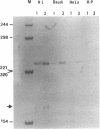
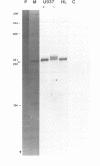
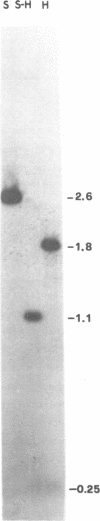
Abstract
A recently reported cDNA clone coding for human promyelocytic L apoferritin, shows some differences with a liver L apoferritin cDNA. We have investigated if these differences are due to the expression of different genes or to an alternative transcription of an unique gene. In this paper we report data suggesting that a single gene is mainly expressed in several tissues examined. This gene has been cloned and characterized. Its sequence shows three introns: the exon sequence is identical to that of cDNA clone isolated from human liver. A minimum of five related pseudogenes have been also analysed. One of them is a processed pseudogene interrupted by an intron-like fragment.
Full text
PDF




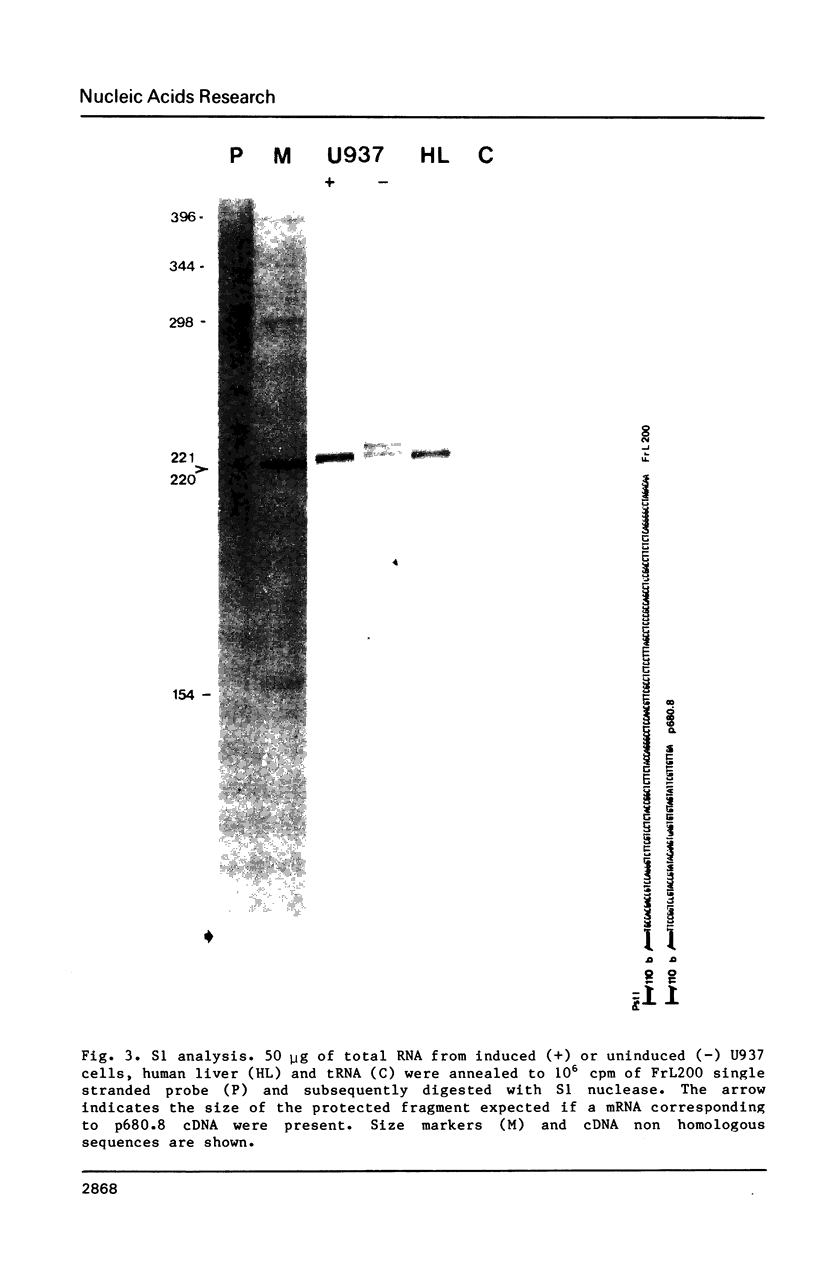








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Fitton J. E., Lewis W. G., May K., Harrison P. M. The amino acid sequence of human liver apoferritin. FEBS Lett. 1983 Nov 28;164(1):139–144. doi: 10.1016/0014-5793(83)80037-4. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Banyard S. H., Stammers D. K., Harrison P. M. Electron density map of apoferritin at 2.8-A resolution. Nature. 1978 Jan 19;271(5642):282–284. doi: 10.1038/271282a0. [DOI] [PubMed] [Google Scholar]
- Bensi G., Raugei G., Klefenz H., Cortese R. Structure and expression of the human haptoglobin locus. EMBO J. 1985 Jan;4(1):119–126. doi: 10.1002/j.1460-2075.1985.tb02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Broxmeyer H. E., Bognacki J., Dorner M. H., de Sousa M. Identification of leukemia-associated inhibitory activity as acidic isoferritins. A regulatory role for acidic isoferritins in the production of granulocytes and macrophages. J Exp Med. 1981 Jun 1;153(6):1426–1444. doi: 10.1084/jem.153.6.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Ciliberto G., Raugei G., Costanzo F., Dente L., Cortese R. Common and interchangeable elements in the promoters of genes transcribed by RNA polymerase iii. Cell. 1983 Mar;32(3):725–733. doi: 10.1016/0092-8674(83)90058-2. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Braunwald A. D., Kuo A. L. Phorbol ester induction of leukemic cell differentiation is a membrane-mediated process. Proc Natl Acad Sci U S A. 1982 May;79(9):2865–2869. doi: 10.1073/pnas.79.9.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Harland R., Melton D. Transcription of tRNA genes in vivo: single-stranded compared to double-stranded templates. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4147–4151. doi: 10.1073/pnas.77.7.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese R., Melton D., Tranquilla T., Smith J. D. Cloning of nematode tRNA genes and their expression in the frog oocyte. Nucleic Acids Res. 1978 Dec;5(12):4593–4611. [PMC free article] [PubMed] [Google Scholar]
- Dörner M. H., Salfeld J., Will H., Leibold E. A., Vass J. K., Munro H. N. Structure of human ferritin light subunit messenger RNA: comparison with heavy subunit message and functional implications. Proc Natl Acad Sci U S A. 1985 May;82(10):3139–3143. doi: 10.1073/pnas.82.10.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gronenborn B., Messing J. Methylation of single-stranded DNA in vitro introduces new restriction endonuclease cleavage sites. Nature. 1978 Mar 23;272(5651):375–377. doi: 10.1038/272375a0. [DOI] [PubMed] [Google Scholar]
- Hu N., Messing J. The making of strand-specific M13 probes. Gene. 1982 Mar;17(3):271–277. doi: 10.1016/0378-1119(82)90143-3. [DOI] [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Koller B., Delius H., Bünemann H., Müller W. The isolation of DNA from agarose gels by electrophoretic elution onto malachite green-polyacrylamide columns. Gene. 1978 Nov;4(3):227–239. doi: 10.1016/0378-1119(78)90020-3. [DOI] [PubMed] [Google Scholar]
- Matzner Y., Hershko C., Polliack A., Konijn A. M., Izak G. Suppressive effect of ferritin on in vitro lymphocyte function. Br J Haematol. 1979 Jul;42(3):345–353. doi: 10.1111/j.1365-2141.1979.tb01142.x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Munro H. N., Linder M. C. Ferritin: structure, biosynthesis, and role in iron metabolism. Physiol Rev. 1978 Apr;58(2):317–396. doi: 10.1152/physrev.1978.58.2.317. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]