Abstract
This study proposes an easy to use in situ device, based on multi-frequency permittivity measurements, to monitor the growth and death of attached Vero cells cultivated on microporous microcarriers, without any cell sampling. Vero cell densities were on-line quantified up to 106 cell mL−1. Some parameters which could potentially impact Vero cell morphological and physiological states were assessed through different culture operating conditions, such as media formulation or medium feed-harvest during cell growth phase. A new method of in situ cell death detection with dielectric spectroscopy was also successfully implemented. Thus, through permittivity frequency scanning, major rises of the apoptotic cell population in bioreactor cultures were detected by monitoring the characteristic frequency of the cell population, fc, which is one of the culture dielectric parameters. Both cell density quantification and cell apoptosis detection are strategic information in cell-based production processes as they are involved in major events of the process, such as scale-up or choice of the viral infection conditions. This new application of dielectric spectroscopy to adherent cell culture processes makes it a very promising tool for risk-mitigation strategy in industrial processes. Therefore, our results contribute to the development of Process Analytical Technology in cell-based industrial processes.
Keywords: Adherent Vero cells, In situ monitoring, Multi-frequency permittivity, Cell density, Apoptosis
Introduction
In the past decade, regulation agencies have encouraged the implementation of new on-line analytical techniques to monitor biotechnological processes. These directives, as the “Process Analytical Technology” (PAT) guidance of the Food and Drug Administration (FDA), have the goal to favour a better understanding of the production processes, to develop the monitoring of the critical parameters and thus, to ensure the final product quality (Mandenius et al. 2009). The animal cell culture processes are particularly concerned by these directives. Indeed, they are mainly implemented for production of therapeutic recombinant proteins or viral vaccines, which are highly sensitive productions (Merten 2000; Knezevic et al. 2008; Liu et al. 2007). Among the parameters which could be strategic for a cell-based process, the cell growth (i.e., viable cell concentration evolution over time) might be one of the most important to monitor in real-time. This is especially the case for viral vaccine productions where the cell density is essential to define the time and multiplicity of viral infection (TOI and MOI) (Le Ru et al. 2010; Al-Rubeai 1998; Souza et al. 2007). Besides, the traditional off-line numeration methods are very time-consuming and operator-dependant.
Numerous techniques for the on-line quantification of animal cell density were proposed over the past thirty years. Indirect methods employed conventional sensors to estimate the cell density from oxygen uptake rate (OUR), carbon dioxide evolution rate (CER) or ATP production rate (APR) (Kamen et al. 1996; Eyer and Heinzle 1996; Zeiser et al. 2000; Konstantinov et al. 1994). Nevertheless, they were based on the hypothesis of a constant correlation between cell density and OUR, CER or APR in the culture. Besides, direct methods based on viable cell density estimation from physical measurements were also implemented. Thus we could mention the nuclear magnetic resonance spectroscopy (NMR) (Bradamante et al. 2004), the acoustic resonance densitometry (ARD) (Kilburn et al. 1989), the absorbance or scattering (Card et al. 2008), the fluorescence (Teixeira et al. 2009), the dielectric spectroscopy (Ansorge et al. 2010) or the real-time imaging (Rudolph et al. 2008) which were used for this purpose. Only few of these methods are now commercialized, most of them being difficult to set up or presenting a poor sensitivity (Konstantinov et al. 1994). Moreover, they have generally been developed for suspension cell cultures. To our knowledge, only three teams reported the on-line monitoring of adherent and/or immobilized cells: hybridoma and CHO cells with dielectric spectroscopy (Noll and Biselli 1998; Ducommun et al. 2002) and fibroblasts with real-time imaging (Rudolph et al. 2008). Therefore, the cell density remains one of the most challenging parameter to be on-line monitored in microcarrier cell cultures.
Reviewing the on-line methods demonstrates that, while the cell growth was widely studied mainly during the exponential growth phase, the cell death detection, quantification or characterization were poorly considered. However, real-time information on cell death could definitely be of interest to better understand and control the cell bioprocesses, especially to manage the critical steps of production i.e., infection time or scale-up. The on-line cell death detection also could help to identify cell stressing operating conditions or to anticipate process failures. Despite the fact that animal cell death has been widely off-line studied, no method has been yet proposed for its real-time in situ detection. Off-line methods mainly focus on the morphological or biochemical characteristics of dead cells, which allow differentiating the two mechanisms of cell death occurring in bioprocesses: necrosis and apoptosis. Necrosis is mainly induced by extreme culture conditions which damage cellular membranes and provoke cell lysis (Szabo et al. 2004; Shah et al. 2006). On the contrary, apoptosis is a part of the cell response to non lethal stress, and results in cell membrane reorganization and cell fragmentation in small apoptotic bodies (Al-Rubeai 1998; Ishaque and Al-Rubeai 1998; Figueroa et al. 2004; Schulze-Horsel et al. 2009).
Among the analytical techniques available nowadays to on-line monitor, cell growth, cell death or cell morphology, dielectric spectroscopy could be a very promising method. Moreover, sterilizable probe could be implemented to apply dielectric spectroscopy through permittivity measurements in the bioreactor. In opposition to other methods based on cell physiological state, the dielectric spectroscopy directly evaluates the volume fraction of cells displaying intact plasma membranes, also called biovolume (Ducommun et al. 2002; Cannizzaro et al. 2003). The measured permittivity is related to the biovolume through an empirical model initially developed for spherical cells (Schwan 1957). Thus, in several studies, the viable cell concentration was assessed by considering a constant cell size all over the culture (Zeiser et al. 1999). Dielectric spectroscopy has been used for the in situ concentration monitoring of insect cell, such as Sf-9 cells or High-5 cells (Zeiser et al. 1999, 2000), and of mammalian cells, such as CHO, HEK293 or hybridoma cells (Ansorge et al. 2010; Ducommun et al. 2001, 2002; Cannizzaro et al. 2003; Siano 1997; Opel et al. 2010). It has been implemented in batch and fed-batch cultures, as well as in packed bed reactors. This method has also been dedicated to monitor production levels of viruses (lentivirus, baculovirus) or proteins, but also evolution of cell size or of processes (Zeiser et al. 2000; Ansorge et al. 2010, 2011; Cannizzaro et al. 2003). A single group applied this method to adherent CHO cells cultivated on macroporous microcarriers (Ducommun et al. 2001, 2002). Moreover, while the dielectric characteristics of dead cells have already been studied off-line by using various methods (electrorotation, dielectrophoresis…) (Wang et al. 2002; Labeed et al. 2006; Patel and Markx 2008), the on-line dielectric spectroscopy has not yet been proposed for quantification or detection of animal cell death.
The Vero cell line, derived from epithelial kidney cells of the African Green Monkey and cultivated on microporous microcarriers at large scale, is one of the main industrial animal cell platform used for the manufacturing of viral vaccines (Liu et al. 2007; Souza et al. 2009; Kistner et al. 1998; Rourou et al. 2007; Toriniwa and Komiya 2007). However, until now, no research paper has reported the on-line real-time monitoring of adherent Vero cell density and death.
In this work, we present the potential of dielectric spectroscopy for in situ monitoring bioreactor cultures of Vero cells attached on microporous microcarriers. We have evaluated the influence of various culture operating conditions, including medium feed-harvest or medium formulation, which are known to impact cell physiology and consequently real-time quantification of Vero cell density. Moreover, we propose to use the on-line dielectric spectroscopy as a new in situ method for the detection of apparition of cell apoptosis.
Theory
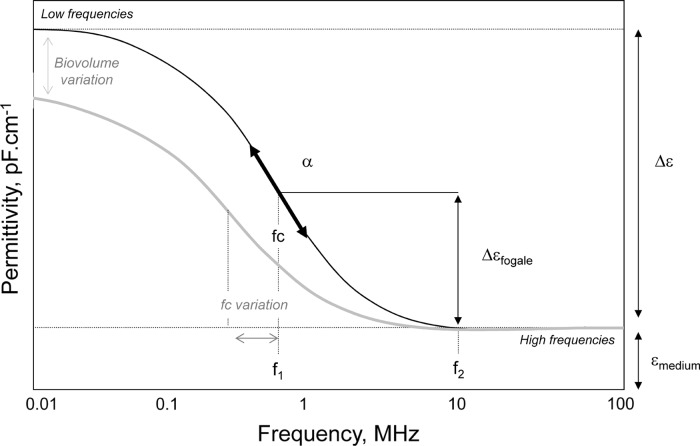
The in situ monitoring of the cell density with the dielectric spectroscopy exploits the cell ability to be polarized by an electrical field. The magnitude of the cell polarization, and more specifically of the membrane polarization, is measured as the permittivity (Δε). If cells are polarized by increasing frequencies from 0.01 to 10 MHz, a drop of the permittivity is observed (Fig. 1). This drop is called the β-dispersion and was described through an empirical model established for spherical cells (Eq. 1) (Schwan 1957). The permittivity increment depends on the volume fraction of cells in the culture medium, called biovolume, (P) (Eq. 2), the cell size (r) and the state of cellular membrane represented by the membrane capacitance (CM) (Markx and Davey 1999). The cell density is then calculated from the biovolume, considering the cells as a spherical structure with a constant radius (Eq. 3).
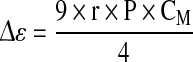 |
1 |
 |
2 |
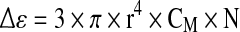 |
3 |
with: Δε: permittivity (F m−1), r: cell radius (m), 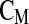 : cell membrane capacitance (F m−2), P: volume fraction of cells in the culture medium (%), N: cell density (cell m−3).
: cell membrane capacitance (F m−2), P: volume fraction of cells in the culture medium (%), N: cell density (cell m−3).
Fig. 1.
Schematical representation of the β-dispersion of permittivity. β-dispersion and variation of its characteristic parameters, Δε, fc and α are presented. The determinations of Δεfogale as well as the influence of a variation of cell characteristics or biovolume on the β-dispersion are also presented
According to the work of Schwan (1957), the β-dispersion can be characterized by two other parameters specific of each cell population: the characteristic frequency (fc), and the empirical factor (α) (Fig. 1). The frequency fc depends on the cell size (r), the cell membrane capacitance (CM), and both conductivities of the medium (σm) and of the cell cytoplasm (σi) (Eq. 4). In general, the intracellular conductivity (si) is considered as negligible (2–4 mS cm−1) in comparison to the culture medium conductivity (15–20 mS cm−1) (Cannizzaro et al. 2003). Thus, Eq. 4 could be reduced to Eq. 5 by suppressing the term 1/2 × σm.
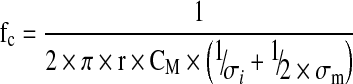 |
4 |
 |
5 |
with: fc: characteristic frequency (Hz), σι: intracellular conductivity (mS cm−1), σm: medium conductivity (mS cm−1).
Materials and methods
Cell culture
The Vero cell line was provided by Sanofi Pasteur (Marcy L’Etoile, France) from a cell bank previously adapted to grow in serum-free conditions. Cells were cultivated in two serum-free culture media. The first one, called reference medium, MR, has been described in a previous work Petiot et al. (2010a). This medium contained 4 mM of glutamine and 22 mM of glucose. The second one, called modified medium, MM, was based on the reference medium in which glutamine was substituted by a same amount of glutamax® (alanine-glutamine dipeptide) (Sigma, USA). Cell expansion was performed in 175 cm2 culture T-flasks (Fisher Bioblock Scientific, France) and on Cytodex 1 microcarriers (GE Healthcare Bioscience-AB, Sweden) in 250 mL spinner flasks, with seeding cell concentrations of 3.2 and 2.75 × 105 cell mL−1, respectively. These cultures were performed in an incubator regulated at 37 °C and 5% CO2 and the spinner flasks were stirred at 45 rpm (Techne, UK). A 2-L bioreactor (Pierre Guérin, France) was set up for the on-line permittivity measurements with an initial cell concentration of 2.75 × 105 cell mL−1. Cultures in bioreactor were controlled at 7.2 pH units, 37 °C and 25% dissolved oxygen. The stirring speed was set at 90 rpm. For a medium exchange, scheduled after 2 days of culture, the agitation was stopped during 20 min to allow the microcarrier settling before the harvest of 80% of the culture supernatant. The bioreactor was then replenished to its original volume with fresh medium pre-warmed at 37 °C. Bioreactor cultures were repeated at least twice and displayed very similar results.
Off-line quantification of cell populations
Adhered and suspension cells
For the numeration of adhered Vero cells, the settled microcarriers of 4 mL culture samples were washed twice with PBS and treated with Crystal Violet solution (Sigma, France) at 37 °C for at least 1 h prior numeration of the released nuclei on a Fuchs-Rosenthal hemacytometer (Preciss, France). The cells in suspension, either viable or necrotic, were evaluated in the culture supernatant with a Trypan blue dye exclusion numeration.
Lysed cells
The lysed cells were quantified in culture supernatant by the analysis of the lactate dehydrogenase (LDH) activity according to the protocol previously described Petiot et al. (2010b). The activity of the LDH released in the cell culture supernatant was analyzed with the enzymatic LDH PAP kit (Ellitech, Salon-de-Provence, France). The intracellular LDH content of viable Vero cells was determined in 90% viability cells sampled in exponential growth phase of a spinner flask culture. This procedure allowed to observe 1.33 × 10−6 LDH Units cell−1.
Apoptotic and necrotic cells
The proportion of apoptotic and necrotic cells was quantified by using the Nexin V kit (Guava Technologies, US). This kit enables to detect the cell apoptosis through the labelling of phosphatidyl-serines translocated at the cell membrane with annexin V coupled to a PE fluorochrome. It also detects porous cells with the fluorescent 7-AAD DNA label allowing the determination of necrotic cell population. Thus, twice a day, 1 mL sample was carefully trypsinized to avoid cell damages. Detached cells were diluted in culture medium to reach cell densities between 1 × 104 and 5 × 105 cell mL−1. 2% BSA were added to the samples to limit non-specific labelling before an half dilution in the Nexin-V reagent. Then, cells were analysed after 20 min of incubation at room temperature by the Guava Easycyte cytometer and data were processed with the Express Pro software (Guava Technologies, US).
In situ permittivity measurements
The cell culture permittivity was measured using the sterilizable Fogale Biomass System® (Fogale Nanotech, France) implemented on the 2-L bioreactor. The measurement method has been well-described by (Ansorge et al. 2007). Briefly, the relative permittivity signal  corresponds to the magnitude of permittivity between a high frequency f2 at 10 MHz and a working frequency f1 (Fig. 1), the working frequency depending on the cell line used. For adherent Vero cells, the f1 frequency was set at 382 kHz as recommended by the manufacturer. It was possible to consider here the
corresponds to the magnitude of permittivity between a high frequency f2 at 10 MHz and a working frequency f1 (Fig. 1), the working frequency depending on the cell line used. For adherent Vero cells, the f1 frequency was set at 382 kHz as recommended by the manufacturer. It was possible to consider here the 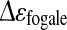 evolution as similar to Δε evolution since the frequency of measure, f1, was very close to the lowest frequency used for Δε calculation (382 and 300 kHz respectively). This particularity allowed to calculate a new parameter called specific permittivity (permittivity per cell, εx), described in Eq. 6.
evolution as similar to Δε evolution since the frequency of measure, f1, was very close to the lowest frequency used for Δε calculation (382 and 300 kHz respectively). This particularity allowed to calculate a new parameter called specific permittivity (permittivity per cell, εx), described in Eq. 6.
 |
6 |
Measurements were performed every 6 min and the baseline was set by recording the permittivity of the medium containing cell-free microcarriers, prior to cell seeding. An additional frequency scan module was provided by Fogale Nanotech, allowing to acquire permittivity at 20 fixed frequencies, ranging from 300 kHz to 10 MHz. Data processing of the multi-frequency scanning acquisitions with a dedicated software allowed the determination of Δε and fc parameters (Fig. 1). With the same device, the medium conductivity was also acquired on-line during every culture to control that its variation was negligible.
Results and discussion
In situ monitoring of adhered Vero cell density
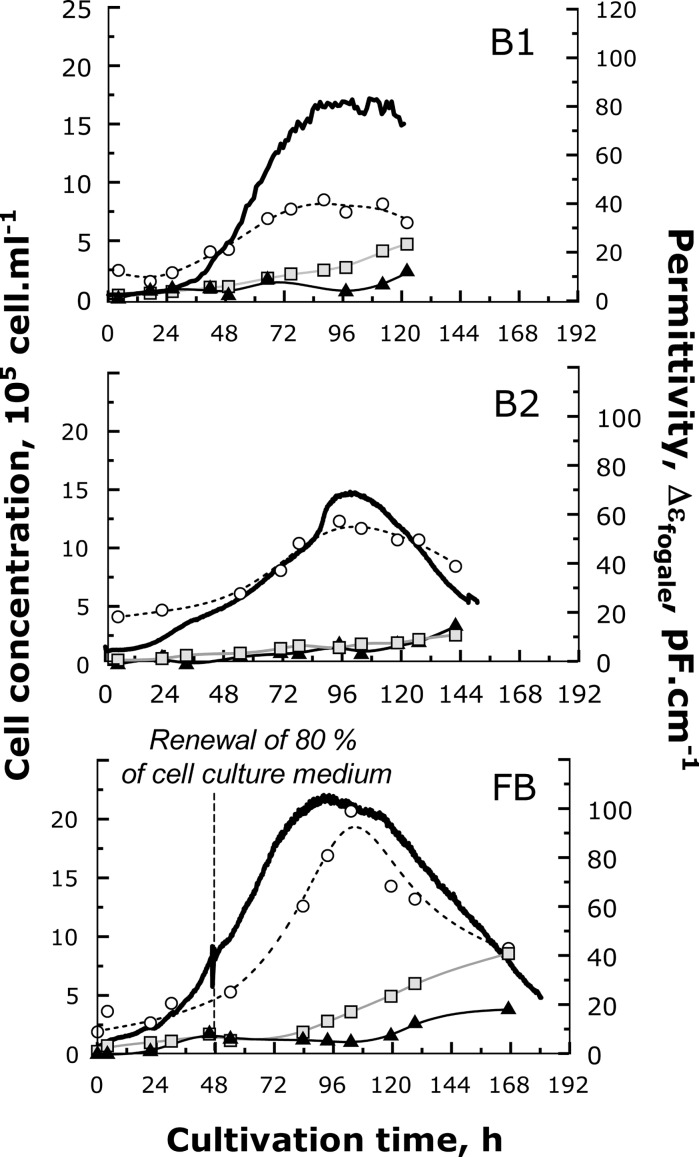
The objective of this part was to demonstrate that Vero cell growth could be easily monitored through real-time permittivity measurements. Three Vero cell cultures were performed in bioreactors with different process operating conditions. The first two cultures were performed in batch mode. Two serum-free media were tested, respectively the reference medium (MR) in Batch 1 (B1), and the modified reference medium (MM) in Batch 2 (B2). A third culture, carried out with MR medium, included a 80% renewal of the culture supernatant after 48 h of culture (FB). All these experiments were in situ monitored with the Fogale Biomass system® while the different cell populations, either adhered or in suspension, were off-line analysed. The evolutions with time of the permittivity measurement  as well as the cell population concentrations (viable, apoptotic and lysed cells) are presented in Fig. 2. It has to be precised that no necrotic cells were detected either on microcarriers or in culture supernatant. Besides, the jump observed in the permittivity at 48 h in FB culture resulted from the medium renewal process, while the probe was staying outside the culture medium for few minutes. So, this shift mainly corresponded to an artefact in the permittivity measurement which has not to be considered. The actual variation of the conductivity measured in the bioreactor due to medium renewal was no more than 0.8 mS cm−1. This is negligible in comparison to the mean value of the medium conductivity measured along the different processes (19.5, 20.5 and 23.5 mS cm−1 for FB, B1 and B2 cultures, respectively).
as well as the cell population concentrations (viable, apoptotic and lysed cells) are presented in Fig. 2. It has to be precised that no necrotic cells were detected either on microcarriers or in culture supernatant. Besides, the jump observed in the permittivity at 48 h in FB culture resulted from the medium renewal process, while the probe was staying outside the culture medium for few minutes. So, this shift mainly corresponded to an artefact in the permittivity measurement which has not to be considered. The actual variation of the conductivity measured in the bioreactor due to medium renewal was no more than 0.8 mS cm−1. This is negligible in comparison to the mean value of the medium conductivity measured along the different processes (19.5, 20.5 and 23.5 mS cm−1 for FB, B1 and B2 cultures, respectively).
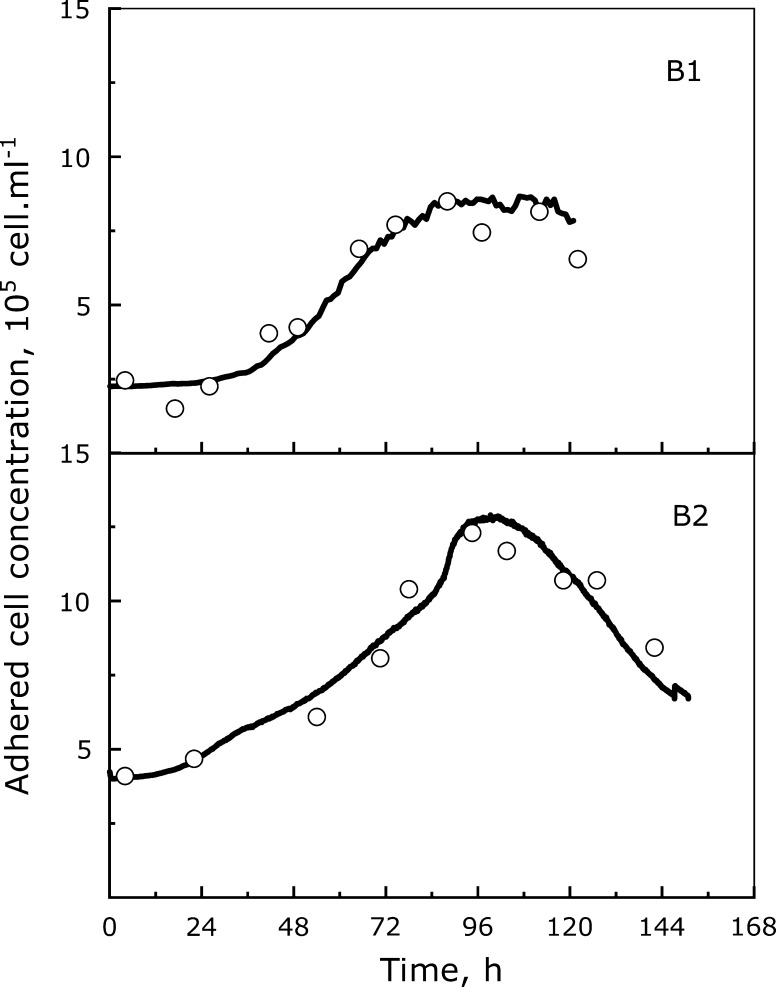
Fig. 2.
Evolution with time of cell populations and on-line permittivity. Viable (open circle), apoptotic (black triangle) and lysed (gray square) Vero cells during batch cultures performed in serum-free conditions with (FB) or without (B1 and B2) medium renewal with in-line permittivity acquisition (continuous black lines). Cultures B1 and FB were performed in MR medium while B2, was performed in MM medium
Adhered cells monitored by permittivity measurements
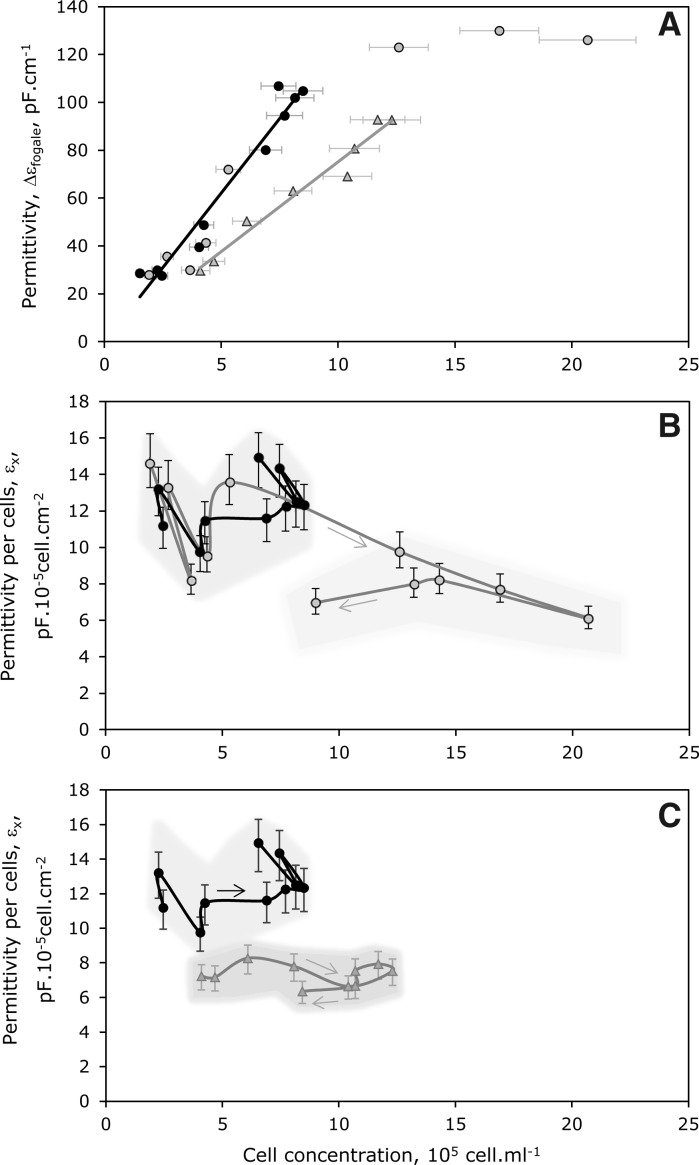
First, permittivity curve profiles were very close to adhered cell growth profiles, leading to linear correlation coefficients (r2) between these two parameters above 0.95, for each culture conditions (Figs. 2, 3a). The cultures performed in reference medium, with (FB) or without (B1) medium renewal, presented a similar correlation (Eq. 7) while, the culture carried out with glutamine-substituted medium MM (B2), displayed a different slope coefficient (Eq. 8). Moreover, the correlation obtained in reference medium was only reliable for cell densities lower than 1 × 106 cell mL−1, whereas MM correlation was valid until a cell density of 1.5 × 106 cell mL−1 (Eq. 8).
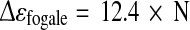 |
7 |
 |
8 |
Fig. 3.
Evolution of the permittivity 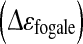 and the specific permittivity, εX, with the adhered Vero cell concentration. A Correlation between permittivity
and the specific permittivity, εX, with the adhered Vero cell concentration. A Correlation between permittivity  and concentration of Vero cells attached on microcarriers, during Batch 1 (B1: black circle), Batch 2 (B2: gray triangle) and Batch with 80% of medium renewal (FB: gray circle). B and C Evolution of specific permittivity, εX, corresponding to permittivity values acquired per cells for B1 (black circle), B2 (grey triangle) and FB (gray circle). The grey area represents the variability of εX for each culture
and concentration of Vero cells attached on microcarriers, during Batch 1 (B1: black circle), Batch 2 (B2: gray triangle) and Batch with 80% of medium renewal (FB: gray circle). B and C Evolution of specific permittivity, εX, corresponding to permittivity values acquired per cells for B1 (black circle), B2 (grey triangle) and FB (gray circle). The grey area represents the variability of εX for each culture
These results suggest that some modifications of the dielectric properties of Vero cells may occur either depending on the culture medium formulation or on the cell density. In the following parts, we have chosen to separately study the influence of these two parameters.
Cell biovolume evolution
Regarding the FB culture, the linear correlation was no longer valid for cell densities higher than 106 cell mL−1 after medium renewal (Fig. 3A). Additionally, different evolutions of the cell specific permittivity (εx) could be observed between batch and fed-batch cultures. In the case of the batch processes, despite an erratic behaviour due to cell count variability, the value of εx remained in the same range all over the cultures (Fig. 3C). On the contrary, a progressive decrease of the specific permittivity could be identified in the fed-batch culture when cell density increased above 106 cell mL−1 (Fig. 3B).
This progressive decrease of εx in FB culture suggested some changes in cell dielectric properties resulting from morphological or physiological evolution of Vero cells. Indeed, going back to the Eq. 6, based on empirical model of the global permittivity (Δε), the specific permittivity could be influenced either by cell size (r) or cell membrane capacitance (CM). A variation of cell size might have a stronger impact than CM change, due to the fact that the cell radius is expressed to the power four. These two cell parameters are difficult to assess in the case of adherent cells. CM is usually measured with very specific dedicated tools as electro-rotation (Pethig et al. 2005) or electrophysiology (Gentet et al. 2000). Measurements are performed on single cells and can not be applied to the study of a cell population. Concerning the size evaluation of adherent cells, it could be compromised by the limiting step of trypsinization which is known to impact the cell morphology and consequently the cell radius (Umegaki et al. 2004; Merten 2009).
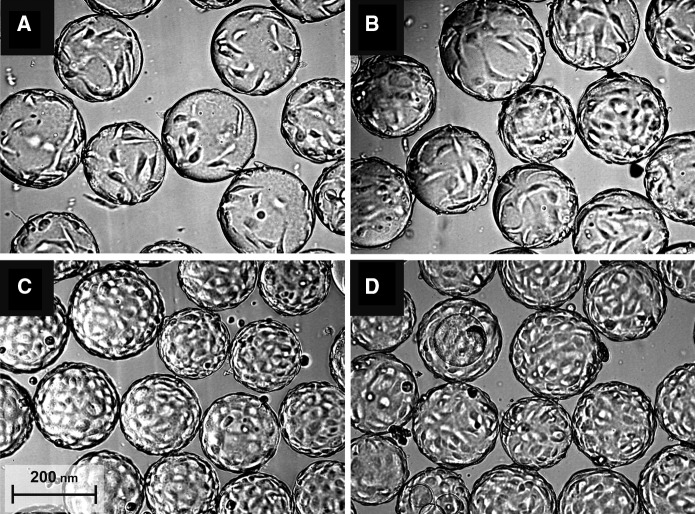
To overcome these difficulties, a microscopic observation of the microcarrier colonisation by Vero cells was performed during the fed-batch culture (Fig. 4). The pictures revealed morphological changes of adhered Vero cells on microcarriers during the culture and allowed to distinguish four different phases. Just after seeding, cells were distributed on the microcarriers surface with large area without cell-to-cell contact (Fig. 4A). Then, after about 50 h of growth, the cells were covering the whole microcarrier surface and reached confluency (Fig. 4B). In the late cell growth phases, from 70 h of culture onward, cell tissue remained confluent while the cell density was increasing (Fig. 4C, D). This suggests a potential decrease of the cell size. This hypothesis was strengthened by a paper dealing with human kidney tumour cells where a clear cell size reduction has been related to microcarrier surface saturation at the end of the exponential growth phase (Pons et al. 1992). In our case, the formation of cell multi-layers and some bead-to-bead adhesions were also observed in the final phase of the FB culture. All these events could potentially affect the cell specific permittivity above 1 × 106 cell mL−1, either by reducing the cell biovolume or by increasing the membrane capacitance. Based on the Eq. 6, the decrease in cell specific permittivity is more likely due to cell size reduction. It is less evident that the cell-to-cell contact could have a dramatic impact on CM values. Indeed Vero cells were reaching confluency on the microcarrier surface after 50 h of culture (0.5 × 106 cell mL−1), while εx began to decrease about 24h after confluency of 1 x 106 cells mL−1 was obtained.
Fig. 4.
Microscopic observation of Vero cells attached on microcarriers. Evolution of Vero cell morphology on microporous microcarriers at 4 h (A), 56 h (B), 70 h (C) and 94 h (D) after cell seeding, during culture performed with 80% of medium renewal after 48 h of culture (FB)
As described in recent papers,  could also theoretically be impacted by a variation of the conductivities (Ansorge et al. 2010; Opel et al. 2010). In our case, the magnitude of change of the culture medium conductivity (σM) along with the different whole culture processes was always lower than 4 mS cm−1, value which was negligible in comparison to σM mean values. Otherwise, the hypothesis of a modified intracellular conductivity (σi) induced by cell physiological state evolution could also be rejected. Indeed, on the one hand, the linear correlation obtained for the batch 1 was reliable until 120 h of culture, despite the occurrence of a plateau growth phase from 72 h onwards, and on the non-linearity of the correlation observed in the FB culture occurred before the end of the exponential cell growth phase.
could also theoretically be impacted by a variation of the conductivities (Ansorge et al. 2010; Opel et al. 2010). In our case, the magnitude of change of the culture medium conductivity (σM) along with the different whole culture processes was always lower than 4 mS cm−1, value which was negligible in comparison to σM mean values. Otherwise, the hypothesis of a modified intracellular conductivity (σi) induced by cell physiological state evolution could also be rejected. Indeed, on the one hand, the linear correlation obtained for the batch 1 was reliable until 120 h of culture, despite the occurrence of a plateau growth phase from 72 h onwards, and on the non-linearity of the correlation observed in the FB culture occurred before the end of the exponential cell growth phase.
Impact of the culture medium composition on adherent cell dielectric properties
The slope of the linear correlation between  and cell density was found lower in the modified medium MM than in reference medium MR (Fig. 3A). Although the initial conductivity values of MR and MM medium without cells were very close (16.7 and 15.5 mS cm−1, respectively), the mean specific permittivity was also lower in MM medium (6–8 pF cm−2 10−6cell) than in MR medium (10–14 pF cm−2 10−6cell) (Fig. 3C). Based on Eq. 6, these data suggested an influence of the culture medium composition either on the cell morphological (r) or physiological characteristics (CM). It has been reported that the medium composition could affect the size of primary rat cells (Conlon et al. 2004). Additionally, the medium formulation is also known to affect the cell membrane structure (Santos-Sacchi and Navarrete 2002) and CM is related to the structure and thickness of the cell membrane (Gentet et al. 2000; Kanapitsas et al. 2006). However, based on microscopic observations all over the cultures, no difference of the Vero cell size was observed between MR and MM media. So, in our case, the only parameter which could affect the permittivity value was the cell membrane capacitance. It could be reasonably assumed that it was initial substitution of glutamine by glutamax® which induced some variations in cellular membrane composition and so in CM. This could explain the fact that cell trypsinization from carriers was more difficult when cells were cultivated in the glutamax® containing medium (data not shown). This indicates that cell dielectric properties were not only cell line specific but also dependent on the conditions of culture operation.
and cell density was found lower in the modified medium MM than in reference medium MR (Fig. 3A). Although the initial conductivity values of MR and MM medium without cells were very close (16.7 and 15.5 mS cm−1, respectively), the mean specific permittivity was also lower in MM medium (6–8 pF cm−2 10−6cell) than in MR medium (10–14 pF cm−2 10−6cell) (Fig. 3C). Based on Eq. 6, these data suggested an influence of the culture medium composition either on the cell morphological (r) or physiological characteristics (CM). It has been reported that the medium composition could affect the size of primary rat cells (Conlon et al. 2004). Additionally, the medium formulation is also known to affect the cell membrane structure (Santos-Sacchi and Navarrete 2002) and CM is related to the structure and thickness of the cell membrane (Gentet et al. 2000; Kanapitsas et al. 2006). However, based on microscopic observations all over the cultures, no difference of the Vero cell size was observed between MR and MM media. So, in our case, the only parameter which could affect the permittivity value was the cell membrane capacitance. It could be reasonably assumed that it was initial substitution of glutamine by glutamax® which induced some variations in cellular membrane composition and so in CM. This could explain the fact that cell trypsinization from carriers was more difficult when cells were cultivated in the glutamax® containing medium (data not shown). This indicates that cell dielectric properties were not only cell line specific but also dependent on the conditions of culture operation.
Our results attest that the empirical model of Schwan, originally dedicated to spherical cells, could be applied to adherent cells such as Vero cells, grown on microcarriers, until the cells reach confluency. Indeed, a very good prediction of Vero cell densities by the Fogale Biomass system was observed, whatever the culture medium and the cell growth phase in both batch cultures (Fig. 5). Taking into account that the usual cell densities targeted for virus infection of Vero cells are mostly below 1 × 106 cell mL−1, such a method for in situ monitoring the Vero cell density could be attractive for vaccine manufacturers (Liu et al. 2007; Petiot et al. 2010b; Toriniwa and Komiya 2008; Butler et al. 2000). In the case of higher Vero cell densities, additional studies would be needed to quantify more precisely the cell size and to modelize the cell morphological modifications appearing after confluency on a spherical surface.
Fig. 5.
Comparison of attached cell kinetics quantified off-line (open circle) and on-line through permittivity measurements (continuous black lines)
In situ monitoring of Vero cell death
Several works have already reported apoptosis induction just after virus infection in large-scale viral productions (Petiot et al. 2011; Ravindra et al. 2008; Chan and Abubakar 2003). Furthermore, the cell viability may affect the viral infection efficiency. So, in addition to viable cell density monitoring during animal cell-based production processes, it would be also strategic to observe the occurrence of cell death in the bioreactor on-line. Consequently, our objective was to investigate the ability to detect the dead cell populations by using on-line permittivity measurements. Three dead cell populations might be present inside bioreactor cell culture; the lysed, necrotic and apoptotic cells. First, the lysed cells could not be detected through dielectric spectroscopy tool as their cell membrane are disrupted. So lysed cells could not contribute to the acquired permittivity (Cannizzaro et al. 2003). Second, different studies assumed that necrotic cells could only partially participate to the global measured permittivity since their membranes are permeabilized (Cannizzaro et al. 2003; Markx and Davey 1999). In the present case, no necrotic cells were observed either on the microcarriers or in the culture supernatant. Thus, no interference from necrotic cell would appear on permittivity signals. Finally, on the contrary, the dielectric properties of apoptotic cells have already been described in several works by using dielectrophoresis or electro-rotation off-line methods (Labeed et al. 2006; Kanapitsas et al. 2006; Huang et al. 2007; Patel et al. 2008). Indeed, apoptotic cells are likely to be detected by an electrical field as they are split into small apoptotic bodies, with dislocated nuclei but intact membranes. That is why in the following section we focused on the in situ detection of the apparition of Vero cell apoptosis by on-line permittivity monitoring.
Cell apoptosis detection through the evolution of the characteristic frequency
During batch culture 1, the adhered Vero cells reached their maximal density after 72 h of culture (Fig. 2). Indeed, the apoptotic cell concentration was close to 0.75 × 105 cell mL−1 at the beginning of the culture and increased from 96 h of culture to reach at 120 h a maximal value of 2.3 × 105 cell mL−1. This corresponded roughly to 36% of the adhered cells. The trend of apoptosis apparition in batch 2 was very similar to the one observed in batch 1. In that case, the maximal apoptotic cell density of 3 × 105 cell mL−1 was reached after 144 h, corresponding only to 16% of adhered cells. Clearly, the substitution of glutamine by glutamax® had a positive influence on the Vero cell physiology. In the culture performed with a medium renewal (FB), the maximal density was attained after 96 h, while the increase of apoptotic cell level occurred later, at about 120 h, to reach 3.8 × 105 cell mL−1 after 168 h. Nevertheless, as the decrease of adhered cell concentration was correctly monitored by permittivity measures during the last phase of cultures B1 and B2, the  alone did not allow identifying the culture time corresponding to the increase of cell apoptosis (Fig. 5).
alone did not allow identifying the culture time corresponding to the increase of cell apoptosis (Fig. 5).
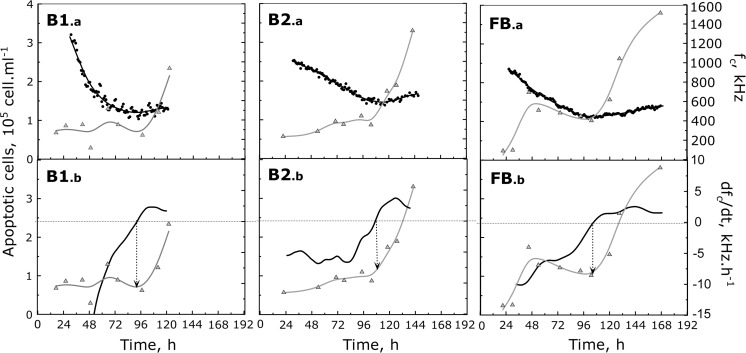
In Fig. 6, the evolution with time of the characteristic frequency (fc) was plotted in parallel to the off-line measurements of apoptotic cell density for the three bioreactor cultures (B1.a, B2.a, FB.a). No data are presented before 24 h since the cell densities were too low to provide a reliable calculation of the parameter. The patterns of the fc curves were found to be similar for the three cultures. During the first phase, corresponding to the increase of attached cell concentration, fc value rapidly decreased. But, during the late phase of the growth curve, fc trend was changing and it increased till the end of the culture. Interestingly, the culture time of the fc evolution shift matched with the important rise of the cell apoptosis. To confirm this observation, the derivative curves of fc were plotted with the apoptotic cell kinetics in Fig. 6 (B1.b, B2.b, FB.b). As expected, whatever the culture conditions used (medium renewal and medium type), the fc derivative reached a value of zero always at the same time as the apoptotic cell population proportion increased. It has to be highlighted that a similar fc increase with a corresponding onset of the cell death has recently been observed in the case of CHO cells (Opel et al. 2010).
Fig. 6.
Detection of Vero cell apoptosis with characteristic frequency monitoring. Evolution with time of apoptotic cell concentration (grey triangle), compared with the characteristic frequency, fc (A) or with its derivative, dfc/dt (b) (both represented with black lines), during batch cultures (B1 and B2) and batch culture performed with 80% medium renewal after 48 h (FB)
Based on Eq. 5, the fc parameter can be a direct indicator of the changes occurring when cells enter in apoptosis thanks to the monitoring of their dielectric properties (Cannizzaro et al. 2003; Markx and Davey 1999; Matanguihan et al. 1994). First, an obvious reduction of the cell size (r) is happening with the formation of apoptotic bodies (Huang et al. 2007). Second, different groups have observed a global increase of the intracellular conductivity (σi) during apoptosis, probably related to the cell size reduction inducing higher cytoplasmic ion concentrations (Labeed et al. 2006; Kanapitsas et al. 2006). An increase of CM was also reported for various cell lines undergoing the apoptotic process such as HL-60, Jurkat E6-1, K-562 and CHO cells (Opel et al. 2010; Labeed et al. 2006; Patel et al. 2008). All these cell evolutions could explain the increase of the characteristic frequency observed when apoptosis rises in the Vero cell proportion. Nevertheless, the fact that the original dielectric model was designed for suspension cells should remind us that adherent cells might also exhibit different response to the electrical field.
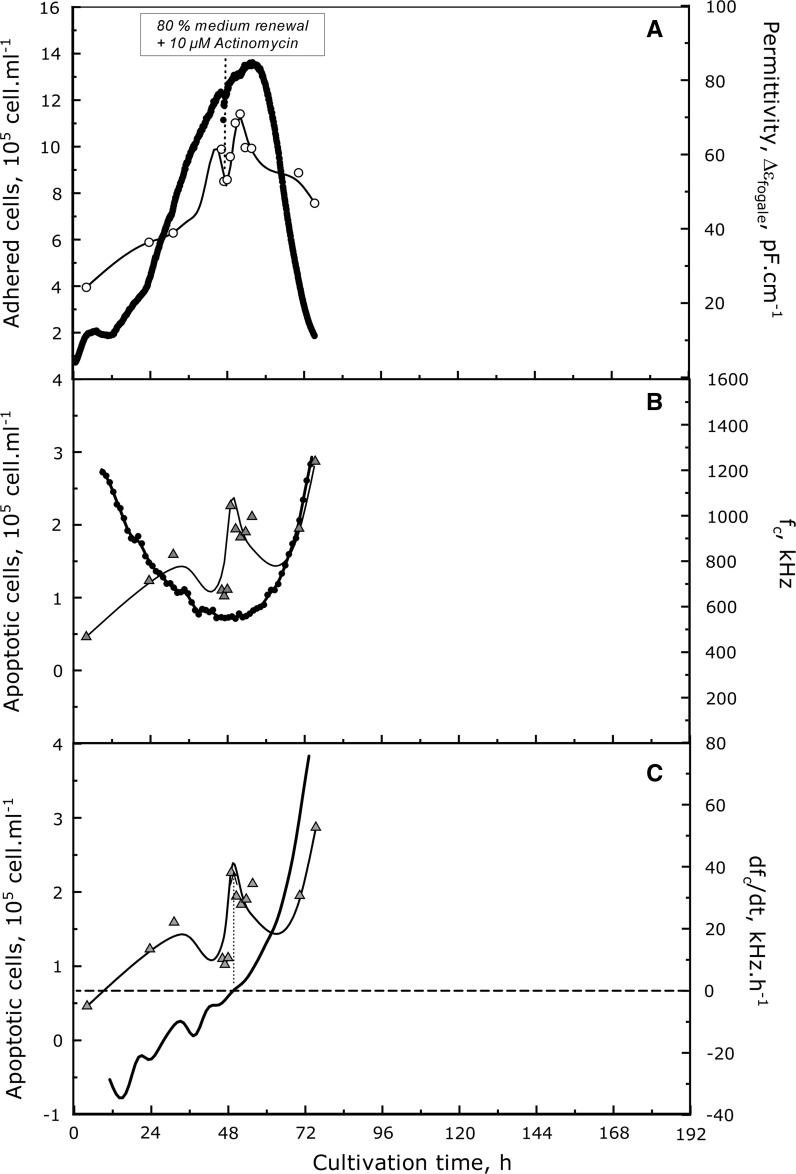
So, to validate the relation between on-line evolution of fc and apoptosis increase, apoptosis was induced in a further culture (FB2) by actinomycin D addition in the middle of the growth phase (at 48 h of culture). The permittivity 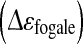 and the characteristic frequency (fc) were acquired on-line all over the culture (Fig. 7). A rise of the apoptotic cell concentration from 1 × 105 to 2.3 × 105 cell mL−1 was observed concomitantly to the fc increase and when its derivative nullifies. This increase of apoptosis at 48 h of culture was not observed when Vero cells were cultivated using the same medium renewal (FB1), which confirmed the artificial apoptosis induction by actinomycin. So, this experiment represents the proof-of-concept that on-line dielectric spectroscopy, through the use of fc parameter monitoring, could be a useful tool for in situ detecting a rise of cell apoptosis. Consequently, the Fogale biomass system present great potential for risk-mitigation strategies of Vero cell-based industrial processes to prevent the apparition of massive cell apoptosis.
and the characteristic frequency (fc) were acquired on-line all over the culture (Fig. 7). A rise of the apoptotic cell concentration from 1 × 105 to 2.3 × 105 cell mL−1 was observed concomitantly to the fc increase and when its derivative nullifies. This increase of apoptosis at 48 h of culture was not observed when Vero cells were cultivated using the same medium renewal (FB1), which confirmed the artificial apoptosis induction by actinomycin. So, this experiment represents the proof-of-concept that on-line dielectric spectroscopy, through the use of fc parameter monitoring, could be a useful tool for in situ detecting a rise of cell apoptosis. Consequently, the Fogale biomass system present great potential for risk-mitigation strategies of Vero cell-based industrial processes to prevent the apparition of massive cell apoptosis.
Fig. 7.
Apoptosis induction during the exponential growth phase of a batch culture of Vero cell with medium renewal. Apoptosis was induced with the addition of 10 μM of actinomycin D at 48 h of culture after 80% of medium renewal. Adhered cell concentration (open circle) is plotted together with the on-line permittivity measurements (continuous black lines) (A). Apoptotic cell concentrations (triangle: B, C) were compared to characteristic frequency fc (B) or its derivative, dfc/dt (C)
Conclusion
Our results first reported the ability of the dielectric spectroscopy to on-line monitor, without any sampling, concentrations up to 1 × 106 cell mL−1 of Vero cells attached on microcarriers and cultivated in different medium formulations. In the linear range of the obtained correlations, we have shown that the empirical relation between permittivity and cell density based on a theoretical background developed for spherical cells, could be used for adherent-dependent cells growing on microporous microcarriers. Consequently, dielectric spectroscopy could be a valuable and reliable tool for the development of a PAT strategy in industrial vaccine processes. It would allow to better control the cell growth, which is essential at critical times of the process, such as trypsinization, infection procedure or scale-up steps. The second major interest of our results was the in situ Vero cell apoptosis detection during bioreactor cultures. For the first time, we were able to on-line identify an important rise of cell apoptosis during a Vero cell culture. This event was related to the evolution with time of the characteristic frequency (fc) recorded in real-time from the monitoring of multi-frequency permittivity. This use of additional data from dielectric spectroscopy represents a major step forward to detect cell apoptosis known to be provoked by viral infection or by a possible failure during the culture process. With this additional in situ and real-time information, such a tool could then participate to risk-mitigation strategy in industrial processes. In conclusion, this new application of dielectric spectroscopy for cell culture processes makes it a very promising tool for further PAT developments.
Acknowledgments
The authors would like to acknowledge Sven Ansorge for his critical review of the manuscript.
References
- Al-Rubeai M. Apoptosis and cell culture technology. Adv Biochem Eng Biotechnol. 1998;59:225–249. doi: 10.1007/BFb0102300. [DOI] [PubMed] [Google Scholar]
- Ansorge S, Esteban G, Schmid G. On-line monitoring of infected Sf-9 insect cell cultures by scanning permittivity measurements and comparison with off-line biovolume measurements. Cytotechnology. 2007;55:115–124. doi: 10.1007/s10616-007-9093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge S, Esteban G, Schmid G. Multifrequency permittivity measurements enable on-line monitoring of changes in intracellular conductivity due to nutrient limitations during batch cultivations of CHO cells. Biotechnol Prog. 2010;26:272–283. doi: 10.1002/btpr.347. [DOI] [PubMed] [Google Scholar]
- Ansorge S, Lanthier S, Transfiguracion J, Henry O, Kamen A (2011) Monitoring lentiviral vector production kinetics using online permittivity measurements. Biochem Eng J 54:16–25
- Bradamante S, Barenghi L, Villa A. Simulated weightlessness in the design and exploitation of a NMR-compatible bioreactor. Biotechnol Prog. 2004;20:1454–1459. doi: 10.1021/bp049816k. [DOI] [PubMed] [Google Scholar]
- Butler M, Burgener A, Patrick M, Berry M, Moffatt D, Huzel N, Barnabe N, Coombs K. Application of a serum-free medium for the growth of vero cells and the production of reovirus. Biotechnol Prog. 2000;16:854–858. doi: 10.1021/bp000110+. [DOI] [PubMed] [Google Scholar]
- Cannizzaro C, Gügerli R, Marison I, Stockar Uv. On-line biomass monitoring of CHO perfusion culture with scanning dielectric spectroscopy. Biotechnol Bioeng. 2003;84:597–610. doi: 10.1002/bit.10809. [DOI] [PubMed] [Google Scholar]
- Card C, Hunsaker B, Smith T, Hirsch J (2008). Near-infrared spectroscopy for rapid, simultaneous monitoring of multiple components in mammalian cell culture. BioProc Int 6:58–67
- Chan YF, Abubakar S. Enterovirus 71 infection induces apoptosis in Vero cells. Malays J Pathol. 2003;25:29–35. [PubMed] [Google Scholar]
- Conlon I, Lloyd A, Raff M (2004) Coordination of cell growth and cell cycle progression in proliferating mammalian cells. In: Hall MN, Raff M, Thomas G (eds) Cell growth: control of cell size. Laboratory Press, Cold Spring Harbor. pp 85–101
- Ducommun P, Bolzonella I, Rhiel M, Pugeaud P, Stockar U, Marison IW. On-line determination of animal cell concentration. Biotechnol Bioeng. 2001;72:515–522. doi: 10.1002/1097-0290(20010305)72:5<515::AID-BIT1015>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ducommun P, Kadouri A, Stockar U, Marison IW. On-line determination of animal cell concentration in two industrial high-density culture processes by dielectric spectroscopy. Biotechnol Bioeng. 2002;77:316–323. doi: 10.1002/bit.1197. [DOI] [PubMed] [Google Scholar]
- Eyer K, Heinzle E. On-line estimation of viable cells in a hybridoma culture at various DO levels using ATP balancing and redox potential measurement. Biotechnol Bioeng. 1996;49:277–283. doi: 10.1002/(SICI)1097-0290(19960205)49:3<277::AID-BIT5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Figueroa B, Chen S, Oyler GA, Hardwick JM, Betenbaugh MJ. Aven and Bcl-xL enhance protection against apoptosis for mammalian cells exposed to various culture conditions. Biotechnol Bioeng. 2004;85:589–600. doi: 10.1002/bit.10913. [DOI] [PubMed] [Google Scholar]
- Gentet LJ, Stuart GJ, Clements JD. Direct measurement of specific membrane capacitance in neurons. Biophys J. 2000;79:314–320. doi: 10.1016/S0006-3495(00)76293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Chen A, Wang L, Guo M, Yu J. Electrokinetic measurements of dielectric properties of membrane for apoptotic HL-60 cells on chip-based device. Biomed Microdevices. 2007;9:335–343. doi: 10.1007/s10544-006-9038-y. [DOI] [PubMed] [Google Scholar]
- Ishaque A, Al-Rubeai M. Use of intracellular pH and annexin-V flow cytometric assays to monitor apoptosis and its suppression by bcl-2 over-expression in hybridoma cell culture. J Immunol Methods. 1998;221:43–57. doi: 10.1016/S0022-1759(98)00166-5. [DOI] [PubMed] [Google Scholar]
- Kamen AA, Bédard C, Tom R, Perret S, Jardin B. On-line monitoring of respiration in recombinant-baculovirus infected and uninfected insect cell bioreactor cultures. Biotechnol Bioeng. 1996;50:36–48. doi: 10.1002/(SICI)1097-0290(19960405)50:1<36::AID-BIT5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Kanapitsas A, Vartzeli-Nikaki P, Konsta AA, Visvardis EE, Sideris EG. Dielectric properties during apoptosis in peripheral blood cells from chronic lymphocytic leukaemia patients. Dielectr Electr Insulation IEEE Trans. 2006;13:1057–1062. [Google Scholar]
- Kilburn DG, Fitzpatrick P, Blake-Coleman BC, Clarke DJ, Griffiths JB. On-line monitoring of cell mass in mammalian cell cultures by acoustic densitometry. Biotechnol Bioeng. 1989;33:1379–1384. doi: 10.1002/bit.260331103. [DOI] [PubMed] [Google Scholar]
- Kistner O, Barrett PN, Mundt W, Reiter M, Schober-Bendixen S, Dorner F. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine. 1998;16:960–968. doi: 10.1016/S0264-410X(97)00301-0. [DOI] [PubMed] [Google Scholar]
- Knezevic I, Stacey G, Petricciani J. WHO Study Group on cell substrates for production of biologicals, Geneva, Switzerland, 11–12 June 2007. Biologicals. 2008;36:203–211. doi: 10.1016/j.biologicals.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Konstantinov K, Chuppa S, Sajan E, Tsai Y, Yoon S, Golini F. Real-time biomass-concentration monitoring in animal-cell cultures. Trends Biotechnol. 1994;12:324–333. doi: 10.1016/0167-7799(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Labeed FH, Coley HM, Hughes MP (2006) Differences in the biophysical properties of membrane and cytoplasm of apoptotic cells revealed using dielectrophoresis. Biochim Biophys Acta 1760: 922–929 [DOI] [PubMed]
- Le Ru A, Jacob D, Transfiguracion J, Ansorge S, Henry O, Kamen A. Scalable production of influenza virus in HEK-293 cells for efficient vaccine manufacturing. Vaccine. 2010;28:3661–3671. doi: 10.1016/j.vaccine.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Liu C–C, Lian W-C, Butler M, Wu S-C. High immunogenic enterovirus 71 strain and its production using serum-free microcarrier Vero cell culture. Vaccine. 2007;25:19–24. doi: 10.1016/j.vaccine.2006.06.083. [DOI] [PubMed] [Google Scholar]
- Mandenius C-F, Graumann K, Schultz TW, Premstaller A, Olsson I-M, Petiot E, Clemens C, Welin M. Quality by design (QbD) for biotechnology-related pharmaceuticals. Biotechnol J. 2009;4:600–609. doi: 10.1002/biot.200800333. [DOI] [PubMed] [Google Scholar]
- Markx GH, Davey CL. The dielectric properties of biological cells at radiofrequencies: applications in biotechnology. Enzym Microb Technol. 1999;25:161–171. doi: 10.1016/S0141-0229(99)00008-3. [DOI] [Google Scholar]
- Matanguihan RM, Konstantinov KB, Yoshida T. Dielectric measurement to monitor the growth and the physiological states of biological cells. Bioproc Biosyst Eng. 1994;11:213–222. [Google Scholar]
- Merten OW. Safety for vaccine(e)s. Cytotechnology. 2000;34:181–183. doi: 10.1023/A:1008160817560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merten O-W (2009) Cell detachment. In: Flickinger MC (ed) Encyclopedia of industrial biotechnology : bioprocess, bioseparation, and cell technology. Wiley, New York. pp 1–22
- Noll T, Biselli M. Dielectric spectroscopy in the cultivation of suspended and immobilized hybridoma cells. J Biotechnol. 1998;63:187–198. doi: 10.1016/S0168-1656(98)00080-7. [DOI] [PubMed] [Google Scholar]
- Opel CF, Li J, Amanullah A. Quantitative modeling of viable cell density, cell size, intracellular conductivity, and membrane capacitance in batch and fed-batch CHO processes using dielectric spectroscopy. Biotechnol Prog. 2010;26:1187–1199. doi: 10.1002/btpr.425. [DOI] [PubMed] [Google Scholar]
- Patel PM, Markx GH. Dielectric measurement of cell death. Enzym Microb Technol. 2008;43:463–470. doi: 10.1016/j.enzmictec.2008.09.005. [DOI] [Google Scholar]
- Patel PM, Bhat A, Markx GH. A comparative study of cell death using electrical capacitance measurements and dielectrophoresis. Enzym Microb Technol. 2008;43:523–530. doi: 10.1016/j.enzmictec.2008.09.006. [DOI] [Google Scholar]
- Pethig R, Jakubek L, Sanger R, Heart E, Corson E, Smith P. Electrokinetic measurements of membrane capacitance and conductance for pancreatic beta-cells. IEE Proc Nanobiotechnol. 2005;152:189–193. doi: 10.1049/ip-nbt:20050040. [DOI] [PubMed] [Google Scholar]
- Petiot E, Fournier F, Gény C, Pinton H, Marc A. Rapid screening of serum-free media for the growth of adherent vero cells by using a small-scale and non-invasive tool. Appl Biochem Biotechnol. 2010;160:1600–1615. doi: 10.1007/s12010-009-8674-0. [DOI] [PubMed] [Google Scholar]
- Petiot E, Guedon E, Blanchard F, Gény C, Pinton H, Marc A. Kinetic characterization of vero cell metabolism in a serum-free batch culture process. Biotechnol Bioeng. 2010;107:143–153. doi: 10.1002/bit.22783. [DOI] [PubMed] [Google Scholar]
- Petiot E, Jacob D, Lanthier S, Lohr V, Ansorge S, Kamen A. Metabolic and kinetic analyses of influenza production in perfusion HEK293 cell culture. BMC Biotechnol. 2011;11:84. doi: 10.1186/1472-6750-11-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M-N, Wagner A, Vivier H, Marc A. Application of quantitative image analysis to a mammalian cell line grown on microcarriers. Biotechnol Bioeng. 1992;40:187–193. doi: 10.1002/bit.260400127. [DOI] [PubMed] [Google Scholar]
- Ravindra PV, Tiwari AK, Ratta B, Chaturvedi U, Palia SK, Subudhi PK, Kumar R, Sharma B, Rai A, Chauhan RS. Induction of apoptosis in Vero cells by Newcastle disease virus requires viral replication, de novo protein synthesis and caspase activation. Virus Res. 2008;133:285–290. doi: 10.1016/j.virusres.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Rourou S, van der Ark A, van der Velden T, Kallel HA. A microcarrier cell culture process for propagating rabies virus in Vero cells grown in a stirred bioreactor under fully animal component free conditions. Vaccine. 2007;25:3879–3889. doi: 10.1016/j.vaccine.2007.01.086. [DOI] [PubMed] [Google Scholar]
- Rudolph G, Lindner P, Gierse A, Bluma A, Martinez G, Hitzmann B, Scheper T. Online monitoring of microcarrier based fibroblast cultivations with in situ microscopy. Biotechnol Bioeng. 2008;99:136–145. doi: 10.1002/bit.21523. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Navarrete E. Voltage-dependent changes in specific membrane capacitance caused by prestin, the outer hair cell lateral membrane motor. Pflügers Archiv Eur J Physiol. 2002;444:99–106. doi: 10.1007/s00424-002-0804-2. [DOI] [PubMed] [Google Scholar]
- Schulze-Horsel J, Schulze M, Agalaridis G, Genzel Y, Reichl U. Infection dynamics and virus-induced apoptosis in cell culture-based influenza vaccine production–Flow cytometry and mathematical modeling. Vaccine. 2009;27:2712–2722. doi: 10.1016/j.vaccine.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Schwan HP. Electrical properties of tissue and cell suspensions. Adv Biol Med Phys. 1957;5:147–208. doi: 10.1016/b978-1-4832-3111-2.50008-0. [DOI] [PubMed] [Google Scholar]
- Shah D, Clee P, Boisen S, Al-Rubai M (2006) NucleoCounter–an efficient technique for the determination of cell number and viability in animal cell culture processes. Cytotechnology 51:39–44 [DOI] [PMC free article] [PubMed]
- Siano SA. Biomass measurement by inductive permittivity. Biotechnol Bioeng. 1997;55:289–304. doi: 10.1002/(SICI)1097-0290(19970720)55:2<289::AID-BIT7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Souza M, Freire M, Castilho L (2007) Cultivation of vero cells on microporous and macroporous microcarriers. In: Smith R (ed) Cell technology for cell products. Springer, Harrogate, UK, pp 753–755
- Souza MCO, Freire MS, Schulze EA, Gaspar LP, Castilho LR. Production of yellow fever virus in microcarrier-based Vero cell cultures. Vaccine. 2009;27:6420–6423. doi: 10.1016/j.vaccine.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Szabo S, Monroe S, Fiorino S, Bitzan J, Loper K. Evaluation of an automated instrument for viability and concentration measurements of cryopreserved hematopoetic cells. Lab Hematol. 2004;10:109–111. doi: 10.1532/LH96.04020. [DOI] [PubMed] [Google Scholar]
- Teixeira AP, Portugal CAM, Carinhas N, Dias JML, Crespo JP, Alves PM, Carrondo MJT, Oliveira R. In situ 2D fluorometry and chemometric monitoring of mammalian cell cultures. Biotechnol Bioeng. 2009;102:1098–1106. doi: 10.1002/bit.22125. [DOI] [PubMed] [Google Scholar]
- Toriniwa H, Komiya T. Japanese encephalitis virus production in Vero cells with serum-free medium using a novel oscillating bioreactor. Biologicals. 2007;35:221–226. doi: 10.1016/j.biologicals.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Toriniwa H, Komiya T. Long-term stability of Vero cell-derived inactivated Japanese encephalitis vaccine prepared using serum-free medium. Vaccine. 2008;26:3680–3689. doi: 10.1016/j.vaccine.2008.04.076. [DOI] [PubMed] [Google Scholar]
- Umegaki R, Kino-oka M, Taya M. Assessment of cell detachment and growth potential of human keratinocyte based on observed changes in individual cell area during trypsinization. Biochem Eng J. 2004;17:49–55. doi: 10.1016/S1369-703X(03)00124-4. [DOI] [Google Scholar]
- Wang X, Becker FF, Gascoyne PRC (2002) Membrane dielectric changes indicate induced apoptosis in HL-60 cells more sensitively than surface phosphatidylserine expression or DNA fragmentation. Biochem Biophys Acta 1564:412–420 [DOI] [PMC free article] [PubMed]
- Zeiser A, Bédard C, Voyer R, Jardin B, Tom R, Kamen AA. On-line monitoring of the progress of infection in Sf-9 insect cell cultures using relative permittivity measurements. Biotechnol Bioeng. 1999;63:122–126. doi: 10.1002/(SICI)1097-0290(19990405)63:1<122::AID-BIT13>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zeiser A, Elias CB, Voyer R, Jardin B, Kamen AA. On-line monitoring of physiological parameters of insect cell cultures during the growth and infection process. Biotechnol Prog. 2000;16:803–808. doi: 10.1021/bp000092w. [DOI] [PubMed] [Google Scholar]