Abstract
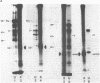
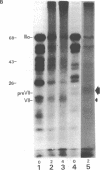
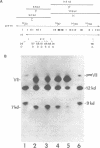
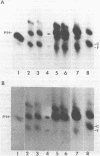
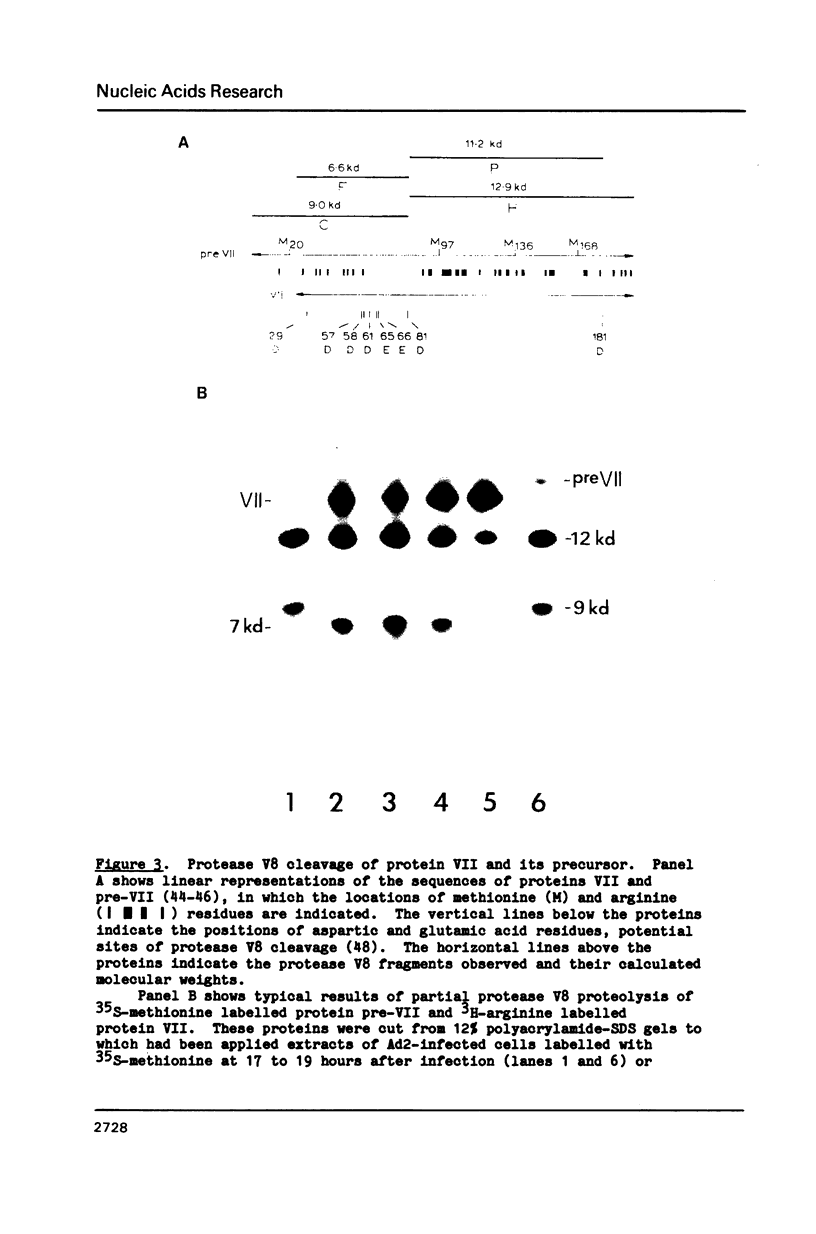
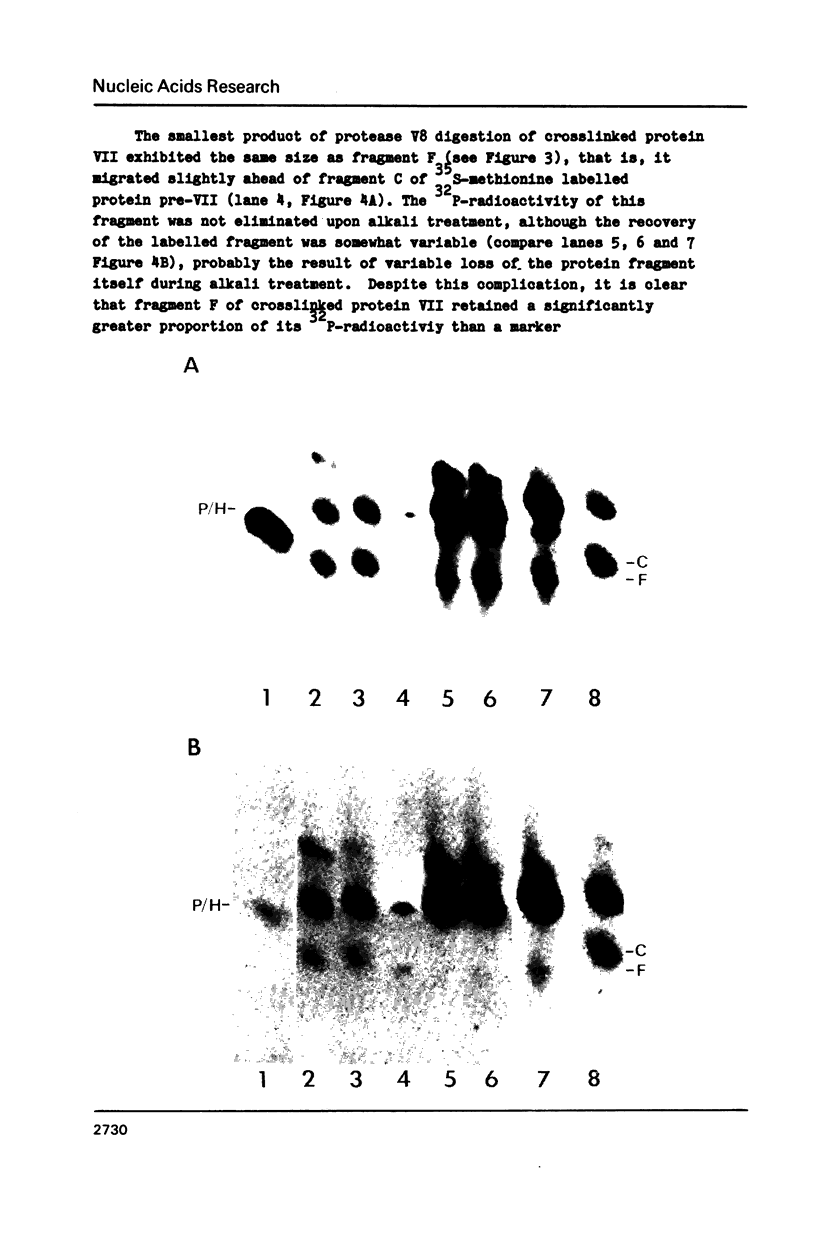
The interactions of the major core protein of adenovirus type 2 (Ad2) protein VII, and its precursor, protein pre-VII, with viral DNA, were studied using UV light induced crosslinking of 32P-labelled oligonucleotides to the proteins. Proteolytic fragments of these two proteins that contain DNA-binding domains were identified by virtue of their covalently attached, alkali-resistant 32P-radioactivity. The overall efficiency of crosslinking of protein pre-VII to DNA, in H2ts1 virions assembled at 39 degrees C, was comparable to that of the crosslinking of protein VII to DNA in Ad2 virions. However, a protease V8 fragment comprising the N-terminal half of protein pre-VII crosslinked to DNA at least ten times more efficiently than the corresponding N-terminal fragment of protein VII, which is truncated by the removal of 23 amino acids from the N-terminus of protein pre-VII during virion maturation.
Full text
PDF

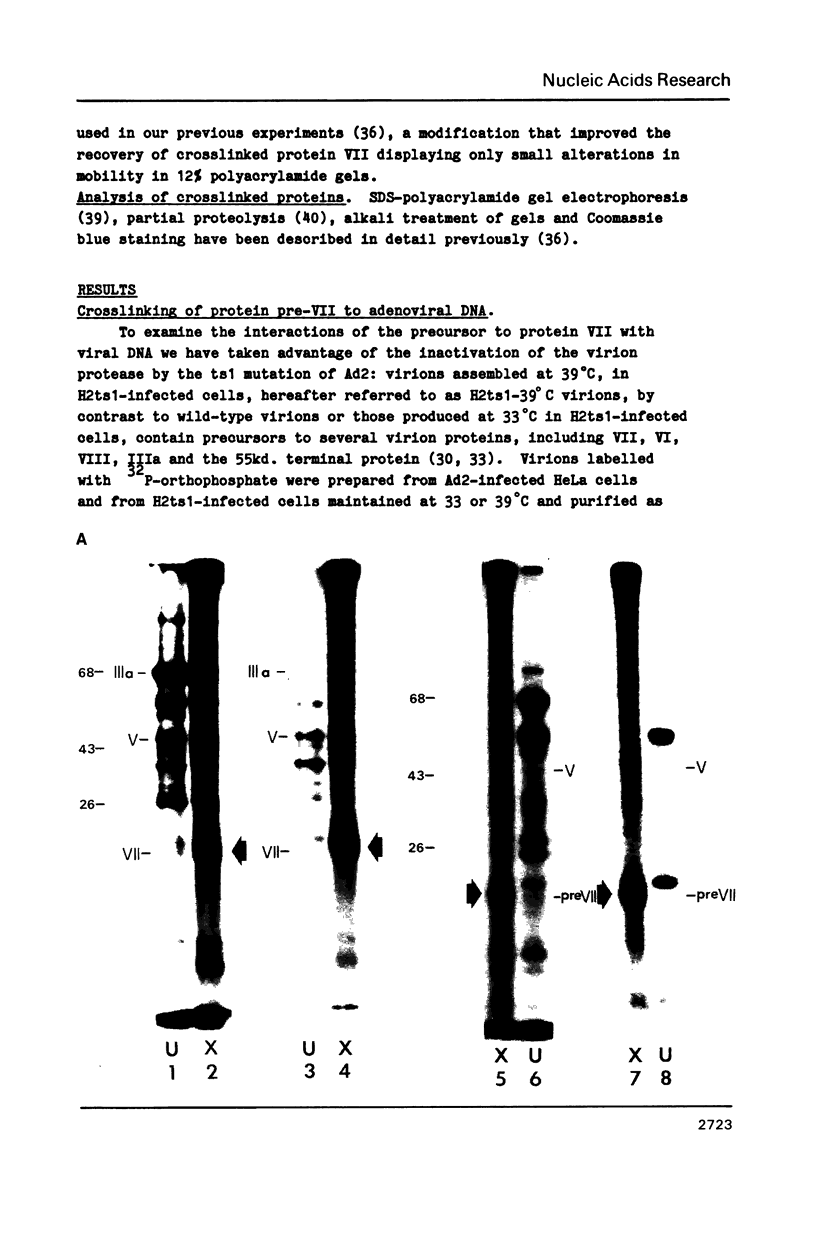
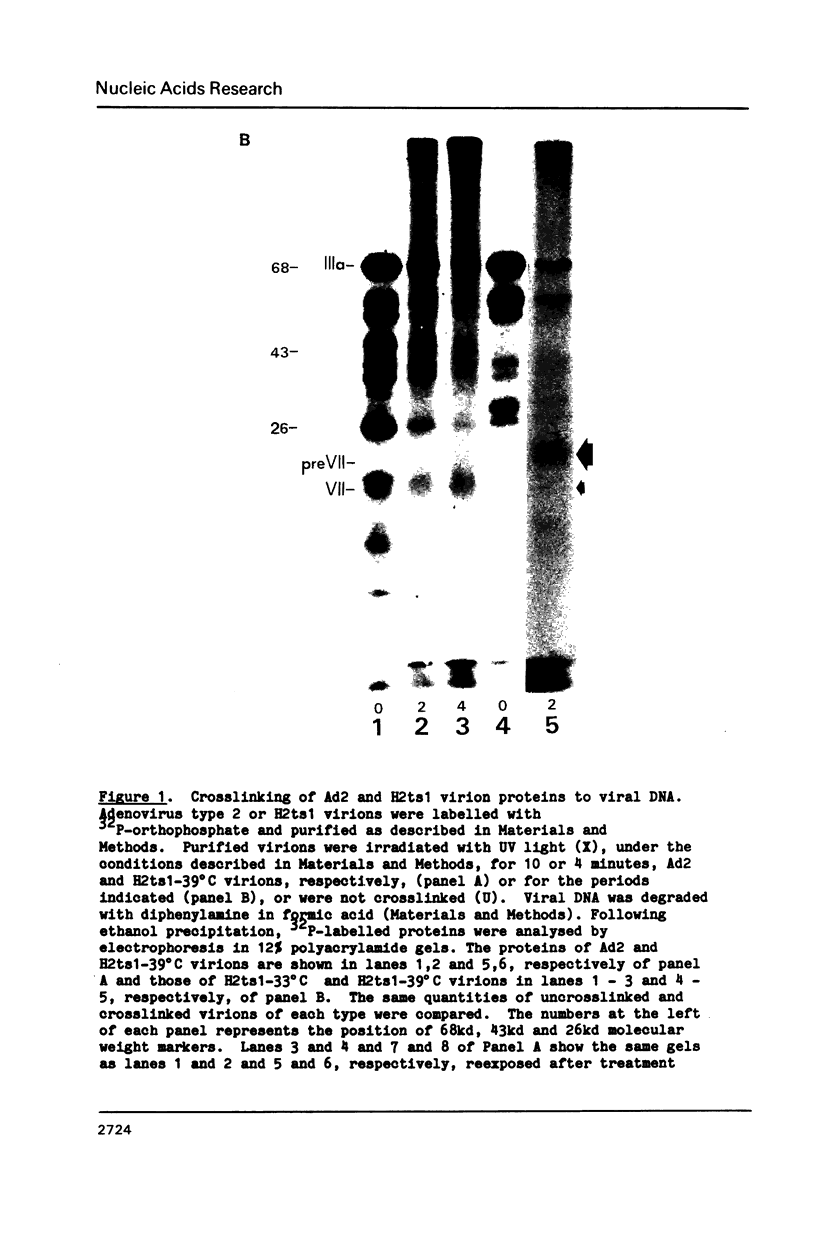

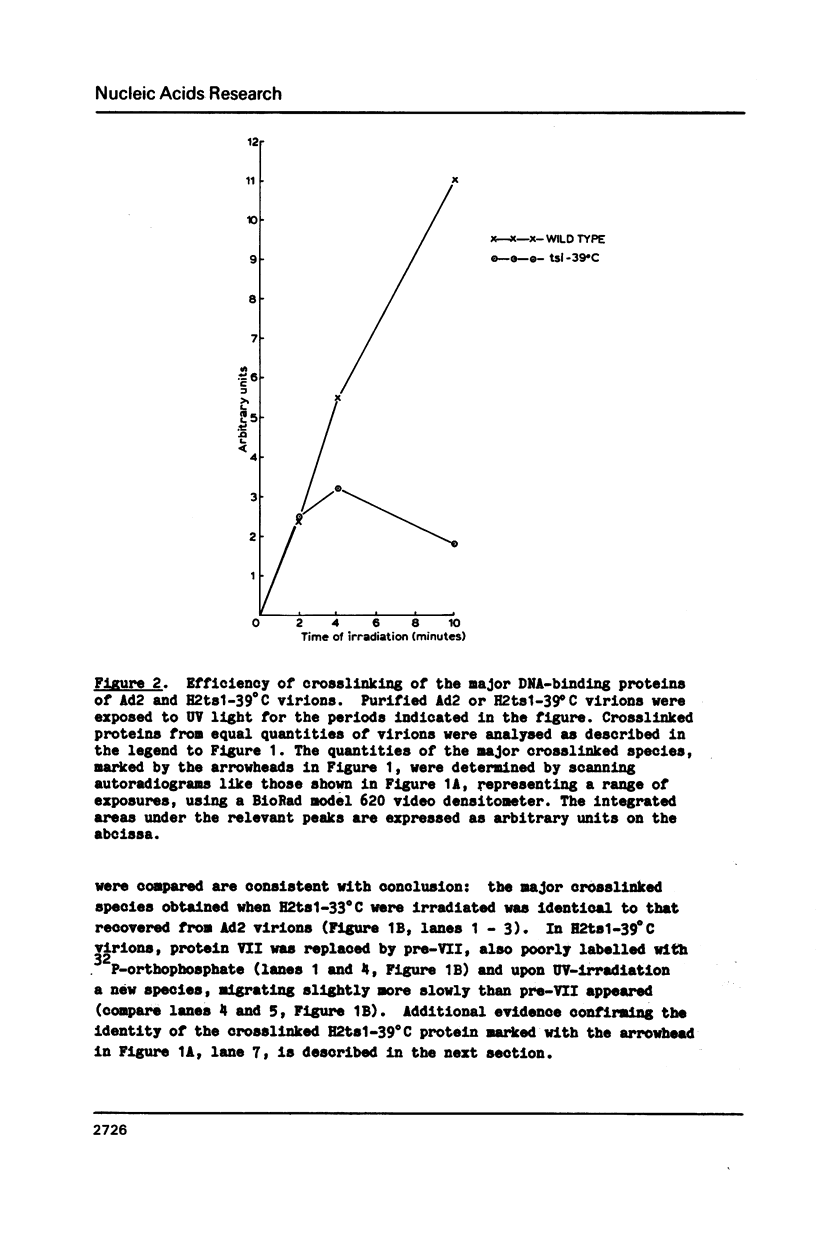







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Akusjärvi G., Lager M., Yeh-kai L., Pettersson U. Genes encoding the core proteins of adenovirus type 2. J Biol Chem. 1984 Nov 25;259(22):13980–13985. [PubMed] [Google Scholar]
- Amin M., Mirza A., Weber J. Genetic analysis of adenovirus type 2. VII. Cleavage-modified affinity for DNA of internal virion proteins. Virology. 1977 Jul 1;80(1):83–97. doi: 10.1016/0042-6822(77)90382-8. [DOI] [PubMed] [Google Scholar]
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Boudin M. L., D'Halluin J. C., Cousin C., Boulanger P. Human adenovirus type 2 protein IIIa. II. Maturation and encapsidation. Virology. 1980 Feb;101(1):144–156. doi: 10.1016/0042-6822(80)90491-2. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Westphal M., Burlingham B. T., Winterhoff U., Doerfler W. Structure and composition of the adenovirus type 2 core. J Virol. 1975 Aug;16(2):366–387. doi: 10.1128/jvi.16.2.366-387.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., Weber J. Virion core-like organization of intranuclear adenovirus chromatin late in infection. Virology. 1980 Nov;107(1):306–310. doi: 10.1016/0042-6822(80)90297-4. [DOI] [PubMed] [Google Scholar]
- Bégin M., Weber J. Genetic analysis of adenovirus type 2. I. Isolation and genetic characterization of temperature-sensitive mutants. J Virol. 1975 Jan;15(1):1–7. doi: 10.1128/jvi.15.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee P. K., Vayda M. E., Flint S. J. Interactions among the three adenovirus core proteins. J Virol. 1985 Aug;55(2):379–386. doi: 10.1128/jvi.55.2.379-386.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corden J., Engelking H. M., Pearson G. D. Chromatin-like organization of the adenovirus chromosome. Proc Natl Acad Sci U S A. 1976 Feb;73(2):401–404. doi: 10.1073/pnas.73.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Martin G. R., Torpier G., Boulanger P. A. Adenovirus type 2 assembly analyzed by reversible cross-linking of labile intermediates. J Virol. 1978 May;26(2):357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniell E., Groff D. E., Fedor M. J. Adenovirus chromatin structure at different stages of infection. Mol Cell Biol. 1981 Dec;1(12):1094–1105. doi: 10.1128/mcb.1.12.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau G. R., Boily Y., Houmard J. Purification and properties of an extracellular protease of Staphylococcus aureus. J Biol Chem. 1972 Oct 25;247(20):6720–6726. [PubMed] [Google Scholar]
- Edvardsson B., Everitt E., Jörnvall H., Prage L., Philipson L. Intermediates in adenovirus assembly. J Virol. 1976 Aug;19(2):533–547. doi: 10.1128/jvi.19.2.533-547.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardsson B., Ustacelebi S., Williams J., Philipson L. Assembly intermediates among adenovirus type 5 temperature-sensitive mutants. J Virol. 1978 Feb;25(2):641–651. doi: 10.1128/jvi.25.2.641-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Flint S. J., Gallimore P. H., Sharp P. A. Comparison of viral RNA sequences in adenovirus 2-transformed and lytically infected cells. J Mol Biol. 1975 Jul 25;96(1):47–68. doi: 10.1016/0022-2836(75)90181-3. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Maizel J. V., Jr The polypeptides of adenovirus. V. Young virions, structural intermediate between top components and aged virions. Virology. 1974 Feb;57(2):409–424. doi: 10.1016/0042-6822(74)90181-0. [DOI] [PubMed] [Google Scholar]
- Kunkel G. R., Martinson H. G. Histone-DNA interactions within chromatin. Isolation of histones from DNA-histone adducts induced in nuclei by UV light. Nucleic Acids Res. 1978 Nov;5(11):4263–4272. doi: 10.1093/nar/5.11.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laver W. G. Isolation of an arginine-rich protein from particles of adenovirus type 2. Virology. 1970 Jul;41(3):488–500. doi: 10.1016/0042-6822(70)90170-4. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Pereira H. G., Russell W. C., Valentine R. C. Isolation of an internal component from adenovirus type 5. J Mol Biol. 1968 Nov 14;37(3):379–386. doi: 10.1016/0022-2836(68)90109-5. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. II. Soluble proteins, cores, top components and the structure of the virion. Virology. 1968 Sep;36(1):126–136. doi: 10.1016/0042-6822(68)90122-0. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Structure of adenovirus chromatin. Biochim Biophys Acta. 1982 Jan 26;696(1):76–86. doi: 10.1016/0167-4781(82)90012-4. [DOI] [PubMed] [Google Scholar]
- Mirza M. A., Weber J. Uncoating of adenovirus type 2. J Virol. 1979 May;30(2):462–471. doi: 10.1128/jvi.30.2.462-471.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V. The architecture of adenoviruses: recent views and problems: Brief review. Arch Virol. 1980;64(3):175–196. doi: 10.1007/BF01322699. [DOI] [PubMed] [Google Scholar]
- Prage L., Pettersson U., Höglund S., Lonberg-Holm K., Philipson L. Structural proteins of adenoviruses. IV. Sequential degradation of the adenovirus type 2 virion. Virology. 1970 Oct;42(2):341–358. doi: 10.1016/0042-6822(70)90278-3. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Blair G. E. Polypeptide phosphorylation in adenovirus-infected cells. J Gen Virol. 1977 Jan;34(1):19–35. doi: 10.1099/0022-1317-34-1-19. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Laver W. G., Sanderson P. J. Internal components of adenovirus. Nature. 1968 Sep 14;219(5159):1127–1130. doi: 10.1038/2191127a0. [DOI] [PubMed] [Google Scholar]
- Russell W. C., McIntosh K., Skehel J. J. The preparation and properties of adenovirus cores. J Gen Virol. 1971 Apr;11(1):35–46. doi: 10.1099/0022-1317-11-1-35. [DOI] [PubMed] [Google Scholar]
- Sato K., Hosokawa K. The structure of adenovirion chromatin revealed by ultraviolet light-induced cross-linking. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1318–1323. doi: 10.1016/0006-291x(81)91591-6. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Cao T. M., Coleman R. T., Budelier K. A. Gene and protein sequences of adenovirus protein VII, a hybrid basic chromosomal protein. Proc Natl Acad Sci U S A. 1983 May;80(10):2902–2906. doi: 10.1073/pnas.80.10.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung M. T., Lischwe M. A., Richards J. C., Hosokawa K. Adenovirus chromatin I. Isolation and characterization of the major core protein VII and precursor Pro-VII. J Biol Chem. 1977 Jul 25;252(14):4981–4987. [PubMed] [Google Scholar]
- Tate V. E., Philipson L. Parental adenovirus DNA accumulates in nucleosome-like structures in infected cells. Nucleic Acids Res. 1979 Jun 25;6(8):2769–2785. doi: 10.1093/nar/6.8.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M. L., Déry C. V., Talbot B. G., Weber J. In vitro cleavage specificity of the adenovirus type 2 proteinase. Biochim Biophys Acta. 1983 Mar 16;743(2):239–245. doi: 10.1016/0167-4838(83)90220-0. [DOI] [PubMed] [Google Scholar]
- Vayda M. E., Rogers A. E., Flint S. J. The structure of nucleoprotein cores released from adenovirions. Nucleic Acids Res. 1983 Jan 25;11(2):441–460. doi: 10.1093/nar/11.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. Genetic analysis of adenovirus type 2 III. Temperature sensitivity of processing viral proteins. J Virol. 1976 Feb;17(2):462–471. doi: 10.1128/jvi.17.2.462-471.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]