Abstract
Although isolation and characterization of embryonic stem cells have been successful in cattle, maintenance of bovine embryonic stem cells in culture remains difficult. In this study, we compared different methods of cell passaging, feeder cell layers and medium conditions for bovine embryonic stem cell-like cells. We found that a murine embryonic fibroblast feeder layer is more suitable for embryonic stem cell-like cells than bovine embryonic fibroblasts. When murine embryonic fibroblasts were used, a mechanical method of passaging led to better cell growth than passaging by trypsin digestion. We also found that exogenous supplementation with leukemia inhibitory factor maintained the embryonic stem cell-like cells in an undifferentiated state, whereas addition of stem cell factor resulted in their differentiation. Our findings provide an experimental basis for the establishment of an effective culture system for bovine embryonic stem cells.
Keywords: Bovine embryonic stem cell-like cells, Feeder layer, Passage, SCF, LIF
Introduction
Embryonic stem cells possess pluripotency and are thus able to differentiate into various tissues in vivo and in vitro. Human, pig, mouse rabbit and cattle embryonic stem cell lines have been established (Thomson et al.1998; Xu et al. 2001; Evans and Kaufman 1981; Miyoshi et al. 2000). Successful establishment of bovine embryonic stem cell lines will promote research into and the application of livestock-related reproductive biotechnology to facilitate and improve cattle and other livestock breeding. The growth of bovine embryonic stem cells in culture is influenced by the type and preparation of the feeder layer, the passage method and the medium.
Embryonic fibroblasts have been used as the feeder layer in most embryonic stem cell studies (Smith and Hooper 1987), because fibroblasts derived from fetuses secrete leukemia inhibitory factor (LIF). Some researchers have used murine embryonic fibroblasts as feeder layers for bovine embryonic stem cell culture (Wang et al. 2005; Hamano et al. 1998); Li et al. (2004) used murine embryonic fibroblast feeder layers for porcine embryonic stem cell culture, whereas Munoz et al. (2008) used bovine embryonic fibroblast for bovine embryonic stem cell culture; Behboodi et al. (2011) and Pawar et al. (2009) used goat fetal fibroblast for Goat Embryonic Stem Cells culture; Strojek et al. (1990) suggested that homologous fibroblasts are better as feeder layers than heterologous murine embryonic fibroblasts. Familari and Selwood (2006a) suggested that other types of cells can be taken as feeder layers as well as mouse fibroblast. However, few researchers have compared the effects of using different fibroblasts as feeder layers for the culture of bovine embryonic stem cell-like cells.
The passage method also affects the growth of bovine embryonic stem cells in culture. Bettiol et al. (2007) treated human embryonic stem cells with collagenase IV and then isolated them using a mechanical method. Cao et al. (2009) used mechanical separation for passaging of bovine embryonic stem cells. Huang et al. (2010) cultured embryonic stem cell-like cells from buffalo embryos after external fertilization, using a mechanical method and trypsin digestion for passaging; they found that the cells could be cultured to the eighth passage with the mechanical method, but differentiated when grown to at most the fourth passage with trypsin digestion. Hamano et al. (1998) obtained bovine embryonic stem cell-like cells with trypsin digestion. However, which method is most suitable for the establishment and maintenance of bovine embryonic stem cell-like cells remains unclear. Stem cell factor (SCF), a cytokine that binds to the c-Kit receptor, plays an important role in hematopoiesis, spermatogenesis and melanogenesis (Manova et al. 1990; Yan et al. 2000). How exogenous cytokines such as SCF and LIF influence embryonic stem cell culture remains to be elucidated.
Some markers indicative of pluripotency of embryonic stem cells have been investigated in mouse and human. Murine and human embryonic stem cells stain positive for alkaline phosphatase activity. The transcription factor Oct-4 appears to be a primary transcription factor in embryonic stem cells. SSEA-1 and SSEA-4 are expressed in specific stage. SSEA-4 is expressed in human embryonic stem cells while murine embryonic stem cells exhibit SSEA-1 (Behboodi et al. 2011). The expression of the makers on the cattle ESC needs more research to prove.
In this study, we compared different methods of cell passaging, types of feeder cell layer and culture conditions for bovine embryonic stem cell-like cells. The findings may provide useful experimental basis for the establishment of an effective culture system for bovine embryonic stem cells.
Materials and methods
Preparation of feeder cell layers
Irradiated primary murine embryonic fibroblasts were used as described previously (Reubinoff et al. 2000). For the preparation of bovine embryonic fibroblast, bovine fetuses at 4–6 weeks were washed with warm saline. After removal of the head, limbs and innards, the carcasses were cut into pieces. The tissue pellets were placed on 100 mm dished (Corning Life Sciences, Corning, NY) and incubated for 30 min at 37 °C under an atmosphere of 5% CO2. Bovine embryonic fibroblast growth medium (Cell Applications, San Diego, CA) was added for further culture. The medium was changed every 2 days and passaged every 4–6 days. The bovine embryonic fibroblasts were inactivated in a medium containing 10 ng/mL mitomycin-C (Zhejiang Haizheng Pharmaceutical Co., Zhejiang, China) for 5 h, washed three times with Ca2+ and Mg2+ free phosphate buffered saline (PBS(-)) and then treated with 0.25% trypsin-0.02% EDTA solution. The trypsinized cells were harvested by centrifugation at 500g.
For preparation of murine embryonic fibroblast and bovine embryonic fibroblast feeder layers, the cell suspension was seeded at a density of 5 × 104 cells per well in a four-well plate coated with 0.1% gelatin (Sigma, St. Louis, MO). Both murine embryonic fibroblasts and bovine embryonic fibroblasts were plated 1 day before inner cell masses (ICMs) were seeded (Munoz et al.2008; Roach et al. 2006; Bettiol et al. 2007; Bryja et al. 2006; Li et al. 2004). Embryonic stem cell-like cells were cultured on each of the two feeder layers using bovine embryonic stem cell-like basic culture medium which consisted of 90%DMEM/F12 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 0.1 mM β-Mercaptoethanol (Chemicon), 0.1 mM nonessential amino acids, 100 IU/mL penicillin, 0.05 mg/mL streptomycin, 20 ng/mL LIF and 10 ng/mL bFGF (Table 2). The effects of the different feeder layers on culture were investigated, including the rate of attachment blastocyst and the clone of the inner cell masse growing morphological features.
Table 2.
Medium conditions for bovine embryonic stem cell-like cell culture
| Medium type | Fundamental ingredient | Cytokine concentrations |
|---|---|---|
| Medium I | Fundamental ingredient | 20 ng/mL LIF + 10 ng/mL bFGF |
| Medium II | Fundamental ingredient | 20 ng/mL LIF + 10 ng/mL SCF + 10 ng/mL bFGF |
| Medium III | Fundamental ingredient | 10 ng/mL SCF + 10 ng/mL bFGF |
Fundamental ingredient which consisted of 90% DMEM/F12 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 0.1 mM β-mercaptoethanol (Chemicon) , 0.1 mM nonessential amino acids, 100 IU/ml penicillin, 0.05 mg/ml streptomycin
ICM preparation
Oocytes collected from cattle ovaries at an abattoir were cultured in TCM 199 medium (Gibco, Gaithersburg, MD) supplemented with 10 mmol/L Hepes (Sigma, St. Louis, MO), 1 μg/mL estradiol (Sigma) and 0.1 mg/ml follicle stimulating hormone (Kawazaki, Tokyo, Japan) for 22–24 h. The mature oocytes were fertilized in vitro using commercial bovine semen (Inner Mongolia Livestock Breeding and Improving Center, Huhhot, China) as described previously (Tervit et al. 1972). The zygotes were cultured in an in vitro production system using SOFaa medium supplemented with 10% fetal bovine serum (Gibco). The blastocysts were hatched in 8–10 days. The hatched blastocysts were cultured on feeder layers with the culture medium I which consisted of 90%DMEM/F12 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 0.1 mM β-Mercaptoethanol (Chemicon), 0.1 mM nonessential amino acids, 100 IU/mL penicillin, 0.05 mg/mL streptomycin, 20 ng/mL LIF, 10 ng/mL bFGF; the culture medium II which consisted of 90%DMEM/F12 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 0.1 mM β-Mercaptoethanol (Chemicon),0.1 mM nonessential amino acids, 100 IU/mL penicillin, 0.05 mg/mL streptomycin, 20 ng/mL LIF, 10 ng/mL bFGF, 10 ng/mL SCF; the culture medium III which consisted of 90%DMEM/F12 (Gibco) supplemented with 10% fetal bovine serum (Gibco), 0.1 mM β-Mercaptoethanol (Chemicon),0.1 mM nonessential amino acids, 100 IU/mL penicillin, 0.05 mg/mL streptomycin, 10 ng/mL bFGF, 10 ng/mL SCF (Table 2). The bovine embryonic stem cell-like cells were also cultured in three types of culture medium which contained different factors. After the hatched blastocysts attached on the feeder layers, inner cell masses that grew well were used for passaging.
Methods of cell passaging
After the ICMs had been cultured on murine embryonic fibroblast feeder layers for 3–5 days, embryonic stem cell-like colonies had grown well. For the mechanical method of passaging, the colonies were dissected in a microdrop by repeated pipetting using a micropipette under a microscope. The disaggregated colony cells were reseeded onto new murine embryonic fibroblast feeder layers. For trypsin digestion, the colonies were washed with PBS and treated with 0.25% trypsin-0.02% EDTA for 4–6 min at 37 °C under an atmosphere of 5% CO2. The digestion was inhibited by culture medium and the cells were harvested by centrifugation. Resuspended cells were reseeded at a density of 1 × 104 cells per well onto new murine embryonic fibroblast feeder layers. The cells were cultured in bovine embryonic stem cell-like basic culture medium.
Alkaline phosphatase staining
Embryonic stem cell-like cells were washed was PBS(-) followed by fixing in 4% paraformaldehyde at 4 °C overnight. After fixation, the cells were washed twice with PBS(-) and stained with alkaline phosphatase (AP) staining solution (Westang, Shanghai, China), according to the manufacturer’s instructions, for 30 min in the dark. They were then observed under a light microscope. The color of embryonic stem cell-like cells were purple under the microscope when the AP staining was positive.
Formation of embryoid bodies
Embryonic stem cell-like cells were detached from the murine embryonic fibroblast feeder layer using 5 mL pipette and placed in a conical tube. The cells were washed twice with PBS(-) and treated with 0.25% trypsin-0.02% EDTA for 3–4 min. Embryonic stem cell-like cells were in the suspension and could be centrifuged and collected when fibroblasts had adhered to the dish 6 h after the cells were seeded in dishes coated with gelatin. The digestion was inhibited by bovine embryonic fibroblast growth medium. The resuspended cells were seeded in a 35 mm dish and cultured in bovine embryonic fibroblast growth medium. After 7–10 days in culture, the embryoid bodies (EBs) were examined under a light microscope.
Immunostaining
Before preparation feeder layers, the sterile coverslip was put into the well in a four-well plate. Embryonic stem cell-like cells grown on coverslips were washed, fixed with 4% paraformaldehyde, permeabilized with PBS containing 0.1% (vol/vol) Triton X-100, and incubated sequentially with 0.4% bovine serum albumin in PBS. The cells were pretreated with 10% normal goat serum-PBS for 30 min. They were then incubated with monoclonal antibodies against OCT-4, SSEA-1 or SSEA-4 (1:50) followed by secondary antibodies (Biosynthesis Biotechnolgy CO. LTD, Beijing, bsf-0368G), using an embryonic stem cell marker sample kit (Chemicon, Temecula, CA) according to the protocol supplied by the manufacturer. Immunofluorescence images were observed under a fluorescence microscope.
Statistical analysis
All values were expressed as means ± SEM. Analysis of variance was used to determine the significance of differences among the groups. Values of p < 0.05 were considered statistically significant.
Results
Effect of feeder cell layer on bovine embryonic stem cell-like cell growth
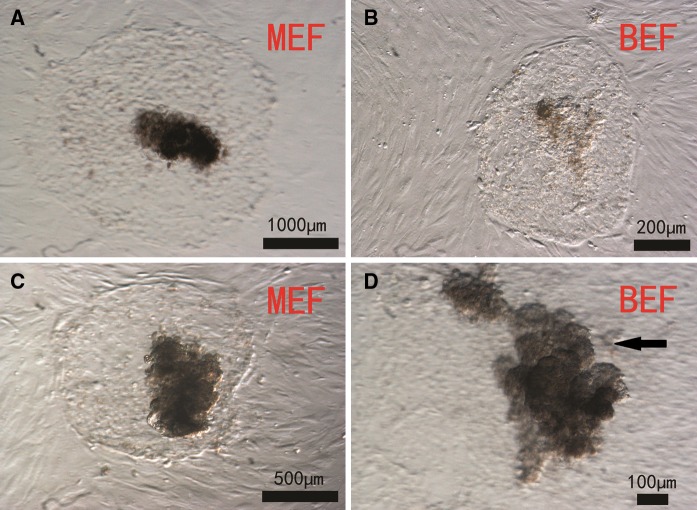
As shown in Table 1, embryonic stem cell-like cells grew better on murine embryonic fibroblast feeder layers than on bovine embryonic fibroblast feeder layers. On murine embryonic fibroblast feeder layers, the inner cell masses grew rapidly (the diameter of the clone is 600 μm, on the third day after passage) and the cells were dark in color and had obvious boundaries with the trophic layer (Fig. 1a), whereas on bovine embryonic fibroblast feeder layers, the ICMs grew slowly (the diameter of the clone is 200 μm, on the third day after passage) and had no obvious boundaries with the trophic layer (Fig. 1b). During subculture, trophoblasts grew well on murine embryonic fibroblast feeder layers (Fig. 1c), but disappeared gradually on bovine embryonic fibroblast feeder layers (Fig. 1d). After one or two passages, EBs were seen clearly in murine embryonic fibroblast feeder layer culture but their numbers were low with bovine embryonic fibroblast feeders.
Table 1.
Effect of different feeder cell layers on bovine embryonic stem cell-like cell culture
| Cell type | The passage No. of feeder cell layers | Attachment time (days) | No. of transplanted blastocyst | The percentage rate of attachment of blastocytes (%) |
|---|---|---|---|---|
| MEF | 2–3 | 2–3 | 48 | 78.5 ± 4.0* |
| BEF | 5–6 | 3–5 | 32 | 56.5 ± 3.3 |
MEF murine embryonic fibroblast; BEF bovine embryonic fibroblast. Data are expressed as mean ± SEM (n = 3), *p < 0.05 versus BEF
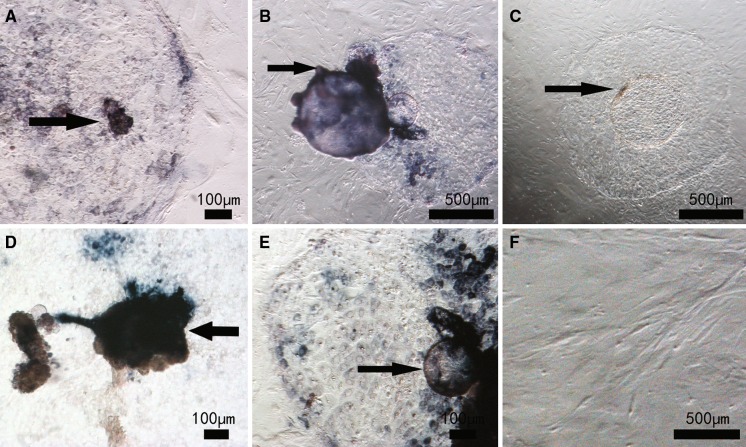
Fig. 1.
Morphology of bovine embryonic stem cell-like cells on mouse embryonic fibroblast (murine embryonic fibroblast - MEF) and bovine embryonic fibroblast (bovine embryonic fibroblast - BEF) layers. Hatched blastocysts were cultured on murine embryonic fibroblast or bovine embryonic fibroblast feeder layers. Inner cell masses that grew well were isolated by a mechanical method. Cells from primary colonies a, b and from the third passage c, d are shown
Effect of feeder cell layer on bovine embryonic stem cell-like cell differentiation
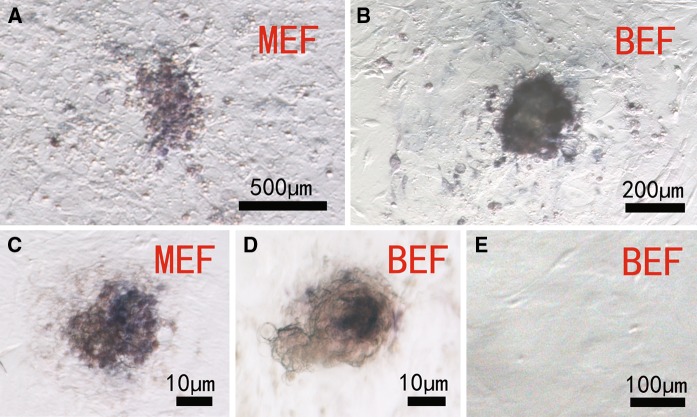
As shown in Fig. 2, in primary culture, embryonic stem cell-like cells on both murine embryonic fibroblast and bovine embryonic fibroblast feeder layers stained positive for AP (Fig. 2a, b), indicating that these cells had features of stem cells. After five passages, cells on murine embryonic fibroblast feeder layers still stained for AP (Fig. 2c) but those on bovine embryonic fibroblast feeder layers did not (Fig. 2d), indicating that the embryonic stem cell-like cells on bovine embryonic fibroblast feeders had differentiated.
Fig. 2.
Alkaline phosphatase staining of bovine embryonic stem cell-like cells on mouse embryonic fibroblast (murine embryonic fibroblast - MEF) and bovine embryonic fibroblast (bovine embryonic fibroblast - BEF) layers. Primary culture a, b, subculture at the fifth passage c, d and bovine embryonic fibroblast cells as control e
Effect of passaging method on embryonic stem cell-like cell growth
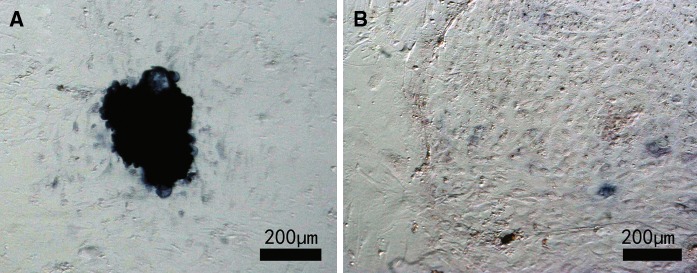
Embryonic stem cell-like cells were cultured on murine embryonic fibroblast feeder layers. As shown in Fig. 3, the cells formed embryonic stem cell-like colonies and were positive for AP (Fig. 3a) staining when the mechanical method was used. With the trypsin method, most cells had differentiated after three passages and AP staining was negative (Fig. 3b).
Fig. 3.
Alkaline phosphatase staining of bovine embryonic stem cell (bES)-like cells using different passaging methods. Bovine embryonic stem cell-like cells were cultured on a mouse embryonic fibroblast feeder layer and passaged using a mechanical method a or an enzyme digested method b. Cells from the third passage are shown
Effect of medium conditions on embryonic stem cell-like cell growth
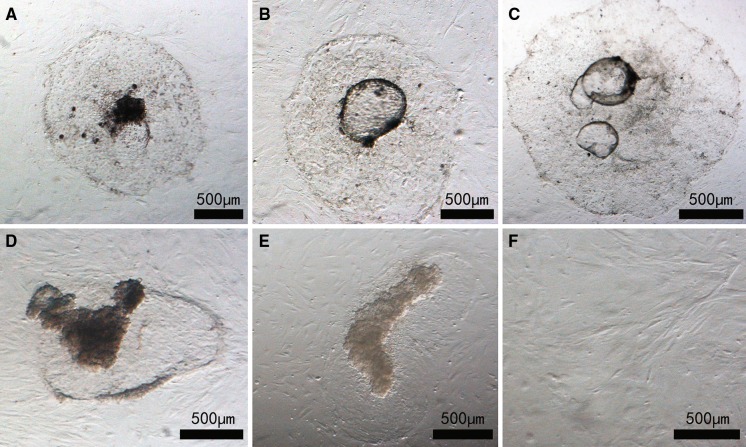
Blastocysts were cultured in three different media (Table 2). The time to cell attachment was not significantly different among the media. However, the attachment rate was significantly higher in medium II than that in medium III (p < 0.05). The mean attachment rate was significantly different between media II and III, but was not significantly different between media I and II, and between media I and III (Table 3). Embryonic stem cell-like colonies grew well in medium I; these colonies were dark in color and had obvious boundaries with the trophic layer (Fig. 4a). The colonies in media II and III had transparent spheres in their center (Fig. 4b, c). The embryonic stem cell-like colonies tended to increase and had undifferentiated features in media I and II (Fig. 4d, e), but tended to decrease and had disappeared after five passages in medium III (Fig. 4f).
Table 3.
Effect of medium conditions on bovine embryonic stem cell-like cell culture
| Medium type | No. of transplanted blastocyst | Attachment time (days) | The percentage rate of attachment of blastocytes (%) |
|---|---|---|---|
| Medium I | 42 | 3–4 | 78.4 ± 6.3# |
| Medium II | 42 | 3–4 | 86.6 ± 15.5* |
| Medium III | 43 | 4–5 | 56.5 ± 14.0 |
Data are expressed as mean ± SEM (n = 3),*p < 0.05 versus Medium III; #p > 0.05 Medium I has no significant difference with Medium II and Medium III
Fig. 4.
Bovine embryonic stem (bES) cells growth in different media. Bovine embryonic stem cell-like cells cultured on a mouse embryonic fibroblast feeder layer in medium I a, II b, or III c at passage 0 and in medium I d, II e or III f at the fifth passage
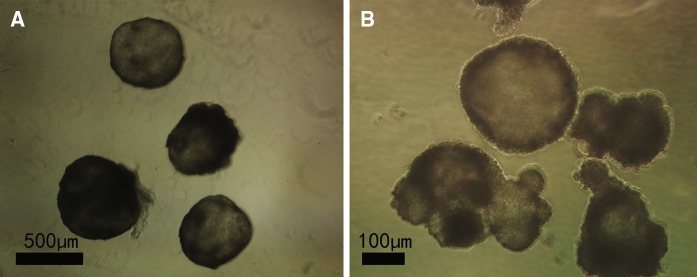
Cell differentiation and EB formation in different media
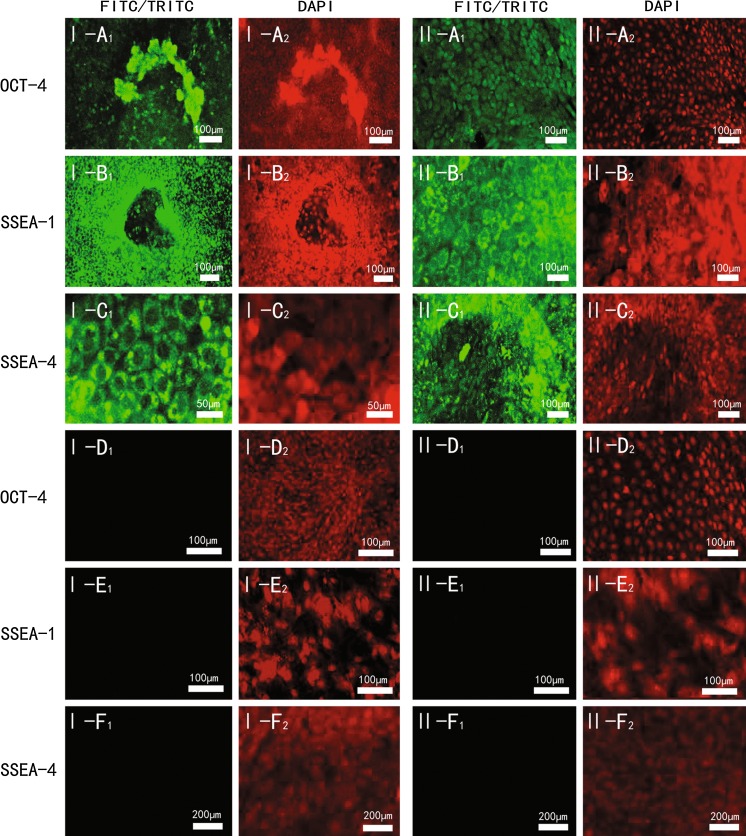
As shown in Fig. 5, in primary culture, embryonic stem cell-like cells stained positive for AP in media I and II (Fig. 5a, b) but were only partially stained in medium III (Fig. 5c). After eight passages, the cells in media I and II still stained for AP (Fig. 5d, e) but those in medium III were not stained (Fig. 5f), indicating that the cells in medium III had differentiated. Bovine embryonic stem cell-like cells cultured for 7–10 days in medium without LIF and a feeder layer differentiated spontaneously into EBs (Fig. 6). To verify that the isolated cells were stem cells, colonies at the fifth passage in media I and II were assessed using an embryonic stem cell marker sample kit. As shown in Fig. 7, cell membranes stained positive for OCT-4 (Fig. 7I-a1, II-a1), SSEA-1 (Fig. 7I-b1, II-b1) and SSEA-4 (Fig. 7I-c1, II-c1).
Fig. 5.
Alkaline phosphatase staining of bovine embryonic stem (bES) cell-like cells in different media. Bovine embryonic stem cell-like cells cultured on a mouse embryonic fibroblast feeder layer in medium I a, II b, or III c at passage 0 and in medium I d or II e at the eighth passage. Bovine embryonic fibroblast cells as control (e)
Fig. 6.
Embryoid bodies (EBs) differentiated in medium without leukemia inhibitory factor and feeder layer. Bovine embryonic stem cell-like cells on a mouse embryonic fibroblast feeder layer at the fifth passage had differentiated spontaneously into EBs in medium I a and II b
Fig. 7.
Representative immunofluorescence staining of bovine embryonic stem (bES) cell-like cells at the fifth passage. Bovine embryonic stem cell-like cells cultured on a mouse embryonic fibroblast (murine embryonic fibroblast) feeder layer in medium I and medium II at the fifth passage. The cells were stained for OCT-4 a, SSEA-1 b or SSEA-4 c. Murine embryonic fibroblasts were stained with OCT-4 d, SSEA-1 e or SSEA-4 f. 1, positive staining; 2, control
Discussion
Two different types of fibroblasts were used in this study, primarily because of their different purification times; murine embryonic fibroblasts are easily purified via passaging, whereas bovine embryonic fibroblasts have a long purification time and need more generations, to a certain extent, which causes their aging.
Bryja et al. (2006) reported that difficulties obtaining an adherent embryo culture caused by low density and poor quality of the feeder layer. To avoid the impact of low density on the feeder layers used in this study, the layers were paved at a density of 2–2.5 × 104 cells/cm2. When the density was lower than this, connections could not be formed among the fibroblasts, and the embryos died and were not adherent when transferred. When the feeder layer density was greater than 2–2.5 × 104 cells/cm2, the embryo cells were compressed by excessive fibroblasts and again embryos were not adherent. A high-quality feeder layer could not be prepared from a long-term fibroblast cell culture because of morphological aging, which also affects embryo adherence (Bryja et al. 2006).
The rate of adherence of bovine embryonic stem cell-like cells differed slightly between the two feeder layers. The reason for this may be that murine embryonic fibroblasts have a shorter growth time and stronger growth, and secrete factors such as LIF that promote embryonic stem cell growth when used as feeder layers. Bovine embryonic fibroblasts grow more slowly and secrete fewer factors conducive to embryonic stem cell growth. Compared to bovine embryonic fibroblasts, murine embryonic fibroblasts were more conducive to adherent culture of bovine embryonic stem cell-like cells. This is consistent with the findings of Hamano et al. (1998). Familari and Selwood (2006b) noted that homologous fibroblasts have been successful as feeder layers for the cultivation of mink, marsupial, human and pig embryonic stem cell-like cells, but have failed in the cultivation of sheep, cattle and chicken embryonic stem cell-like cells. Gjørret and Maddox-Hyttel (2005) also proposed the use of heterologous bovine fibroblasts. They found that heterologous bovine fibroblasts were better than homologous fibroblasts for the culture of bovine embryonic stem cell-like cells. As a homologous feeder layer, bovine fibroblasts prevented the disappearance of bovine embryonic stem cell-like cells by fusion with the trophoblast, and only the middle part of the fifth generation of bovine embryonic stem cell-like cells stained positive for AP. By this time, the bovine embryonic stem cell-like cells had fused with the bovine embryonic fibroblasts and differentiation had occurred. Murine embryonic fibroblasts show better growth and secrete factors promoting embryonic stem cell growth, but as heterologous cells it is difficult for them to fuse with the trophoblast of bovine embryonic stem cell-like cells and they are thus not conducive to the induction of bovine embryonic stem cell differentiation.
In this study, mechanical passaging was more conducive than trypsin digestion to the cultivation of bovine embryonic stem cell-like cells; the cells could be cultivated to at least the 13th generation, whereas with trypsin digestion the cells had differentiated when cultivated at most to the third generation, consistent with the findings of Huang et al. (2010). Trypsin may activate the differentiation pathways of bovine embryonic stem cell-like cells, and the characteristics of bovine embryonic stem cell-like cells differ significantly from those of mouse stem cells in culture.
Culture medium II contained SCF, whereas medium I did not. The two culture media showed no significant difference in mean adherence rate or adherence time, but differed somewhat in morphology and speed of growth of primary bES-like cells. In the latter part of cultivation, the morphologies were similar, but the bovine embryonic stem cell-like cells cultured in medium II grew relatively quickly.
LIF inhibits the differentiation of embryonic stem cell-like cells and is secreted by embryonic fibroblasts. bES-like cells cultured in medium III without LIF had differentiated completely when cultured at most to the fifth generation; a relatively small amount of LIF was secreted from the murine embryonic fibroblasts that did not meet the needs for indefinite bovine embryonic stem cell-like cell proliferation. The average adherence rate of blastocysts in culture medium II was significantly higher than that in medium III, and bovine embryonic stem cell-like cells grown in medium II were significantly increased in the latter part of the passaging. This indicates that a certain concentration of LIF promotes the adherence of bovine embryos and inhibits the differentiation of bovine embryonic stem cell-like cells.
Primary bovine embryonic stem cell-like cells cultured in media I, II and III showed some differences in morphology. Those cultured in medium II or III formed bubble-like structures and trophoblast growth was faster; because c-kit proteins present in the trophoblast cells expanded in vitro, the combination of SCF and c-kit promoted the expansion of the blastocyst surface area (Eckary et al. 2002). SCF induced relatively fast growth of the trophoblast after embryo adherence, and large globular structures were seen in the cultivation system.
It has been noted that embryonic stem cells are mainly from the ICM or embryonic primitive ectoderm (also called the ectoderm) (Cao et al. 2009; Brook and Gardner 1997; Boiani and Scholer 2005). Many cells in the ICM and trophoblast of primary bovine embryonic stem cell-like cells stained positive for AP in both medium I and medium II, and the vesicular body of bovine embryonic stem cell-like cells in medium II was also stained. bES-like cells were less strongly stained in medium III, and the staining was relatively shallow. Studies have shown that only embryonic stem cells with high AP activity are widely differentiated (Sulston et al. 1983; Tada et al. 2001; Chambers et al. 2003; Darr et al. 2006; Wilmut et al. 1997). Bovine embryonic stem cell-like cells in media I and II showed AP activity, whereas medium III contained fewer factors for inhibiting differentiation the primary bovine embryonic stem cells had begun slow differentiation. The eighth generation of bovine embryonic stem cell-like cells cultured in medium I or II stained positive for AP. This was consistent with the results of the in vitro differentiation experiments and three immunofluorescence staining experiments (OCT-4, SSEA-1 and SSEA-4), indicating that embryonic stem cell-like cells cultured in both media maintained the characteristics of embryonic stem cell-like cells.
This study shows that the interaction of appropriate concentrations of 20 ng/mL LIF and 10 ng/mL SCF promotes the adherence of bovine embryos and the later growth of bovine embryonic stem cell-like cells. A certain concentration of LIF promotes the adherence of bovine embryos and inhibits the differentiation of bovine embryonic stem cell-like cells, while an appropriate amount of SCF increases bovine embryonic stem cell-like cells.
Acknowledgments
This work was supported by grants from the National High Technology Research and Development Program of China (“863” Program) (2008AA101005). We gratefully thank Dr. Cang Ming (Inner Mongolia University) and Dr. Guo Xudong (Inner Mongolia University) for providing the directions.
References
- Behboodi E, Bondareva A, Begin I, Rao K, Neveu N. Establishment of goat embryonic stem cells from in vivo produced blastocyst-stage embryos. Mol Reprod Dev. 2011;78:202–211. doi: 10.1002/mrd.21290. [DOI] [PubMed] [Google Scholar]
- Bettiol E, Sartiani L, Chicha L, Krause KH, Cerbai E, Jaconi ME. Fetal bovine serum enables cardiac differentiation of human embryonic stem cells. Int Soc Differ. 2007;75:669–681. doi: 10.1111/j.1432-0436.2007.00174.x. [DOI] [PubMed] [Google Scholar]
- Boiani M, Scholer HR. Regulatory networks in embryo derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci USA. 1997;94:5709–5712. doi: 10.1073/pnas.94.11.5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryja V, Bonilla S, Arenas E. Derivation of mouse embryonic stem cells [J] Nat Protoc. 2006;1:2082–2087. doi: 10.1038/nprot.2006.355. [DOI] [PubMed] [Google Scholar]
- Cao S, Wang F, Chen Z, Liu Z, Mei C, Wu H, Huang J, Li C, Zhou L, Liu L. Isolation and culture of primary bovine embryonic stem cell colonies by a novel method. J Exp Zool. 2009;311:368–376. doi: 10.1002/jez.535. [DOI] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of nanog a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Darr H, Mayshar Y, Benvenisty N. Overexpression of NANOG in human ES cells enables feeder-free growth while inducing primitive ectoderm features. Development. 2006;133:1193–1201. doi: 10.1242/dev.02286. [DOI] [PubMed] [Google Scholar]
- Eckary DC, Lawrence SB, Juengel JL, Greenwood P, McNatty KP, Fidler AE. Gene expression of the tyrosine kinase receptor c-kit during ovarial development in the brush tail possum. Biol Reprod. 2002;66:346–353. doi: 10.1095/biolreprod66.2.346. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Familari M, Selwood L. The potential for derivation of embryonic stem cells in vertebrates. Mol Reprod Dev. 2006;73:123–131. doi: 10.1002/mrd.20376. [DOI] [PubMed] [Google Scholar]
- Familari M, Selwood L. The potential for derivation of embryonic stem cells in vertebrates. Mol Reprod Dev. 2006;73:123–131. doi: 10.1002/mrd.20376. [DOI] [PubMed] [Google Scholar]
- Gjørret JO, Maddox-Hyttel P. Attempts towards derivation and establishment of bovine embryonic stem cell-like cultures. Reprod Fertil Dev. 2005;17:113–124. doi: 10.1071/rd04117. [DOI] [PubMed] [Google Scholar]
- Hamano S, Watanabe Y, Azuma S. Establishment of embryonic stem (ES) cell-like cell lines derived from bovine blastocysts obtained by in vitro culture of oocytes matured and fertilized in vitro. J Reprod Dev. 1998;44:297–303. doi: 10.1262/jrd.44.297. [DOI] [Google Scholar]
- Huang B, Li T, Wang XL, Xie TS, Lu YQ, da Silva FM, Shi DS. Generation and characterization of embryonic stem-like cell lines derived from in vitro fertilization buffalo (Bubalus bubalis) Embryos. 2010;45:122–128. doi: 10.1111/j.1439-0531.2008.01268.x. [DOI] [PubMed] [Google Scholar]
- Li M, Li YH, Hou Y, Sun XF, Sun Q, Wang WH. Isolation and culture of pluripotent cells from in vitro produced porcine embryos. Camb Univ Press. 2004;12:43–48. doi: 10.1017/s0967199404002679. [DOI] [PubMed] [Google Scholar]
- Manova K, Nocka K, Besmer P, Bachvarova RF. Gonadal expression of c-kit encoded at the W locus of the mouse. Development. 1990;110:1057–1069. doi: 10.1242/dev.110.4.1057. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Taquchi Y, Sendai Y, Hoshi H, Sato E. Establishment of a porcine cell line from in vitro-produced blastocysts and transfer of the cells into enucleated oocytes. Biol Reprod. 2000;62:1640–1646. doi: 10.1095/biolreprod62.6.1640. [DOI] [PubMed] [Google Scholar]
- Munoz M, Rodriguez A, Frutos C, Caamano JN, Diez C, Facal N, Gomez E. Conventional pluripotency markers are unspecific for bovine embryonic-derived cell-lines. Theriogenology. 2008;69:1159–1164. doi: 10.1016/j.theriogenology.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Pawar SS, Malakar D, De AK, Akshey YS. Stem cell-like outgrowths from in vitro fertilized goat blastocysts. Indian J Exp Biol. 2009;47:635–642. [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bonso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Roach M, Wang L, Xiang zhong Yang X, Tian C. Bovine embryonic stem cells. Methods Enzymol. 2006;418:21–37. doi: 10.1016/S0076-6879(06)18002-7. [DOI] [PubMed] [Google Scholar]
- Smith AG, Hooper ML. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev Biol. 1987;121:1–9. doi: 10.1016/0012-1606(87)90132-1. [DOI] [PubMed] [Google Scholar]
- Strojek RM, Reed MA, Hoover JL, Waqner TE. A method for cultivation morphological undifferentiated embryonic stem cells from porcine blastocysts. Theriogenology. 1990;33:901–913. doi: 10.1016/0093-691X(90)90825-E. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Tada M, Takahama Y, Abe K, Nakatsuji N, Tada T. Nuclear reprogramming of somatic cells by in vitro hybridization with ES cells. Curr Biol. 2001;11:1553–1558. doi: 10.1016/S0960-9822(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Tervit HR, Wittingham DG, Rowson LE. Successful culture in vitro of sheep and cattle ova. J Reprod Fertil. 1972;30:493–497. doi: 10.1530/jrf.0.0300493. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swierqiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Wang L, Duan E, Sung LY, Jeong BS, Yang X, Tian XC. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol Reprod. 2005;73:149–155. doi: 10.1095/biolreprod.104.037150. [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J. Feeder-free growth of undifferentiated human embryonic stem cells. Nature. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Yan W, Suominen J, Toppari J. Stem cell factor protects germ cells from apoptosis in vitro. J Cell Sci. 2000;113:161–168. doi: 10.1242/jcs.113.1.161. [DOI] [PubMed] [Google Scholar]