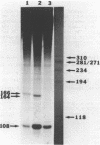
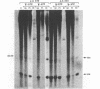
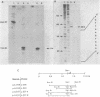
Abstract
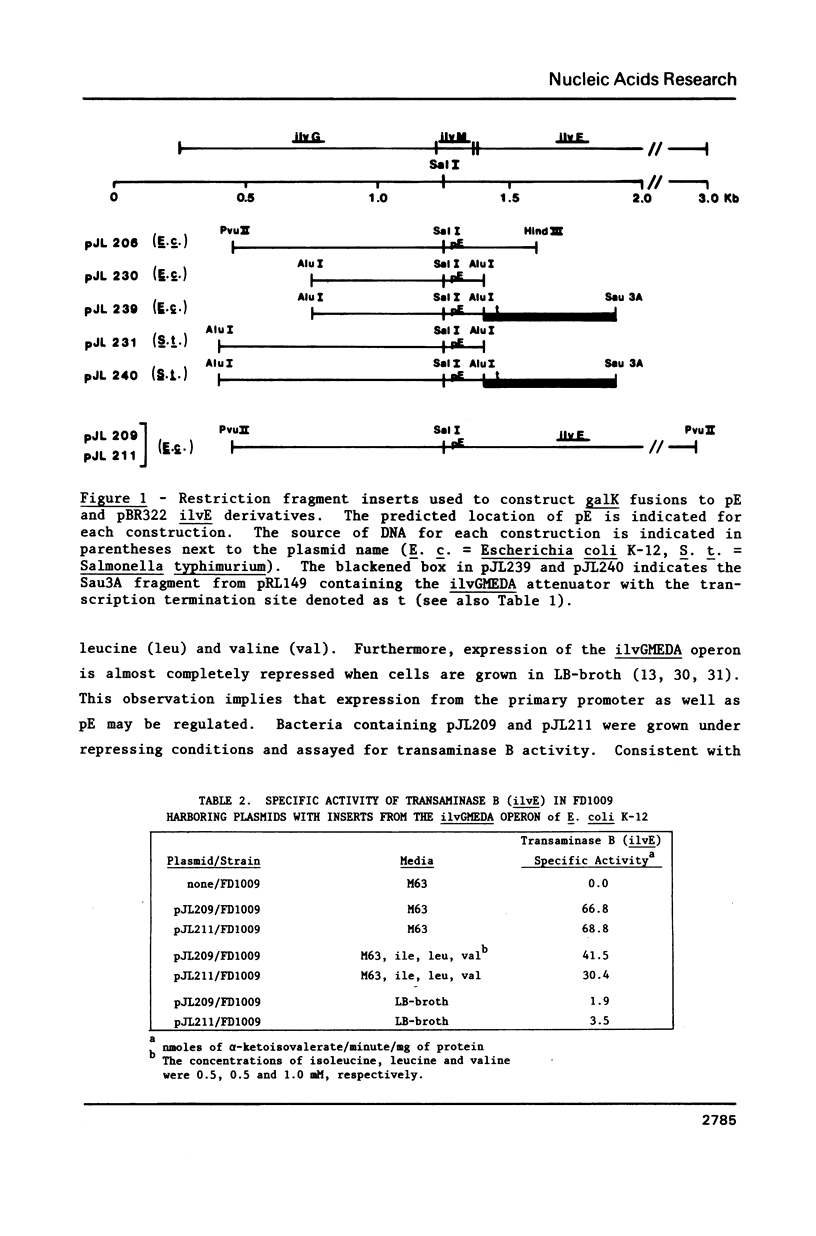
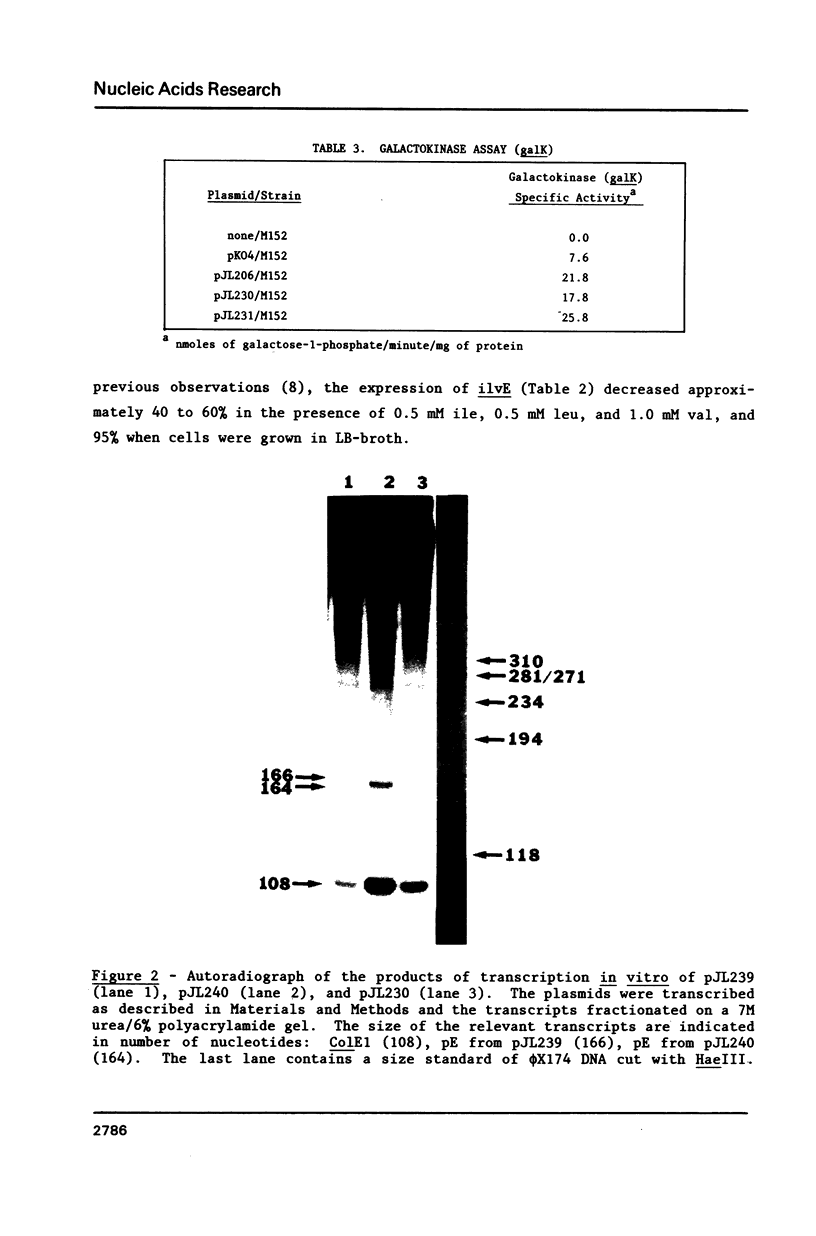
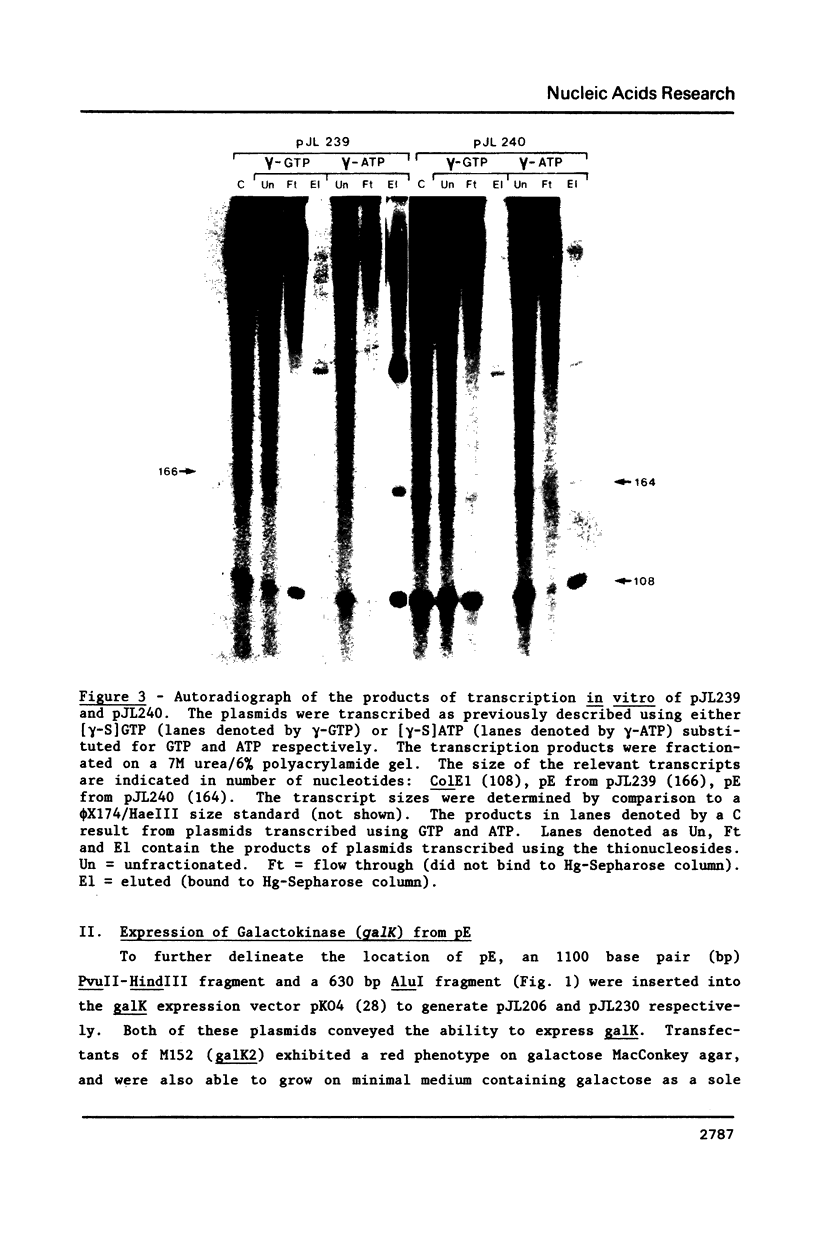
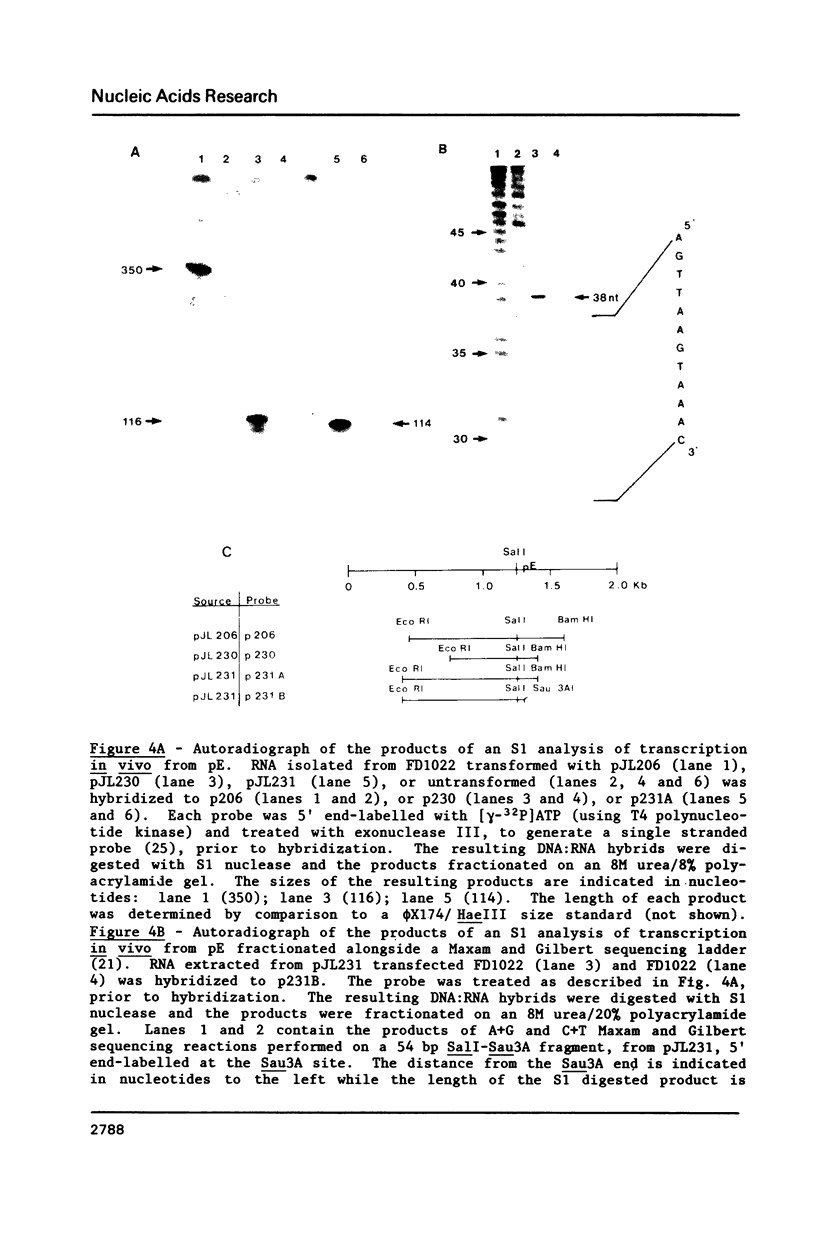
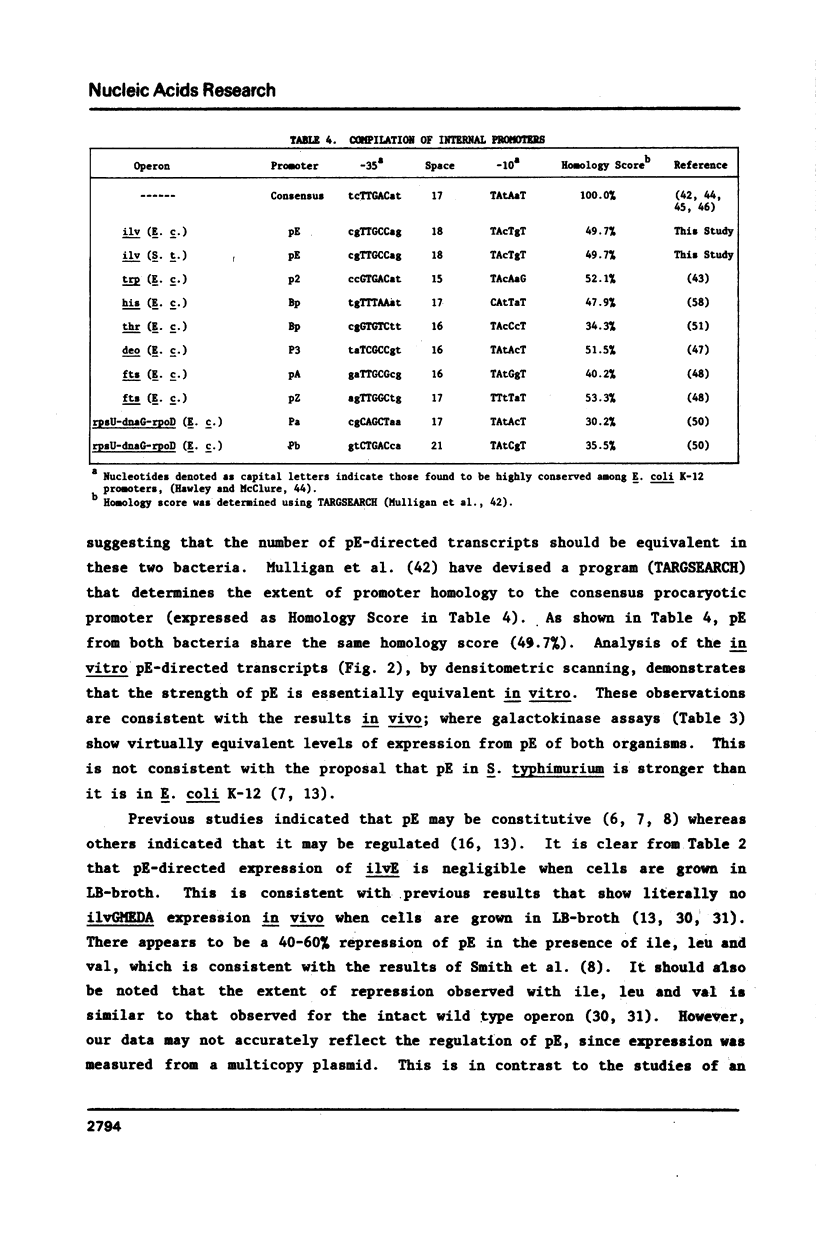
It was previously determined that the distal portion of the ilvGMEDA operon was expressed despite the insertion of transposons into ilvG and ilvE. This observation suggested the existence of internal promoters upstream of ilvE (pE) and ilvD (pD). The internal promoter pE, responsible for part of ilvEDA expression, has been analyzed both in vivo and in vitro. Our results indicate that: pE exists in both E. coli K-12 and S. typhimurium; pE is located in the distal end of the ilvM coding sequence; the pE sequence is highly conserved in the two bacteria; the amino acid sequence of the ilvM gene product is 93% homologous between the two bacteria; transcription from pE can be demonstrated both in vivo and in vitro; the efficiency of pE is essentially equivalent in the two bacteria.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Hatfield G. W. Effects of promoter strengths and growth conditions on copy number of transcription-fusion vectors. J Biol Chem. 1984 Jun 25;259(12):7399–7403. [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. Evidence for two sites for initiation of gene expression in the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1967 Jun 28;26(3):423–436. doi: 10.1016/0022-2836(67)90313-0. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J. Organization and regulation of the ilvGEDA operon in Salmonella typhimurium LT2. J Bacteriol. 1981 Feb;145(2):984–989. doi: 10.1128/jb.145.2.984-989.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg C. M., Shaw K. J., Vender J., Borucka-Mankiewicz M. Physiological characterization of polar Tn5-induced isoleucine-valine auxotrophs in Escherichia coli K.12: evidence for an internal promoter in the ilvOGEDA operon. Genetics. 1979 Oct;93(2):308–319. [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Genetic organization of the Salmonella typhimurium ilv gene cluster. Mol Gen Genet. 1979;177(1):1–11. doi: 10.1007/BF00267247. [DOI] [PubMed] [Google Scholar]
- Blazey D. L., Burns R. O. Transcriptional activity of the transposable element Tn10 in the Salmonella typhimurium ilvGEDA operon. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5011–5015. doi: 10.1073/pnas.79.16.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazey D. L., Kim R., Burns R. O. Molecular cloning and expression of the ilvGEDAY genes from Salmonella typhimurium. J Bacteriol. 1981 Aug;147(2):452–462. doi: 10.1128/jb.147.2.452-462.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calhoun D. H., Wallen J. W., Traub L., Gray J. E., Kung H. F. Internal promoter in the ilvGEDA transcription unit of Escherichia coli K-12. J Bacteriol. 1985 Jan;161(1):128–132. doi: 10.1128/jb.161.1.128-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver R. P., Lawther R. P. Physical analysis of deletion mutations in the ilvGEDA operon of Escherichia coli K-12. J Bacteriol. 1985 May;162(2):598–606. doi: 10.1128/jb.162.2.598-606.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver R. P., Lawther R. P. Restriction endonuclease analysis of the ilvGEDA operon of members of the family Enterobacteriaceae. J Bacteriol. 1985 Jun;162(3):1317–1319. doi: 10.1128/jb.162.3.1317-1319.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayda D. J., Leathers T. D., Noti J. D., Smith F. J., Smith J. M., Subrahmanyam C. S., Umbarger H. E. Location of the multivalent control site for the ilvEDA operon of Escherichia coli. J Bacteriol. 1980 May;142(2):556–567. doi: 10.1128/jb.142.2.556-567.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisolia V., Riccio A., Bruni C. B. Structure and function of the internal promoter (hisBp) of the Escherichia coli K-12 histidine operon. J Bacteriol. 1983 Sep;155(3):1288–1296. doi: 10.1128/jb.155.3.1288-1296.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz H., Platt T. Initiation in vivo at the internal trp p2 promoter of Escherichia coli. J Biol Chem. 1983 Jul 10;258(13):7890–7893. [PubMed] [Google Scholar]
- Jackson E. N., Yanofsky C. Internal promoter of the tryptophan operon of Escherichia coli is located in a structural gene. J Mol Biol. 1972 Aug 21;69(2):307–313. doi: 10.1016/0022-2836(72)90232-x. [DOI] [PubMed] [Google Scholar]
- Jones H. M., Brajkovich C. M., Gunsalus R. P. In vivo 5' terminus and length of the mRNA for the proton-translocating ATPase (unc) operon of Escherichia coli. J Bacteriol. 1983 Sep;155(3):1279–1287. doi: 10.1128/jb.155.3.1279-1287.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Largen M., Belser W. The apparent conservation of the internal low efficiency promoter of the tryptophan operons of several species of Enterobacteriaceae. Genetics. 1973 Sep;75(1):19–22. doi: 10.1093/genetics/75.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Gray J., Adams C. W., Hauser C. A., Hatfield G. W. DNA sequence fine-structure analysis of ilvG (IlvG+) mutations of Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):294–298. doi: 10.1128/jb.149.1.294-298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. A site of action for tRNA mediated regulation of the ilvOEDA operon of Escherichia coli K12. Mol Gen Genet. 1978 Nov 29;167(2):227–234. doi: 10.1007/BF00266916. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield G. W. Effects of altered rho gene product on the expression of the Escherichia coli histidine operon. J Bacteriol. 1978 Dec;136(3):1201–1204. doi: 10.1128/jb.136.3.1201-1204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Hatfield W. Biochemical characterization of an Escherichia coli hisT strain. J Bacteriol. 1977 Apr;130(1):552–557. doi: 10.1128/jb.130.1.552-557.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Nichols B., Zurawski G., Hatfield G. W. The nucleotide sequence preceding and including the beginning of the ilvE gene of the ilvGEDA operon of Escherichia coli K12. Nucleic Acids Res. 1979 Dec 20;7(8):2289–2301. doi: 10.1093/nar/7.8.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutkenhaus J. F., Wolf-Watz H., Donachie W. D. Organization of genes in the ftsA-envA region of the Escherichia coli genetic map and identification of a new fts locus (ftsZ). J Bacteriol. 1980 May;142(2):615–620. doi: 10.1128/jb.142.2.615-620.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miller K. G., Sollner-Webb B. Transcription of mouse rRNA genes by RNA polymerase I: in vitro and in vivo initiation and processing sites. Cell. 1981 Nov;27(1 Pt 2):165–174. doi: 10.1016/0092-8674(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Hawley D. K., Entriken R., McClure W. R. Escherichia coli promoter sequences predict in vitro RNA polymerase selectivity. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):789–800. doi: 10.1093/nar/12.1part2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nargang F. E., Subrahmanyam C. S., Umbarger H. E. Nucleotide sequence of ilvGEDA operon attenuator region of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1823–1827. doi: 10.1073/pnas.77.4.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Auger E. A., Vermersch P. S., Bennett G. N. Role of DNA regions flanking the tryptophan promoter of Escherichia coli. I. Insertion of synthetic oligonucleotides. Gene. 1984 Dec;32(3):337–348. doi: 10.1016/0378-1119(84)90009-x. [DOI] [PubMed] [Google Scholar]
- Saint Girons I., Margarita D. Evidence for an internal promoter in the Escherichia coli threonine operon. J Bacteriol. 1985 Jan;161(1):461–462. doi: 10.1128/jb.161.1.461-462.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salganik R. I., Dianov G. L., Ovchinnikova L. P., Voronina E. N., Kokoza E. B., Mazin A. V. Gene-directed mutagenesis in bacteriophage T7 provided by polyalkylating RNAs complementary to selected DNA sites. Proc Natl Acad Sci U S A. 1980 May;77(5):2796–2800. doi: 10.1073/pnas.77.5.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. V., Van Dyk D. E., Vasta J. F., Kutny R. M. Purification and properties of Salmonella typhimurium acetolactate synthase isozyme II from Escherichia coli HB101/pDU9. Biochemistry. 1985 Aug 27;24(18):4952–4959. doi: 10.1021/bi00339a034. [DOI] [PubMed] [Google Scholar]
- Schmincke C. D., Herrmann K., Hausen P. Size of primary transcripts in Ehrlich ascites cells as measured by tetraphosphate determination. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1994–1998. doi: 10.1073/pnas.73.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Smith F. J., Umbarger H. E. Mutations affecting the formation of acetohydroxy acid synthase II in Escherichia coli K-12. Mol Gen Genet. 1979 Feb 1;169(3):299–314. doi: 10.1007/BF00382276. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Subrahmanyam C. S., Noti J. D., Umbarger H. E. Regulation of ilvEDA expression occurs upstream of ilvG in Escherichia coli: additional evidence for an ilvGEDA operon. J Bacteriol. 1980 Oct;144(1):279–290. doi: 10.1128/jb.144.1.279-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillon M. P., Gotto D. A., Lawther R. P. The DNA sequence of the promoter-attenuator of the ilvGEDA operon of Salmonella typhimurium. Nucleic Acids Res. 1981 Jul 24;9(14):3419–3432. doi: 10.1093/nar/9.14.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. E., Straus D. B., Grossman A. D., Burton Z. F., Gross C. A., Burgess R. R. Transcription from a heat-inducible promoter causes heat shock regulation of the sigma subunit of E. coli RNA polymerase. Cell. 1984 Sep;38(2):371–381. doi: 10.1016/0092-8674(84)90492-6. [DOI] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. Control of ColE1 plasmid replication: the process of binding of RNA I to the primer transcript. Cell. 1984 Oct;38(3):861–870. doi: 10.1016/0092-8674(84)90281-2. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer K., Løve Larsen J. E., Svendsen I. The internal regulated promoter of the deo operon of Escherichia coli K-12. Nucleic Acids Res. 1984 Jul 11;12(13):5211–5224. doi: 10.1093/nar/12.13.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi Q. M., Rockenbach S., Ward J. E., Jr, Lutkenhaus J. Structure and expression of the cell division genes ftsQ, ftsA and ftsZ. J Mol Biol. 1985 Aug 5;184(3):399–412. doi: 10.1016/0022-2836(85)90290-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Y., Thompson E. A., Stallcup M. R. Hormonal regulation of transcription of rDNA: use of nucleoside thiotriphosphates to measure initiation in isolated nuclei. Nucleic Acids Res. 1984 Nov 12;12(21):8115–8128. doi: 10.1093/nar/12.21.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]