at two phases of the life cycle can arise and spread in relatively small populations on such a short timescale, then we should expect ample standing variation for these effects in natural populations.
There are some technical issues with measuring cheating in D. discoideum that are worthy of consideration. First, in many experiments investigating cheating, mixtures are set up at 1 : 1 ratios initially, and spores are counted in the resulting fruiting bodies. Departures from a 1 : 1 ratio in the spores are then considered cheating or resistance to cheating [12]. However, this is not necessarily true. For example, clones could simply present better or worse aggregation competency, and in the extreme case, putative cheaters might actually be social losers. Another method of measuring cheating avoids this problem by measuring prespore and prestalk regions of aggregates, but this presents its own issues because prespore and prestalk proportions have been shown to not always reflect resulting spore and stalk contributions [13]. Additionally, if strains are mixed at a 1 : 1 ratio, persist at this ratio in aggregates, and 20 per cent of the cells end up as stalk (as is assumed in classic mixing experiments), the maximum proportion any one genotype can obtain in the spores of the fruiting body is 62.5 per cent. This number comes from considering that even in an extreme case, when one of the clones in a mixture composes the entire stalk, 60 per cent of its cells must still end up as spores. Both of the aforementioned methods consistently yield estimates of exploitation that are beyond this number, meaning some other process must be invoked (late-stage cannibalism or cell sloughing, for example, or additional rounds of cell division inside the aggregate). The approach employed here avoids these problems by measuring the slug after migration but immediately before culmination, providing a baseline expectation for subsequent proportions. The departure from this baseline in the resulting spores provides a realistic measure of exploitation, and estimates of cheating in all of the evolved populations reported here (which take into account the proportion of each type in the migrated slug) fall within a range requiring no other explanatory mechanism beyond exclusion from the sorus.
If spontaneous mutation is rapidly creating antagonistic variation in clones in the wild, as these results would suggest is likely, then we might expect to detect an arms race in nature. There is some evidence suggesting that Dictyostelium have adapted to a history of evolutionary conflict. Genetically distant strains exclude one another from aggregates more often than genetically similar individuals [7], and at least one set of genes involved in this kin discrimination is known [17]. Although Flowers et al. [8] did not find this relationship between genetic divergence and kin discrimination in a later study, they did show that clones discriminate better against non-self when the competitor was collected from the same geographical location. This raises the tantalizing possibility that wild D. discoideum are locally adapted to the competitors in their area.
Determining the extent of conflict in nature is a challenge, and will be greatly aided by more estimates of relatedness and a better understanding of spore dispersal. Previous work has shown that cheaters readily evolve in populations in which relatedness is maintained at a low level by the experimenter [9]. At higher levels of relatedness such as those observed in the wild (r = 0.875–0.98; [2]), a low cost to clonal development is critical for cheaters to spread. For example, if single cell bottlenecks occur every 100 generations, then a 10 per cent cost to clonal development will keep the frequency of a modest cheater at around 5 per cent [9]. Strains that clear this hurdle by cheating while still sporulating as efficiently as the wild-type do exist [6], although other costs (e.g. smaller spores or a shorter fruiting body) cannot be ruled out. Considering both the evidence for facultative cheating mutants [6] and how rapidly targeted antagonism evolved in this study, it is likely that repeated rare encounters would be sufficient to yield substantial antagonism between strains. A major determinant of the intensity of this arms race in the wild is the extent that cheater development in isolation is compromised. This is an open question and involves a careful dissection of functional differences at all stages of the life cycle. As just one example, it is possible that all spores are not created equal, and counting the number of spores produced during development in isolation is a poor way to uncover fitness costs to cheaters. With many cheater and cheater-resistant strains available to the research community, it should be possible to answer this question.
The approaches employed here to study antagonism in D. discoideum have several advantages. Differences between strains in social fitness readily evolve and are robustly measured by examining both the slug and fruiting body phases of the life cycle together. Natural extensions to the experimental evolution design should allow one to address how conflict drives evolution in Dictyostelium populations by, for example, following adaptation to moving instead of stationary targets, confining competition to either the vegetative or social development portions of the life cycle, examining the frequency dependence of exploitation, requiring periods of clonal development and spore dispersal, or exploring the dynamics of antagonistic coevolution with time shift experiments.
Acknowledgements
I thank David Houle, Tom Miller, Tadeusz Kawecki, Laurent Keller, Christopher Zarpentine, Daniel Deen and also the History and Philosophy of Science discussion group at Florida State University for both valuable advice and logistical support in completing this project. The Dicty Stock Center at Northwestern University kindly provided the D. discoideum and E. coli strains used in the experiments. The project would not have been possible without the tremendous assistance of Ruth Didier and the FSU Flow Cytometry Laboratory. This work was funded by NSF grant no. SES 0724686.
References
- 1.Fortunato A., Strassmann J. E., Santorelli L., Queller D. C. 2003. Co-occurrence in nature of different clones of the social amoeba, Dictyostelium discoideum. Mol. Ecol. 12, 1031–1038 10.1046/j.1365-294X.2003.01792.x (doi:10.1046/j.1365-294X.2003.01792.x) [DOI] [PubMed] [Google Scholar]
- 2.Gilbert O. M., Foster K. R., Mehdiabadi N. J., Strassmann J. E., Queller D. C. 2007. High relatedness maintains multicellular cooperation in a social amoeba by controlling cheater mutants. Proc. Natl Acad. Sci. USA 104, 8913–8917 10.1073/pnas.0702723104 (doi:10.1073/pnas.0702723104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buss L. W. 1982. Somatic-cell parasitism and the evolution of somatic tissue compatibility. Proc. Natl Acad. Sci. USA 79, 5337–5341 10.1073/pnas.79.17.5337 (doi:10.1073/pnas.79.17.5337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strassmann J. E., Zhu Y., Queller D. C. 2000. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature 408, 965–967 10.1038/35050087 (doi:10.1038/35050087) [DOI] [PubMed] [Google Scholar]
- 5.Ennis H. L., Dao D. N., Pukatzki S. U., Kessin R. H. 2000. Dictyostelium amoebae lacking an F-box protein form spares rather than stalk in chimeras with wild type. Proc. Natl Acad. Sci. USA 97, 3292–3297 10.1073/pnas.050005097 (doi:10.1073/pnas.050005097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santorelli L. A., et al. 2008. Facultative cheater mutants reveal the genetic complexity of cooperation in social amoebae. Nature 451, 1107–1110 10.1038/nature06558 (doi:10.1038/nature06558) [DOI] [PubMed] [Google Scholar]
- 7.Ostrowski E. A., Katoh M., Shaulsky G., Queller D. C., Strassmann J. E. 2008. Kin discrimination increases with genetic distance in a social amoeba. PLoS Biol. 6, e287 10.1371/journal.pbio.0060287 (doi:10.1371/journal.pbio.0060287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flowers J. M., Li S. I., Stathos A., Saxer G., Ostrowski E. A., Queller D. C., Strassmann J. E., Purugganan M. D. 2010. Variation, sex, and social cooperation: molecular population genetics of the social amoeba Dictyostelium discoideum. PLoS Genet. 6, e1001013 10.1371/journal.pgen.1001013 (doi:10.1371/journal.pgen.1001013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuzdzal-Fick J. J., Fox S. A., Strassmann J. E., Queller D. C. 2011. High relatedness is necessary and sufficient to maintain multicellularity in Dictyostelium. Science 334, 1548–1551 10.1126/science.1213272 (doi:10.1126/science.1213272) [DOI] [PubMed] [Google Scholar]
- 10.Ponte E., Bracco E., Faix J., Bozzaro S. 1998. Detection of subtle phenotypes: the case of the cell adhesion molecule csA in Dictyostelium. Proc. Natl Acad. Sci. USA 95, 9360–9365 10.1073/pnas.95.16.9360 (doi:10.1073/pnas.95.16.9360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster K. R., Shaulsky G., Strassmann J. E., Queller D. C., Thompson C. R. L. 2004. Pleiotropy as a mechanism to stabilize cooperation. Nature 431, 693–696 10.1038/nature02894 (doi:10.1038/nature02894) [DOI] [PubMed] [Google Scholar]
- 12.Khare A., Santorelli L. A., Strassmann J. E., Queller D. C., Kuspa A., Shaulsky G. 2009. Cheater-resistance is not futile. Nature 461, 980–982 10.1038/nature08472 (doi:10.1038/nature08472) [DOI] [PubMed] [Google Scholar]
- 13.Fortunato A., Queller D. C., Strassmann J. E. 2003. A linear dominance hierarchy among clones in chimeras of the social amoeba Dictyostelium discoideum. J. Evol. Biol. 16, 438–445 10.1046/j.1420-9101.2003.00545.x (doi:10.1046/j.1420-9101.2003.00545.x) [DOI] [PubMed] [Google Scholar]
- 14.Fiegna F., Velicer G. J. 2005. Exploitative and hierarchical antagonism in a cooperative bacterium. PLoS Biol. 3, e370 10.1371/journal.pbio.0030370 (doi:10.1371/journal.pbio.0030370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manhes P., Velicer G. J. 2011. Experimental evolution of selfish policing in social bacteria. Proc. Natl Acad. Sci. USA 108, 8357–8362 10.1073/pnas.1014695108 (doi:10.1073/pnas.1014695108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxer G., Brock D. A., Queller D. C., Strassmann J. E. 2010. Cheating does not explain selective differences at high and low relatedness in a social amoeba. BMC Evol. Biol. 10, 76 10.1186/1471-2148-10-76 (doi:10.1186/1471-2148-10-76) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benabentos R., et al. 2009. Polymorphic members of the lag gene family mediate kin discrimination in Dictyostelium. Curr. Biol. 19, 567–572 10.1016/j.cub.2009.02.037 (doi:10.1016/j.cub.2009.02.037) [DOI] [PMC free article] [PubMed] [Google Scholar]