Abstract
The tetrazolium salts (MTT, XTT, MTS, WST) based colorimetric assay or resazurin based fluorimetric assay are currently typical methods for cell sensitivity determination to anticancer compounds. We presented here a new rapid method for this purpose. This method uses a fluorescent dye named DCFH-DA which is previously taken as a intracellular probe for measurement of H2O2 levels within a cell. The application basis for this method lies in two facts: the membrane permeable feature of the final metabolite of DCFH-DA inside a cell, and the linearity relationship between cell number and H2O2 level. The results showed that there was a perfect association between cell number and fluorescent intensity determined by the DCFH-DA method, no matter whether using resuspended or adherent cells, and further 50% concentration of inhibition (IC50) comparison between data obtained by DCFH-DA method or MTT method using a positive known anticancer compound Baicalin showed that there were no significant differences in cellular sensitivity determination to compound Baicalin though there existed a relatively higher coefficient of variation of IC50 by the DCFH-DA method than that by the MTT method. Thus our data indicate that DCFH-DA might not only be a fine reagent for determination of H2O2 levels in cells but also an ideal fluorescent dye for cellular sensitivity test of anti-cancer compounds, and may be suitable for primary high-throughput drugs screening.
Electronic supplementary material
The online version of this article (doi:10.1007/s10616-011-9423-0) contains supplementary material, which is available to authorized users.
Keywords: DCFH-DA, MTT, Anti-cancer compound, Cell sensitivity, Baicalin
Introduction
High throughput screening with simple, rapid and cost-saving measurement for cell sensitivity is an ideal tool for new drug discovery. Tetrazolium salts (MTT, XTT, MTS, WST) based colourimetric assay is the most typically used method for this purpose (Mosmann 1983; Scudiero et al. 1988; Cory et al. 1991; Ishiyama et al. 1996). This kind of assay involves the ability of viable cells to convert a soluble tetrazolium salt into purple-coloured formazan product whose optical density of the resulting solution could be measured on a multiwell scanning spectrophotometer. The standard incubation time for the assay needs at least 4 h and the result will become inaccurate when detecting reaction solution with higher optical density. Another popularized method for new compound screening is the resazurin based fluorimetric assay (Page et al. 1993; O’Brien et al. 2000). Resazurin is a redox dye commonly used as an indicator of chemical cytotoxicity in cultured cells and metabolically active cells have the ability to reduce blue and nonfluorescent resazurin to pink and highly fluorescent resorufin which is further reduced to uncoloured and nonfluorescent hydroresorufin. Though this dye presents numerous advantages over other cytotoxicity or proliferation tests, it still has several inherent drawbacks for the routine use: the incubation time still needs at least 4 h and easily produce nonfluorescent end-product.
We report here a new screening method for cellular sensitivity to anti-cancer compounds. This method is based on a fluorimetric assay using a dye called DCFH-DA which is widely used as an intracellular probe for direct measurement of the cell redox state (Tammariello et al. 2000; Ottonello et al. 2001). DCFH-DA is a compound readily diffusing into cells where it can be hydrolysed by intracellular esterases to cell membrane-impermeable and non-fluorescent 2′,7′-dichlorofluorescein (H2DCF) which does not permeate membranes, H2DCF is then oxidized by the intracellular H2O2 to a highly fluorescent compound DCF which is membrane permeable and can leak out of cells over time. Accumulation of DCF in cells or extracellular supernatant may be measured by an increase in fluorescence at 530 nm when the sample is excited at 485 nm. The fluorescent intensity is assumed to be proportional to the concentration of hydrogen peroxide in the cells (Bass et al. 1983; Royall and Ischiropoulos 1993).
Materials and methods
Fluorimetric assay of serial dilution of cells via DCFH-DA
A549 and MCF-7 cells in logarithmic growth phase were plated in a 10-cm tissue culture dishes with Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS), 100 unit/mL penicillin and 100 μg/mL streptomycin in a humid atmosphere of 5% CO2 at 37 °C. After reaching 100% confluence, these cells were trypsinized and suspended in PBS. After counted by haemocytometer, cells were added to a non-transparent white 96-well plate (Corning) by a serial dilution of cells in a total of 200 μL of PBS containing 25 μM of DCFH-DA (Sigma). The stock solution of DCFH-DA is at 5 mM in DMSO. Each serial dilution was performed in triplicate and a corresponding serial dilution in PBS without DCFH-DA dye were set as negative control. The blank controls were PBS plus DCFH-DA and PBS only, respectively. Completed 96-well plates were placed at 37 °C for incubation, and fluorimetric assay was executed at 30, 60, 90 and 120 min, respectively, using 485 nm excitation/530 nm emission wave length on a Varioskan Flash Multimode Microplate Reader (Thermo Scientific).
Fluorimetric assay of serial dilution of adherent cells via DCFH-DA
D407 cells line was used in this test. The procedures of cell preparation and the negative/blank controls setting were the same as described above. Serial dilutions of D407 cells were plated in two non-transparent white 96-well plates. After culturing in a humid atmosphere of 5% CO2 at 37 °C for 6 and 48 h, respectively, media were removed and 200 μL of PBS containing 25 μM of DCFH-DA (Sigma) was used for each corresponding well. The fluorimetric assays were carried out after incubation at 37 °C for 30 min.
Cellular growth inhibition test by DCFH-DA based fluorimetric assay versus MTT-Microculture Tetrazolium Assay
A549 and D407 cell lines were used in this part of experiments and Baicalin (Sigma) was used as a known positive compound which could inhibit cell growth. Rapidly growing A549 and D407 cells were harvested, counted, and inoculated at the appropriate concentrations (200-μL volume) into 96-well microtiter plates using a multichannel pipet, respectively. Non-transparent white plates were used for fluorimetric assay. After 24 h, serial dilutions of Baicalin were applied to triplicate culture wells, and cultures were incubated for further 24 h at 37 °C. The stock solution of Baicalin was 10 mg/mL in DMSO and stored at −20 °C. DCFH-DA based fluorimetric assays were carried out as described above. MTT (Sigma) assays were performed as previously described elsewhere (Mosmann 1983). In brief, MTT was prepared at 5 mg/mL in PBS and stored at 4 °C, on test day, MTT was diluted 1:5 in medium without serum, and 200 μL were added to microculture wells. Negative controls and blank controls were also included in each experiment. After 4-h incubation at 37 °C under 5% CO2 in a humidified incubator, the 200 μL were removed from each well, and 150 μL of 100% DMSO were added to solubilize the MTT-formazan product. After thorough dissolving at room temperature, absorbance at 490 nm was measured with a Varioskan Flash Multimode Microplate Reader (Thermo Scientific). Each experiment was repeated three times.
Statistical Analysis
Results were expressed as means ± standard errors of three determinations. Linear correlation and regression analysis and Student’s t test were carried out by Microsoft Excel, Office 2003. 50% concentration of inhibition (IC50) calculations were analysed by the profit program using SPSS 11.5 software. Statistically significant difference was considered at p < 0.05.
Results
Linear relationship of increasing fluorescent intensity with increase in detached cell number determined by DCFH-DA based fluorimetric assay
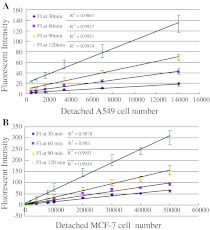
Serial dilutions of A549 cells in triplacte were generated by adding 1, 2, 4, 8, 16 and 32 μL of cells at a concentration of 4.4 × 105/mL in PBS to wells, presenting 440, 880, 1,760, 3,520, 7,040 and 14,080 cells per well, respectively. The final volume of each well was 200 μL of PBS. A similar dilution in PBS in triplicate was prepared for MCF-7 cells at the following cell numbers: 1 × 102, 2 × 102, 4 × 102, 8 × 102, 2 × 103, 4 × 103, 8 × 103, 1 × 104, 2 × 103, 3 × 104, 4 × 104 and 5 × 104, respectively. The value of net fluorescence intensity (FI) = FI(ex) − FI(neg) − FI(blk) + FI(pbs) in which FI(ex) indicates the mean value of fluorescence intensity in the experimental group, and FI(neg) the mean value of fluorescence intensity in negative control, FI(blk) the mean value of fluorescence intensity in PBS and DCFH-DA blank control, FI(pbs) the mean value of fluorescence intensity in PBS blank control. Consistent increases in fluorescent intensity with increased A549 or MCF-7 cell number per well, were observed after 30, 60, 90, or 120 min incubation, respectively. The linearized relationship between detached cells number and fluorescent intensity was visible even at the lowest number of 100 cells per well or at the highest number of 5 × 104 cells per well, and the fluorescent intensity began to increase rapidly over 90 min incubation. Data are presented in Fig. 1.
Fig. 1.
Linear relationship of increasing fluorescent intensity with increase in detached cell number determined by DCFH-DA based fluorimetric assay. a 1, 2, 4, 8, 16, 32 μL of A549 cells at a 4.4 × 105/mL concentration in PBS were added in triplicate that resulted in a serial dilution of 440, 880, 1,760, 3,520, 7,040, 14,080 cells per well; b a serial dilution was prepared at 1 × 102, 2 × 102, 4 × 102, 8 × 102, 2 × 103, 4 × 103, 8 × 103, 1 × 104, 2 × 104, 3 × 104, 4 × 104 and 5 × 104 MCF-7 cells in PBS with three repeats. FI fluorescent Intensity
Linear relationship of increasing fluorescent intensity with increase in adherent cell number determined by DCFH-DA based fluorimetric assay
D407 cells were plated at 2 × 102, 4 × 102, 8 × 102, 1.6 × 103, 3.2 × 103, 6.4 × 103, 1.28 × 104, 2.5 × 104 cells, respectively, in triplicate per culture wells. The value of fluorescent intensity was calculated as described above, Increases in fluorescence intensity consistent with increasing cell number plated per well was also observed, no matter if culturing for 6 h or for 48 h. The linear relationship between fluorescent intensity and cell number was obtained after incubation for only 30 min, even at the lowest number of 200 plating cells per well or at the highest number of 2.5 × 104 plated cells per well. Cell proliferation during 48 h culturing was also observed, indicating that this kind of method may be practicable in cell doubling time calculation. Data are presented in Fig. 2.
Fig. 2.
Linear relationship of increasing fluorescence intensity with the increase in the number of adherent D407 cells determined by the DCFH-DA based fluorimetric assay. D407 cells were plated at 2 × 102, 4 × 102, 8 × 102, 1.6 × 103, 3.2 × 103, 6.4 × 103, 1.28 × 104, 2.5 × 104 cells in triplicate per culture wells. FI fluorescent intensity
Comparison of DCFH-DA based fluorimetric assay versus MTT- microculture tetrazolium assay in detecting ability for cellular sensitivity to anti-cancer compounds
2 × 103 A549 cells or D407 cells in 200 μL medium were inoculated into each well, respectively. The known anticancer compound Baicalin was applied to these two cell lines using a serial dilution of 1, 3, 9, 27 and 81 μg/mL in culture wells with three independent experimental repeats. With increase of the Baicalin concentration, the inhibition rate determined by the DCFH-DA method was increased in parallel with that determined by the MTT method, no matter whether using A549 cells or using D407 cells in the experiments (Fig. 3). But A549 cells growth inhibition determined by DCFH-DA method were significantly higher with Baicalin concentration at 1 μg/mL or 3 μg/mL while significantly lower at 27 μg/mL or 81 μg/mL in comparison to that determined by MTT method (Fig. 3a). This phenomenon was similarly observed in D407 cells, though there were no significant differences except for Baicalin concentration at 1 μg/mL (Fig. 3b). Nevertheless, the 50% inhibition concentration (IC50) of Baicalin calculated from three independent experiments between the DCFH-DA method and the MTT method was not significantly different, either for A549 cells or for D407 cells, though the mean value of IC50 calculated from data determined by the MTT method was lower than that from data determined by the DCFH-DA method, respectively (Table 1). The coefficient of variation (CV) for IC50 obtained for the DCFH-DA method, either for A549 cells or D407 cells, was higher than that obtained for the MTT method (0.225 vs. 0.144, 0.244 vs. 0.08, respectively), indicating that the DCFH-DA method may be selected for use in primary screening experiments though not fit for IC50 calculation due to its relatively broad variation in comparison with the MTT method.
Fig. 3.
Comparison of DCFH-DA based fluorimetric assay versus MTT based colorimetric assay in detecting cellular sensitivity to anticancer compounds. a compounds of inhibition rate using A549 cells; b comparison of inhibition rate using D407 cells
Table 1.
Comparison of 50% inhibition concentration (IC50) calculated from data determined by the DCFH-DA and the MTT method
| IC50 by DCFH-DA method (n = 3) | IC50 by MTT method (n = 3) | |
|---|---|---|
| A549 cells | 22.84 ± 5.15 μg/mL | 18.97 ± 2.74 μg/mL |
| D407 cells | 31.11 ± 7.60 μg/mL | 22.95 ± 1.86 μg/mL |
Discussion
Initially DCFH-DA was used for the detection of reactive oxygen species, especially the H2O2 levels inside cells (Hockenbery et al. 1993; Zamzami et al. 1995). We found in our pre-experiment that the final metabolite of DCFH-DA in cells may permeate and may leak out of the cells, this finding which was also reported by Royall and Ischiropoulos (1993) and Halliwell and Whitemann (2004) sheds light on a new application of DCFH-DA for cell proliferation and cytotoxicity detection. The data presented here show that DCFH-DA is not only a fine reagent for determination of H2O2 level in cells but also an ideal fluorescent dye for use in a cellular sensitivity test to anti-cancer compounds, and may be suitable for primary drugs screening. Each single cell maintains a certain level of H2O2 concentration which forms a substantial basis of application for this method (Wood et al. 2003; Rhee et al. 2010). The higher the cell number is, the higher is the total H2O2 level; the linear relationship between them is theoretically and practically proven by logical reasoning and the experimental data (Figs. 1, 2).
The principles of application between DCFH-DA based fluorimetric assay and Tetrazolium salts (MTT, XTT, MTS, WST) based colourimetric assay or resazurin based fluorimetric assay are totally different. Concerning MTT-like methods, the yellow tetrazolium salts are reduced to form a water-insoluble (for MTT) or soluble (for XTT, MT, WST) crystallized purple formazan via the cleavage of the tetrazolium ring by the succinate dehydrogenase within the mitochondria (Mosmann 1983; Twentyman and Luscombe 1987; Scudiero et al. 1988; Cory et al. 1991; Ishiyama et al. 1996; Goodwin et al. 1995; Marshall et al. 1995). Enzymes that may be involved in resazurin reduction are also various dehydrogenases of mitochondria, cytosol and microsomes (Page et al. 1993; O’Brien et al. 2000; Gonzalez and Tarloff 2001). Thus both tetrazolium salts and resazurin based assays are readily affected by these enzymes activity. As for the new method, though the DCFH-DA needs to be hydrolysed by intracellular esterase once diffusing into cells, the production of the final fluorescent substance is determined by the oxidation of H2O2 whose level is proportional to the cell number (Bass et al. 1983; Royall and Ischiropoulos 1993).
So the prominent feature of DCFH-DA method is the perfect linearity between the cell number and the fluorescence intensity. The feature is consistently presented from a cell density ranging from 500 cells/mL to 2.5 × 105 cells/mL (Figs. 1, 2), even for an overnight incubation (data not shown). The final concentration of DCFH-DA was 25 μM in this study. This concentration was the standard concentration used elsewhere for the determination of intracellular H2O2 levels (Hockenbery et al. 1993; Zamzami et al. 1995). This new application of DCFH-DA enabling the use of a broad range of plating cell densities for culture initiation can be applied for the observation of cell growth affected by compounds to be assessed. The tetrazolium salt method usually leads to inaccurate reading when the plated cell numbers are too high because of exceeding the measurement limit of the photometer used (Marshall et al. 1995).
However there are two problems when applying this method in practices. Firstly, DCFH-DA diffusing into cells at a concentration of 25 µM might be toxic to the cells since we have observed the phenomenon that most of the adherent cells turned round in situ when incubated with DCFH-DA for 2 h, and were unable to keep proliferating in further culture (data not shown). Secondly, the level of H2O2 determined by the DCFH-DA method might fluctuate slightly between the same number of cells with drug function and cells without drug function if the screened compound has an antioxidative effect. Baicalin, for example, an anticancer compound used in this study, is a potent antioxidant primarily extracted from a kind of chinese herb called Scutellaria baicalensis in Latin (Gao et al. 2011; Takahashi et al. 2011). It is a reactive oxygen species scavenger when the concentration of Baicalin in the medium is at low level, while it turns to be a cytotoxic reagent by dramatically increasing intracellular level of reactive oxygen species when the concentration of Baicalin in the medium is at high levels (Du et al. 2010). The result of the former situation will lead to decreased level of H2O2 detected than that of the same cells without Baicalin functioning (control group), while the result of the latter will lead to increased level of H2O2 detected than that of actuals (control group). Even so, A549 cells and D407 cells sensitivity to the anticancer compound Baicalin determined by these two methods were not significantly different (Table 1), though the coefficient of variation of the DCFH-DA method was relatively higher in comparison with that of the MTT method.
In brief, DCFH-DA has many advantages over other techniques developed. It is very easy to use, extremely sensitive to changes in cell numbers, inexpensive and can be used to follow changes in cell proliferation or cytotoxicity over time, thus could be used in cellular sensitivity determination to primary high-throughput screening of drugs.
Electronic supplementary material
Acknowledgments
The authors are grateful to Dr. Dawei Li’s lab, Shanghai Jiaotong University, for providing the Varioskan Flash Multimode Microplate Reader.
Glossary
- MTT
3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide
- XTT
3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzenesulfonic acid hydrate
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- WST-1
4-(3-4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio)-1,3-benzenedisulfonate
- DCFH-DA
2′,7′-dichlorfluorescein-diacetate
- H2DCF
2′,7′-dichlorodihydrofluorescin
- DCF
2′,7′-dichlorofluorescein
- DMSO
Dimethyl sulfoxide
- PBS
Phosphate-buffered saline
References
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130:1910. [PubMed] [Google Scholar]
- Cory AH, Owen TC, Barltrop JA, Cory JG. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Du G, Han G, Zhang S, Lin H, Wu X, Wang M, Ji L, Lu L, Yu L, Liang W. Baicalin suppresses lung carcinoma and lung metastasis by SOD mimic and HIF-1alpha inhibition. Eur J Pharmacol. 2010;630:121–130. doi: 10.1016/j.ejphar.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Gao J, Morgan WA, Sanchez-Medina A, Corcoran O. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including upregulation of p53 and Bax in human lung cancer cells. Toxicol Appl Pharmacol. 2011;254:221–228. doi: 10.1016/j.taap.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez RJ, Tarloff JB. Evaluation of hepatic subcellular fractions for Alamar blue and MTT reductase activity. Toxicol In Vitro. 2001;15:257–259. doi: 10.1016/S0887-2333(01)00014-5. [DOI] [PubMed] [Google Scholar]
- Goodwin CJ, Holt SJ, Downes S, Marshall NJ. Microculture tetrazolium assays: a comparison between two new tetrazolium salts, XTT and MTS. J Immunol Methods. 1995;179:95–103. doi: 10.1016/0022-1759(94)00277-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whitemann M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and do the results mean? Br J Pharmacol. 2004;142:231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518–1520. doi: 10.1248/bpb.19.1518. [DOI] [PubMed] [Google Scholar]
- Marshall NJ, Goodwin CJ, Holt SJ. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995;5:69–84. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000;267:5421–5426. doi: 10.1046/j.1432-1327.2000.01606.x. [DOI] [PubMed] [Google Scholar]
- Ottonello L, Frumento G, Arduino N, Dapino P, Tortolina G, Dallegri F. Immune complex stimulation of neutrophil apoptosis: investigating the involvement of oxidative and nonoxidative pathways. Free Radic Biol Med. 2001;30:161–169. doi: 10.1016/S0891-5849(00)00453-6. [DOI] [PubMed] [Google Scholar]
- Page B, Page M, Noel C. A new fluorometric assay for cytotoxicity measurements in vitro. Int J Oncol. 1993;3:473–476. [PubMed] [Google Scholar]
- Rhee SG, Chang TS, Jeong W, Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol Cells. 2010;29:539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- Royall JA, Ischiropoulos H. Evaluation of 2, 7-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, Hines OJ, Gukovskaya AS, Go VL, Eibl G. Baicalein a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochim Biophys Acta. 2011;1813:1465–1474. doi: 10.1016/j.bbamcr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammariello SP, Quinn MT, Estus S (2000) NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci 20:RC53 [DOI] [PMC free article] [PubMed]
- Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56:279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere JL, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.