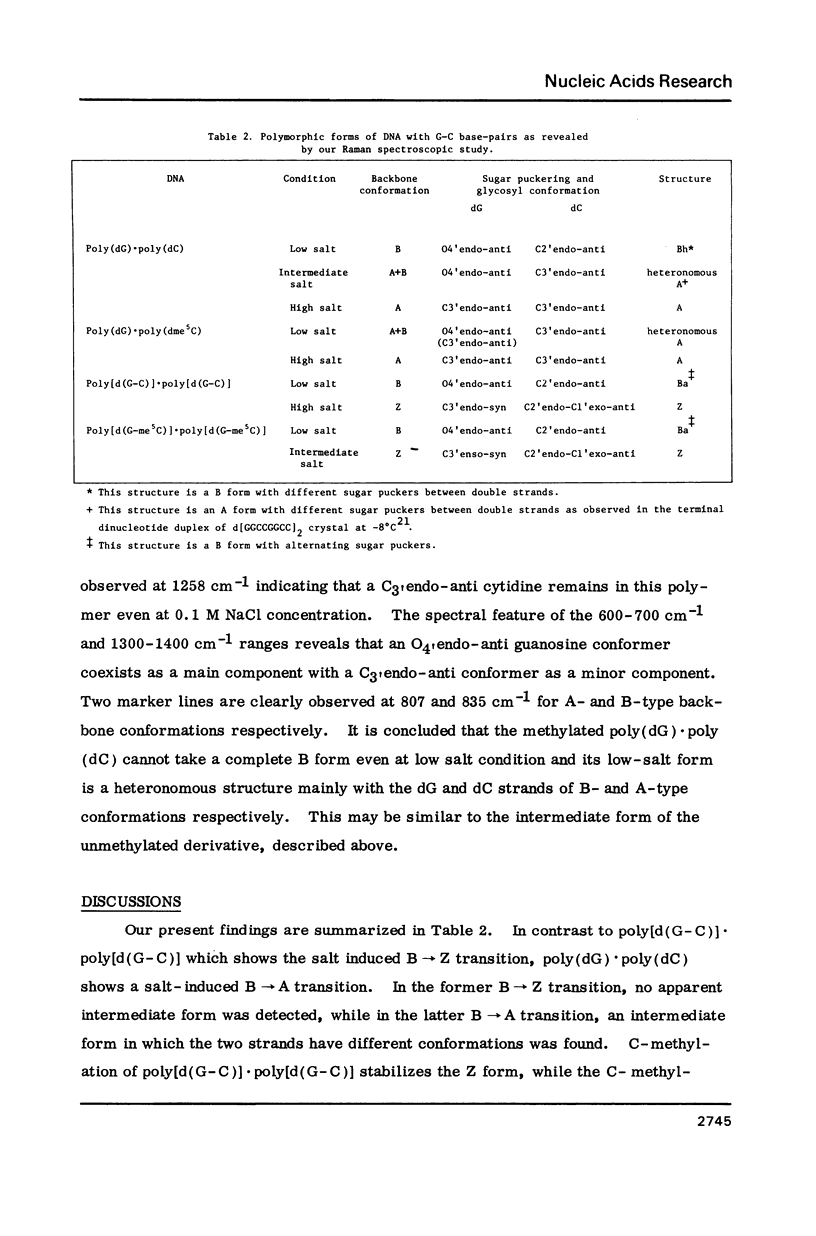
Abstract
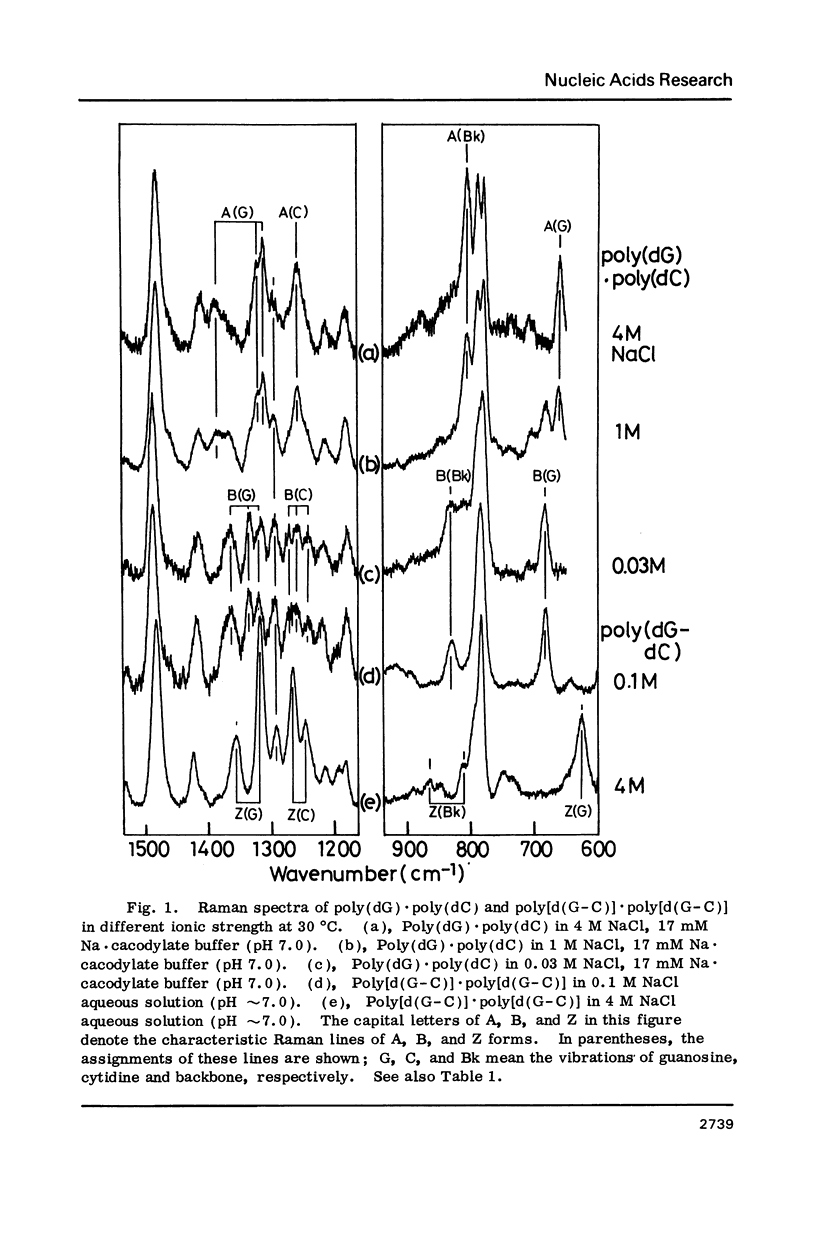
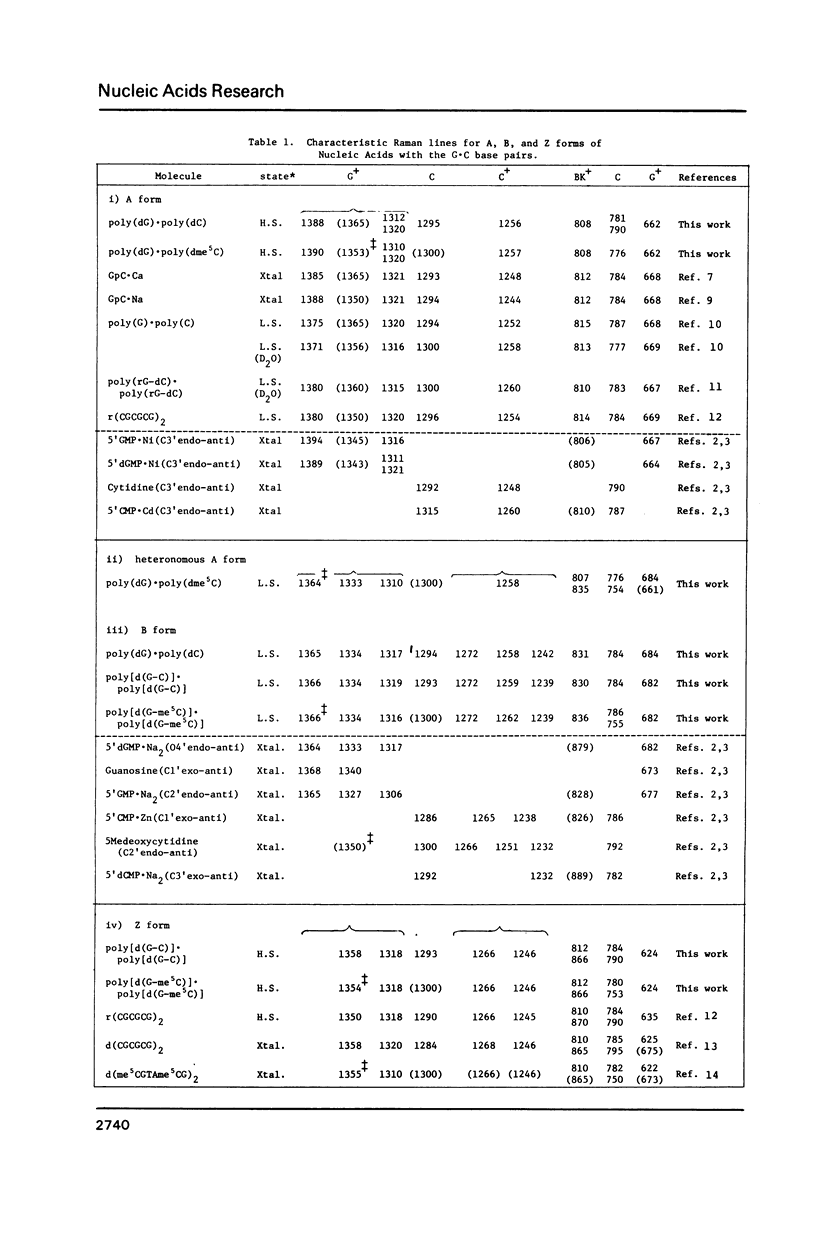
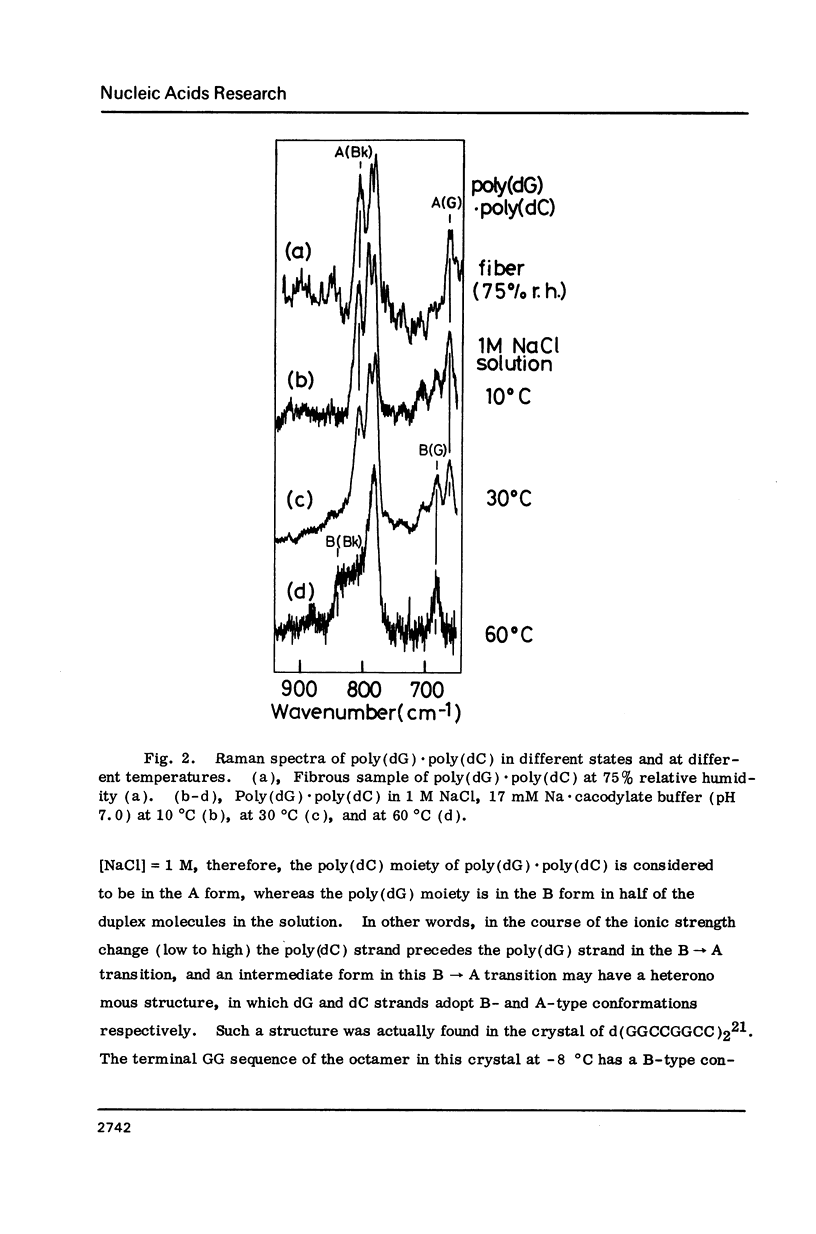
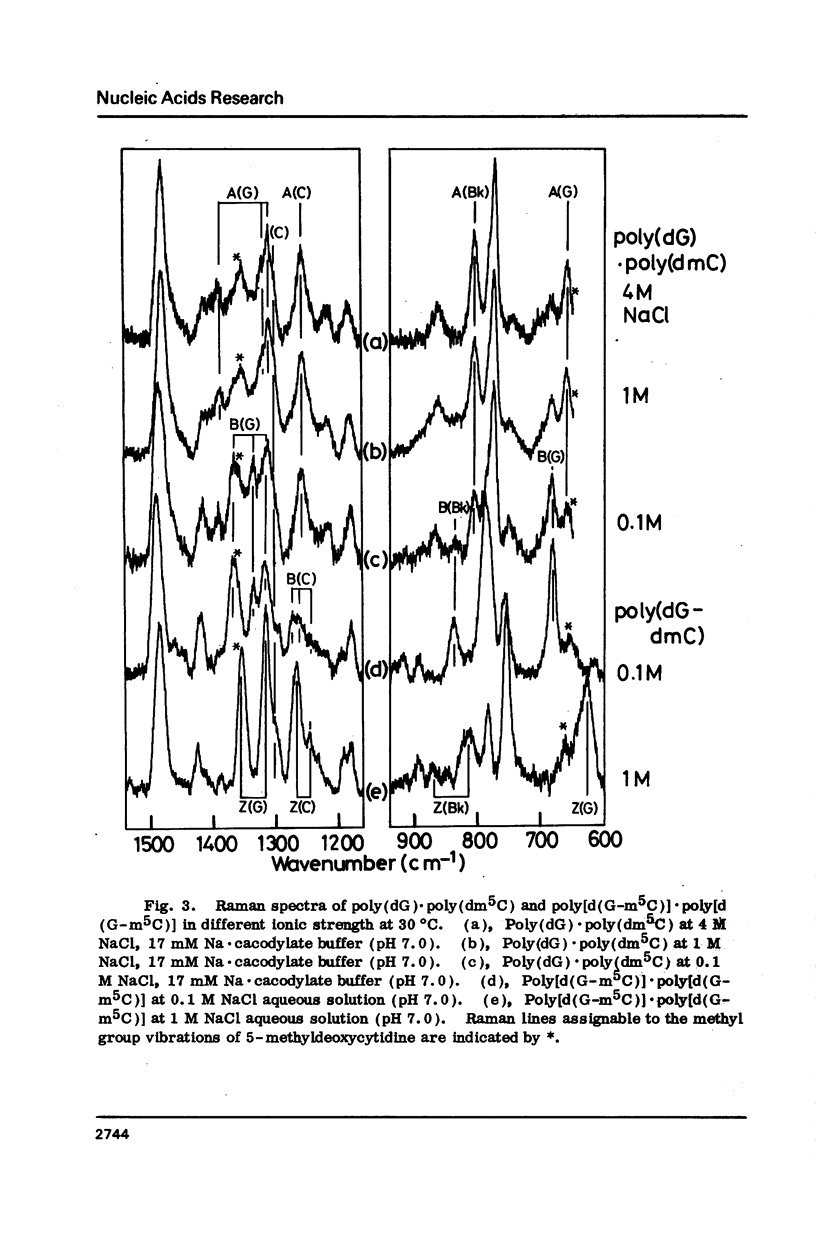
Raman spectra of poly(dG).poly(dC) have been observed in aqueous solutions at various ionic strengths, [NaCl] = 0.03 to 4 M, and at different temperatures, 10 to 60 degrees C. At 30 degrees C, and at [NaCl] = 0.03 M, it was found to have a B-form (with O4'endo-anti guanosine and C2'endo-anti cytidine), whereas, at [NaCl] = 4 M, an A form (with C3'endo-anti guanosine and C3'endo-anti cytidine). At 30 degrees C and [NaCl] = 1 M, namely at an intermediate state, a fraction of this molecules was considered to have a "heteronomous A" form (with O4'endo-anti guanosine and C3' endo-anti cytidine). At 60 degrees C and [NaCl] = 1 M, it assumes the B form, and at 10 degrees C and [NaCl] = 1 M, the A form. Cytosine-5-methylation was found to cause a marked stabilization of the A form. Even at [NaCl] = 0.1 M (at 30 degrees C), a substantial portion of poly(dG).poly(dm5C) was found to have a heteronomous form, in which the dG atrand is in the B form and the dC an A form; it never assumes a complete B form.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Arnott S., Selsing E. Letter: The structure of polydeoxyguanylic acid with polydeoxycytidylic acid. J Mol Biol. 1974 Sep 15;88(2):551–552. doi: 10.1016/0022-2836(74)90502-6. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevides J. M., Wang A. H., van der Marel G. A., van Boom J. H., Rich A., Thomas G. J., Jr The Raman spectra of left-handed DNA oligomers incorporating adenine-thymine base pairs+. Nucleic Acids Res. 1984 Jul 25;12(14):5913–5925. doi: 10.1093/nar/12.14.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahms S., Vergne J., Brahms J. G., Di Capua E., Bucher P., Koller T. Natural DNA sequences can form left-handed helices in low salt solution under conditions of topological constraint. J Mol Biol. 1982 Dec 5;162(2):473–493. doi: 10.1016/0022-2836(82)90539-3. [DOI] [PubMed] [Google Scholar]
- Bram S. Polynucleotide polymorphism in solution. Nat New Biol. 1971 Oct 6;233(40):161–164. doi: 10.1038/newbio233161a0. [DOI] [PubMed] [Google Scholar]
- Conner B. N., Takano T., Tanaka S., Itakura K., Dickerson R. E. The molecular structure of d(ICpCpGpG), a fragment of right-handed double helical A-DNA. Nature. 1982 Jan 28;295(5847):294–299. doi: 10.1038/295294a0. [DOI] [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Erfurth S. C., Kiser E. J., Peticolas W. L. Determination of the backbone structure of nucleic acids and nucleic acid oligomers by laser Raman scattering. Proc Natl Acad Sci U S A. 1972 Apr;69(4):938–941. doi: 10.1073/pnas.69.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Lafleur L., Rice J., Thomas G. J., Jr Raman studies of nucleic acids. VII. Poly A-poly U and poly G-poly C. Biopolymers. 1972;11(12):2423–2437. doi: 10.1002/bip.1972.360111205. [DOI] [PubMed] [Google Scholar]
- Marck C., Thiele D. Poly(dG).poly(dC) at neutral and alkaline pH: the formation of triple stranded poly(dG).poly(dG).poly(dC). Nucleic Acids Res. 1978 Mar;5(3):1017–1028. doi: 10.1093/nar/5.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall M., Brown T., Kennard O. The crystal structure of d(G-G-G-G-C-C-C-C). A model for poly(dG).poly(dC). J Mol Biol. 1985 Jun 5;183(3):385–396. doi: 10.1016/0022-2836(85)90009-9. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Torigoe C., Tsuboi M. An A-form poly(dG).poly(dC) in H2O solution. Biopolymers. 1985 Sep;24(9):1841–1844. doi: 10.1002/bip.360240913. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Tsuboi M., Nakano T., Higuchi S., Sato T., Shida T., Uesugi S., Ohtsuka E., Ikehara M. Raman diagnosis of nucleic acid structure: sugar-puckering and glycosidic conformation in the guanosine moiety. Nucleic Acids Res. 1983 Mar 11;11(5):1579–1588. doi: 10.1093/nar/11.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y., Tsuboi M., Sato T. Structure-spectrum correlations in nucleic acids. I. Raman lines in the 600-700 cm-1 range of guanosine residue. Nucleic Acids Res. 1984 Sep 11;12(17):6901–6908. doi: 10.1093/nar/12.17.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Sequence dependence of the helical repeat of DNA in solution. Nature. 1981 Jul 23;292(5821):375–378. doi: 10.1038/292375a0. [DOI] [PubMed] [Google Scholar]
- Prescott B., Steinmetz W., Thomas G. J., Jr Characterization of DNA structures by laser Raman spectroscopy. Biopolymers. 1984 Feb;23(2):235–256. doi: 10.1002/bip.360230206. [DOI] [PubMed] [Google Scholar]
- Shakked Z., Rabinovich D., Kennard O., Cruse W. B., Salisbury S. A., Viswamitra M. A. Sequence-dependent conformation of an A-DNA double helix. The crystal structure of the octamer d(G-G-T-A-T-A-C-C). J Mol Biol. 1983 May 15;166(2):183–201. doi: 10.1016/s0022-2836(83)80005-9. [DOI] [PubMed] [Google Scholar]
- Thamann T. J., Lord R. C., Wang A. H., Rich A. The high salt form of poly(dG-dC).poly(dG-dC) is left-handed Z-DNA: Raman spectra of crystals and solutions. Nucleic Acids Res. 1981 Oct 24;9(20):5443–5457. doi: 10.1093/nar/9.20.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Fujii S., van Boom J. H., Rich A. Molecular structure of the octamer d(G-G-C-C-G-G-C-C): modified A-DNA. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3968–3972. doi: 10.1073/pnas.79.13.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]



