Abstract
Although green fluorescent protein (GFP) labeling is widely accepted as a tracking method, much remains uncertain regarding the retention of injected GFP-labeled cells implanted in ischemic organs. In this study, we evaluate the effectiveness of GFP for identifying and tracking implanted bone marrow- mesenchymal stem cells (BM-MSCs) and the effect of GFP on the paracrine actions of these cells. MSCs isolated from rat femur marrow were transduced with a recombinant adenovirus carrying GFP. After transplantation of the GFP-labeled BM-MSCs into the infarct zone of rat hearts, the survival, distribution, and migration of the labeled cells were analyzed at 3, 7, 14, and 28 days. To evaluate the effect of GFP on the paracrine actions of BM-MSCs, Western blot analysis was performed to detect the expression of vascular endothelial growth factor (VEGF), b fibroblast growth factor (b FGF), tissue inhibitor of metalloproteinase-1 (TIMP-1) and matrix metalloproteinases-2 (MMP-2). GFP was successfully expressed by BM-MSCs in vitro. At 14 days after cell transplantation the GFP-positive cells could not be detected via confocal microscopy. By using a GFP antibody, distinct GFP-positive cells could be seen and quantitative analysis showed that the expression volume of GFP was 6.42 ± 0.92 mm3 after 3 days, 1.24 ± 0.76 mm3 after 7 days, 0.33 ± 0.03 mm3 after 14 days, and 0.09 ± 0.05 mm3 after 28 days. GFP labeling did not adversely affect the paracrine actions of BM-MSCs. GFP labeling could be used to track MSC distribution and their fate for at least 28 days after delivery to rat hearts with myocardial infarction, and this stem cell tracking strategy did not adversely affect the paracrine actions of BM-MSCs.
Keywords: Cell tracking, Green fluorescent protein, Mesenchymal stem cells, paracrine, Myocardial infarction
Introduction
There have been rapid advances in the use of mesenchymal stem cells (MSCs) for tissue regeneration and repair of the heart in recent years. Their ease of isolation, capacity for large-scale expansion, plasticity and alleged immunoprivileged status (Beggs et al. 2006; Keyser et al. 2007; Le Blanc and Pittenger 2005) are likely to generate more clinical applications in the future. Although interest in stem cell (SC) transplantation grows, not enough is known about the distribution and fate of the delivered cells to address important concerns over safety and efficacy. The ability to track exogenous MSCs after delivery is important for understanding how these SCs achieve the observed therapeutic benefits and to determine the fate of the cells after implantation.
The current noninvasive approaches that are mostly used for in vivo tracking of SCs include direct, nonspecific labeling of cells with magnetic particles or radionuclides and indirect specific labeling with reporter genes. Among these techniques, green florescence protein (GFP) is unique and easy to use, in that it is biocompatible and does not require an added substrate for signal emission, and GFP has been used by other researchers for cell tracking in vivo (Hatzistergos et al. 2010; Huang et al. 2010). Although GFP labeling is widely accepted as a tracking method, little data have been presented regarding retention of injected GFP-labeled cells implanted in ischemic organs over time. Further studies are still necessary to acquire this information.
Mesenchymal stem cells have the potential to differentiate into mature cardiac cells or promote native repair through angiogenesis, recruitment of host SCs, or induction of myocytes into the cell cycle (Kruglyakov et al. 2006; Ohnishi et al. 2007; Amado et al. 2005; Mangi et al. 2003). It is believed that the multifaceted paracrine actions of MSCs explain, at least in part, their tissue-healing properties (Caplan and Dennis 2006; Dai et al. 2005; Gnecchi et al. 2006; Kinnaird et al. 2004). However, so far there was no data relating the relationship between GFP and the paracrine actions of MSCs in vivo. This question is particularly relevant because some labels may affect host gene expression in vivo, as observed in a previous report (Leiker et al. 2008).
In this study, we transplanted bone marrow derived MSCs transduced with a recombinant replication-defective adenovirus vector carrying GFP (Ad-GFP) into ischemic rat hearts. In order to provide more data about this SC tracking method, we evaluated the effectiveness of GFP for identifying and tracking the implanted cells and the effects of GFP on the paracrine actions of the implanted MSCs.
Materials and methods
Rats and establishment of myocardial infarction
Adult Sprague–Dawley (SD) rats matched for weight (200–250 g) were anaesthetized with chloral hydrate (2.5 mg/kg), intubated, and ventilated with room air at 75 breaths per minute with a pressure-cycled rodent ventilator. Rats underwent surgery to induce anterior-wall myocardial infacrtion (MI), which was induced in 60 rats by ligation of the left anterior descending artery as described earlier (Guo et al. 2008). The MI rats were randomly divided into the control (n = 30) and Ad-GFP (n = 30) groups. The tissue specimens obtained from the heart were fixed in ethanol, embedded in paraffin, and sectioned. Tissue sections were stained with hematoxylin and eosin to observe histological changes in myocardial infarction border zone. The investigation protocol was approved by the Institutional Animal Care Committee of the Chinese PLA General Hospital, Chinese PLA Postgraduate Medical School, China.
BM-MSCs culture
The rat bone marrow derived MSCs were cultured as previously described (Guo et al. 2008). Briefly, bone marrow cells were aspirated from the femur and tibia of 80–100 g SD rats. The cell suspension was loaded on a Percoll gradient (Sigma–Aldrich, St. Louis, MO, USA) and centrifuged. The top two-thirds of the total volume was then transferred into a tube and washed with phosphate-buffered saline (PBS) to remove the Percoll. The cells were then cultured in Dulbecco’s modified Eagle’s medium (Gibco BRL, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; Thermo Scientific HyClone, Logan, UT, US). After 24 h, nonadherent debris was removed, and adherent cells were cultured.
Flow cytometry assays for cell surface antigens CD90, CD105, CD29, CD34, CD44, and CD45 of rat BM-MSCs were performed as described (Conget and Minguell 1999; Dominici et al. 2006) (anti-rat-CD90-PE, anti-rat-CD105-PE were purchased from Santa Cruz Biotechnology, Inc., USA; anti-rat-CD29-PE, anti-rat-CD34-PE, anti-rat-CD44-FITC, anti-rat-CD45-FITC, anti-rat-IgG1-FITC, and anti-rat-IgG1-PE were purchased from Becton, Dickinson and Company, Franklin Lakes, NJ, USA). A total of 5 × 105 cells were suspended in 0.25% Trypsin and 1 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA) and then washed in 3 mL of PBS. After centrifugation, the pellet was suspended in 200 μL of the primary antibody solution for 30 min at room temperature in the dark. The analysis was performed with a flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Passage 5 cells were seeded on cover slips in six well plates and cultured in complete medium up to confluency. At confluency, the cells were switched to an adipogenic medium (OriCell™ Sprague–Dawley (SD) Rat Mesenchymal Stem Cell Adipogenic Differentiation Medium Catalog No. RASMX-90031,Cyagen Biosciences, USA), osteogenic medium (OriCell™ Sprague–Dawley (SD) Rat Mesenchymal Stem Cell Osteogenic Differentiation Medium Catalog No. RASMX-90021, Cyagen Biosciences, USA), and chondrogenic Medium (OriCell™ Sprague–Dawley (SD) Rat Mesenchymal Stem Cell chondrogenic Differentiation Medium Catalog No. RASMX-90041, Cyagen Biosciences, USA), respectively, and then further cultured according to the product protocol. Before light microscope and capture images, the adipogenic cultures, osteogenic cultures, and chondrogenic cultures were stained with fresh oil Red-O working solution, alizarin red working solution, and alcian blue working solution, respectively.
Ad-GFP labeling of BM-MSCs
To ensure healthy cells prior to transduction, the culture medium was change when the BM-MSCs had reached 60% confluence. The medium containing 10% FBS was removed prior to transduction. The Ad-GFP (a gift from Chinese Academy of Military Medical Sciences) (Duan et al. 2003; Lu et al. 2006) was then added along with 200 μL of serum-free medium, and the flask was shaken gently every 15 min for 2 h. The passage 5 BM-MSCs were transduced with the recombinant replication-defective adenovirus at different multiplicities of infection (MOI) in the range 0–200 units. After incubation with Ad-GFP for 2 h, culture medium containing 10% FBS was added into the flask. The transduction efficiency was determined by flow cytometry after 48 h. The optimal MOI for transduction of BM-MSCs was used in the following steps.
Intracardiac transplantation of BM-MSCs into ischemic myocardium
After the left anterior descending coronary artery was ligated for 2 weeks, passage 5 BM-MSCs were harvested with trypsin, washed twice in PBS to remove free viral particles, and reconstituted immediately before transplantation. About 5 × 106 BM-MSCs transduced with Ad-GFP (Ad-GFP group) or a control adenovirus, replication-deficient recombinant adenoviral vectors, (control group) were injected into each animal at five locations within the infarct zone. During injections, the needle was left in place for several seconds in the injection location to prevent leakage of BM-MSCs. The same spatial distribution for cell injections was used in each animal. After the chest was closed, a breathing machine was used to assist until the rats resumed independent breathing.
Immunofluorescence staining of heart tissues
Rat hearts were harvested at 3, 7, 14, and 28 days after cell transplantation, washed with PBS through the aorta, and the left ventricle (LV) was then separated. Myocardial specimens were frozen in liquid nitrogen and then cut into 5 μm sections. In two adjacent sections, one was used to detected GFP-positive cells via fluorescence confocal microscopy (TCS SP-2; Leica Microsystems, Wetzlar, Germany) and the other one was stained with an antibody against GFP (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and visualized using an affinity-purified rhodamine or FITC-conjugated secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The survival of the implanted BM-MSCs was quantified by assaying the level of GFP expression. A medical image analysis and management system was used to perform semi-quantitative analyses of the GFP-positive area within the infarct zone. The expression volume of GFP in the infarct zone was calculated according to the formula: V = ΣA × d. ΣA: the area of positive expression in each section, d: the distance between two adjacent stained slices.
To identify the origin of the GFP-positive cells within the infarct zone, we used double-labeled immunohistochemical staining. Antibodies to von Willebrand factor (vWF) (1:1,000; Sigma–Aldrich, St. Louis, MO, USA) and cardiac myosin heavy chain (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used to detect endothelial cells and cardiac myocytes, respectively. Corresponding affinity-purified rhodamine or FITC conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used. Nuclei were labeled using 4′, 6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; Sigma-Aldrich, St. Louis, MO, USA). Images were obtained by confocal microscopy.
Immunoprecipitation and Western-blot analysis of ischemic heart tissues
At 7, 14, and 28 days after cell transplantation, small myocardial specimens for Western blotting were carefully dissected from the infarct zone areas and snap-frozen in liquid nitrogen. A total of 100 μg of each specimen was used to analyze on a 10% SDS-polyacrylamide gel, and immunoblotting was performed using antibodies against vascular endothelial growth factor (VEGF) (1:500), fibroblast growth factor (FGF-2) (1;200), tissue inhibitor of metalloproteinase-1 (TIMP-1) (1:400), matrix metalloproteinases-2 (MMP-2) (1:400), Bcl-2 (1:500), and β-actin (1:800) (all from Santa Cruz Biotechnology, Santa Cruz, CA, USA). Quantification was performed using the AlphaEaseFC Image program (Cell Biosciences, Santa Clara, CA, USA) in a blinded fashion.
Statistical analysis
All data are expressed as mean ± SEM. SPSS 11.5 software (SPSS, Chicago, IL, USA) was used for all analyses. Differences between two groups were evaluated using an unpaired Student’s t test. Values of P < 0.05 were considered statistically significant.
Results
The histology of myocardium infarction
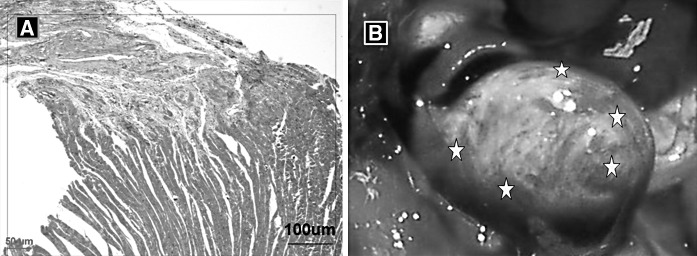
Two weeks after coronary ligation the infarct area of the rat hearts was replaced by fibrotic scar tissue. The distribution of surviving cardiac myocytes in the infarct border was diffuse (Fig. 1a). Animals displayed significant LV free wall thinning and LV dilatation, which are characteristics of LV remodeling. GFP cells localized in the heart area of SD rats are shown as Fig. 1b.
Fig. 1.
The histology of myocardium infarction and the site of cell transplantation. a Histological changes in myocardial infarction border zone. HE staining, Magnification ×200. b MSCs were injected in five locations per animal. The same spatial distribution for cell injections was used in each animal: two injections each along the infarct border zones of the anterior-septal and posterior-lateral walls and one near the apex of the infarct zone. The stars represent the sites of cell transplantation
Characterization of BM-MSCs
To characterize the BM-MSCs, we performed flow cytometry analysis for CD29, CD44, CD34 and CD45. The flow cytometry data demonstrate that these cells were positive for CD29 and CD44 but negative for CD34 and CD45, as described in our previous study (Guo et al. 2008).
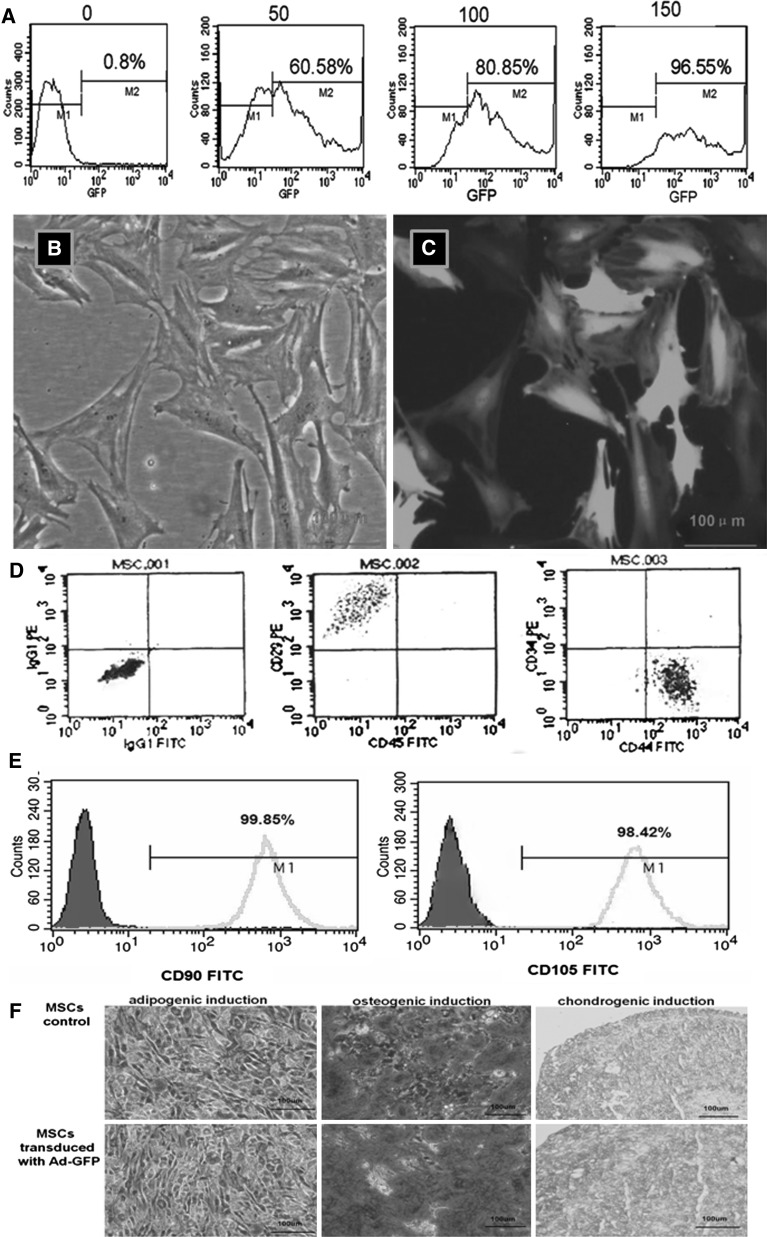
Labeling efficiency of BM-MSCs with Ad-GFP in vitro
The BM-MSCs were transduced with Ad-GFP at different MOIs (Fig. 2a). The MOI of 150 was determined to be ideal for transduction of BM-MSCs with high efficiency (96.55%) and low toxicity. The biological characteristics of the BM-MSCs did not change after transduction. Therefore, BM-MSCs were transduced with Ad-GFP at an MOI of 150 for the following experiments. Control BM-MSCs were transduced with a control adenoviral vector at the same MOI. After Ad-GFP transduction for 48 h, the BM-MSCs were green and spindle-shaped with round nuclei and clear nucleoli, as observed using a fluorescence microscope (Fig. 2b, c). Flow cytometry analysis for CD90, CD105, CD29, CD44, CD34 and CD45 is shown as Fig. 2d, e. BM-MSCs were differentiated in vitro using adipogenic, oesteogenic and chondrogenic induction media. Following 14 days of adipogenic induction, the cells stained oil red ‘O’ positive showing lipid laden adipocyte phenotype. Similarly, when induced with oesteogenic induction medium for 21 days, these cells showed oesteogensis upon staining with alizarin red for calcium deposits. When induced with chondrogenic induction medium for 21 days, these cells showed chondrogensis upon staining with alcian blue (Fig. 2f).
Fig. 2.
Transduction of MSCs with Ad-GFP for 48 h. a The transduction efficiency of the adenovirus vector carrying GFP in function of different MOIs as detected by flow cytometry. b and c Transduction of MSCs with Ad-GFP at an MOI of 150 for 48 h. The same field viewed under an ordinary inverted microscope and fluorescence microscope, respectively. Magnification ×400. d and e Phenotype characterization of MSCs. MSCs were positive for CD90, CD105, CD29 and CD44, but negative for CD34 and CD45. f Adipogenic induction, osteogenic induction and chondrogenic induction of rat bone marrow mesenchymal stem cells before or after transduction are shown
In vivo tracking GFP labeled BM-MSCs in ischemic heart tissues
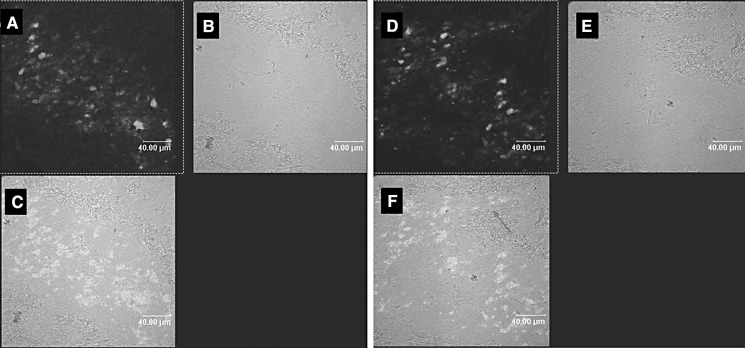
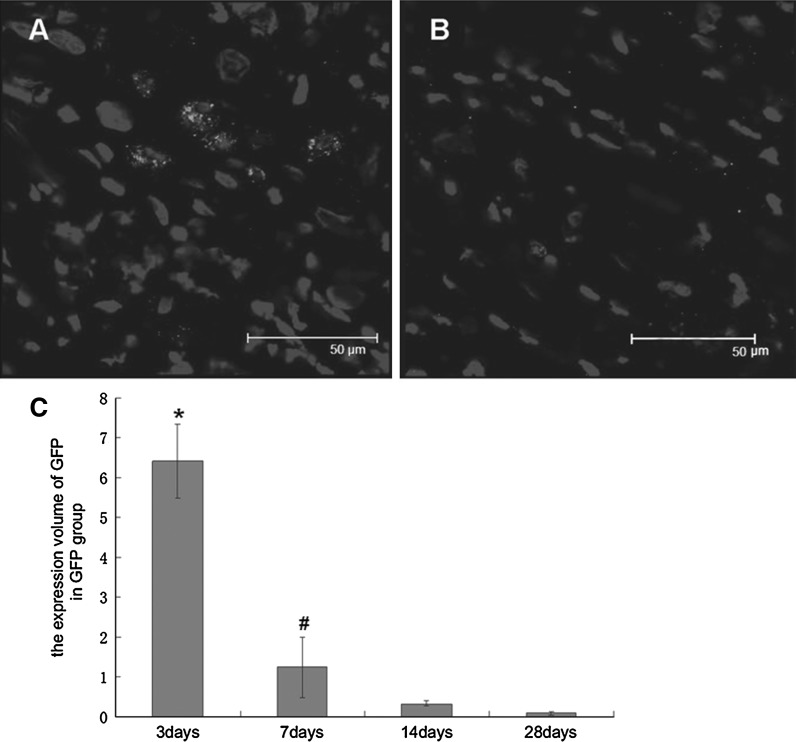
Three days after cell transplantation, we observed GFP-positive cells distributed along the path of the injection needles in the Ad-GFP group as shown in confocal micrograph (Fig. 3). At 7 days after cell transplantation, we detected vague GFP fluorescence and could not detect GFP fluorescence after 14 days by confocal microscope. A GFP antibody was used to detect GFP and was visualized with a rhodamine or FITC-conjugated secondary antibody. Distinct GFP-positive cells were observed with this method, and the number of positive cells was shown to have decreased significantly after 7 days. Quantitative analysis showed that the expression volume of GFP in the Ad-GFP group was 6.42 ± 0.92 mm3 after 3 days and 1.24 ± 0.76 mm3 after 7 days (Fig. 4). The implanted BM-MSCs demonstrated migratory behavior, as some GFP-positive cells were present in the nearby cardiac muscle. At 14 and 28 days after cell transplantation, the number of GFP positive cells was further decreased, and the expression volume of GFP was 0.33 ± 0.03 and 0.09 ± 0.05 mm3, respectively. Most of the GFP-positive cells were observed in the nearby cardiac muscle. The shape of the GFP-positive cells was round or elliptic, and the DAPI-labeled nuclei were intact, not cracked or dissolved.
Fig. 3.
Distribution of GFP positive cells along the track of the injection needle in the infarct zone of hearts at 3 days after transplantation in the Ad-GFP group. Different fields viewed under an ordinary invert microscope (a–c) and confocal microscope (d–f) are presented. a and d GFP positive MSCs, b and e heart tissue, c and f merge of the two images. Big arrow: GFP positive cells along the track of the injection needle; Small arrow: GFP positive cells present in the nearby cardiac muscle
Fig. 4.
A GFP antibody was used to detect the GFP labeled cells in situ at 7 days (a) and 28 days (b) after transplantation, and distinct GFP-positive cells can be seen. The number of positive cells was observed to have decreased gradually (c). * P < 0.05 vs. 7 days or 14 days or 28 days; # P < 0.05 vs. 3 or 14 or 28 days
We defined the identity of the GFP-positive cells within the tissue samples, and most were vWF positive (Fig. 5). The percentage of double stained cells was 2.43 ± 2.04. vWF and cardiac myosin heavy chain staining showed that GFP-positive cells had differentiated into endothelial cells at 28 days after transplantation. No GFP-positive cells were found to have differentiated into cardiac myocytes.
Fig. 5.
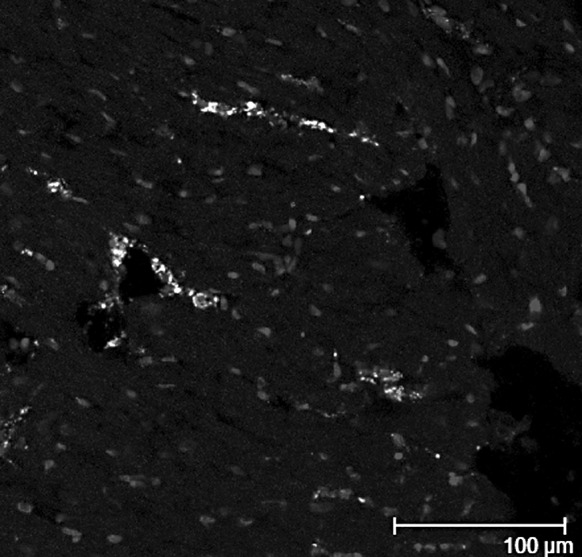
Distribution of GFP and vWF positive cells in the infarct zone of the heart at 28 days after implantation. Nuclei are stained with DAPI. Arrow: GFP and vWF positive cells
GFP labeling did not affect the paracrine actions of BM-MSCs
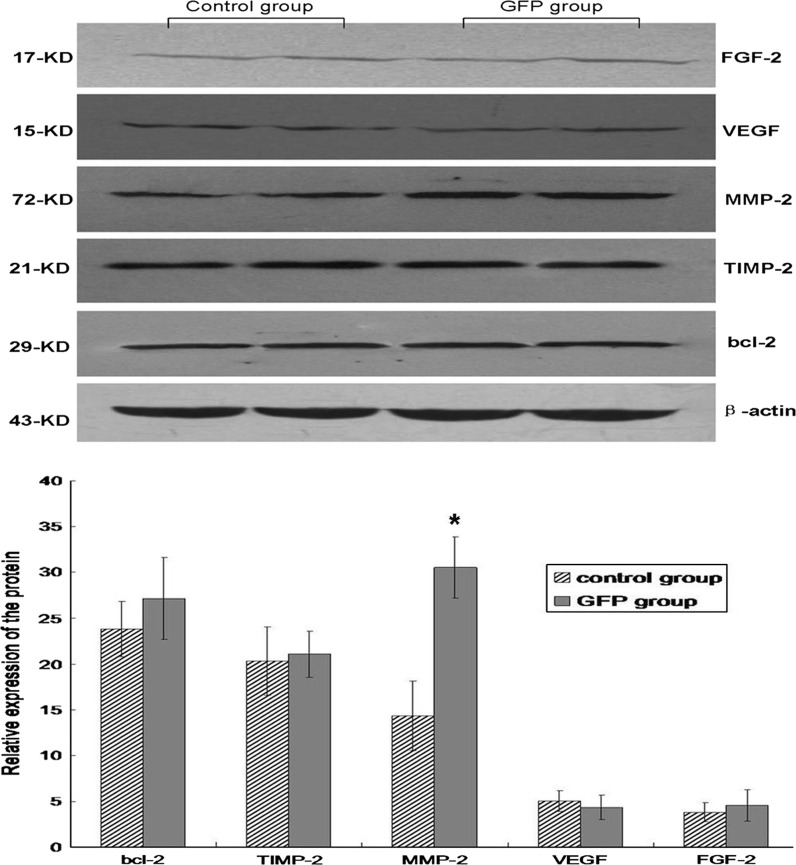
We examined the expression of several growth factors and cytokine proteins in the GFP-labeled cells. The results showed that the labeled BM-MSCs remained fully active for expressing key growth factors and cytokines, and the expression of FGF, VEGF, TIMP-2, and Bcl-2 were not significantly affected by GFP labeled BM-MSCs transplantation. Interestingly, expression of MMP-2 was upregulated approximately twofold in the Ad-GFP group. These results indicated that expression of key growth factors and cytokine proteins was not affected by GFP labeling and that the cell migration and remodeling potential of BM-MSCs in response to tissue injury may be enhanced by GFP labeled BM-MSCs (Fig. 6).
Fig. 6.
Comparison of protein levels in each group via Western blot analysis. The expression of FGF-2, VEGF, TIMP-2, and Bcl-2 were not significantly affected by GFP labeling. MMP-2 was significantly increased in the Ad-GFP group at 7 days after transplantation. Western blots were quantitated from six separate experiments by densitometric analysis. *P < 0.05 versus control group
Discussion
Our results showed that GFP labeling can be stably retained in implanted BM-MSCs for at least 1 month after implantation and that these labeled BM-MSCs can be detected by histological analysis. Further data indicated that GFP did not affect most proteins expressed by the transplanted BM-MSCs.
Stem cells have shown promise for the repair of damaged cardiac tissue, and clinical trials are already underway (Hare et al. 2009; Meyer et al. 2006). To understand the mechanism of cell-based cardiac regeneration therapy, and to improve safety and efficacy, monitoring of the fate and biodistribution of cardiac-delivered SCs is necessary. GFP has become the marker of choice for many types of cell transplantation and lineage marking experiments. It is biocompatible, safe, nontoxic, is detected with high sensitivity and specificity in suspended cells by flow cytometry, and is readily detected in live, frozen, or fixed cells using established methods (Lippincott-Schwarhz and Patterson 2003; Rizzo et al. 2009). The sensitivity and relative simplicity of GFP detection make it appealing. At present, GFP has already been used as an agent for tracking SCs (Duan et al. 2003; Xu et al. 2010); however, little information is known about the retention of injected GFP-labeled cells implanted in the ischemic heart over time.
In the present study, we transduced BM-MSCs for 48 h with a recombinant adenovirus encoding GFP. The transduction of BM-MSCs with the adenoviral vector demonstrated high efficiency (96.55%) and low toxicity, and the biological characteristics of the BM-MSCs did not change after transduction.
After the BM-MSCs were transplanted in the rat hearts, we directly observed BM-MSCs expressing GFP in situ in sliced tissue sections by confocal microscopy. The GFP labeled BM-MSCs could be traced for 7 days before the fluorescence became vague and intangible. This labeling and tracking technique is simple, but the labeling duration is not particularly long lived. We found that the method using a GFP antibody to detect GFP in situ was more effective and precise. Using this method, GFP labeled BM-MSCs could be traced for at least 28 days after transplantation. We observed that the numbers of GFP-positive SCs in the ischemic heart tissue were approximately 20% at 7 days, 5% at 14 days, and 1% at 28 days relative to the total BM-MSCs administered. In a previous study (Wu et al. 2007), BM-MSCs were injected around skin wounds or in the wound beds of normal and diabetic mice. The survival rate of the GFP-expressing BM-MSCs in the wounds was shown to be 27% at 7 days, 7.6% at 14 days, and 2.5% at 28 days relative to the total BM-MSCs administered. Therefore, the survival rate of BM-MSCs in the myocardium is lower than that in the skin.
In this study we found that GFP expression was gradually lost. One reason for this may be decreased cell survival, cell migration, and/or cell differentiation; another reason maybe the transgene transferred by the adenoviral vectors is only transiently expressed, thus GFP will disappear after a certain time. In addition, transduced BM-MSCs will also express some adenoviral proteins that may be ‘seen’ as foreign by the immune system of the MI rats and be eliminated within a short time.
Using GFP to track BM-MSCs after delivery to rat hearts with MI, we could monitor the survival and migration as well as the differentiation of the implanted BM-MSCs. Employing immunostaining and specific antibodies to detect surface marker expression, we were able to monitor the differentiation of the implanted BM-MSCs. In our study we found that GFP-positive cells had differentiated into endothelial cells instead of cardiac myocytes.
Although MSCs possess multilineage potentials, the therapeutic effects of MSCs in cardiac tissues are currently thought to be largely mediated by their paracrine actions (i.e., their ability to produce a multitude of growth factors and cytokines) (Caplan and Dennis 2006; Dai et al. 2005; Gnecchi et al. 2006; Kinnaird et al. 2004), and FGF and VEGF are well known for their regulatory and therapeutic effects on the cardiovascular system (Suzuki et al. 2005; Wang et al. 2006). It is therefore critical to determine whether GFP labeling may adversely affect the paracrine actions of MSCs. We examined the expression of several growth factors and cytokine proteins in the GFP-labeled BM-MSCs. Our study demonstrated that expression of key growth factors and cytokine genes were not affected by GFP labeling. However, we found that the expression of MMP-2 was upregulated in the Ad-GFP group. This result indicated that the migration potential of BM-MSCs in response to ischemic may be enhanced by GFP labeling.
Currently cell tracing techniques involve 5-bromodeoxyuridine (5-BrdU) labeling, fluorescent labeling, genetic labeling, and magnetic labeling, etc. Although 5 BrdU, the dyes DAPI, CM-DiI, CFSE and other labels have a property of high efficiency labeling, these markers will decay or disappear gradually with time. Markers, such as Liposome, are used for short-term tracing of living cells, and have a relatively low transfection efficiency. Magnetic labeling is applied to clinical and experimental study of large animals and may have many side effects (Hamm et al. 2002; Stroh et al. 2009; Yan et al. 2007). Here, we presented results showing that the GFP labeling strategy could be reliably used for tracking BM-MSCs in an MI rat model. GFP labeling could also be used to track MSC distribution and fate after delivery in rat hearts with MI for at least 28 days, and this SC tracking strategy did not adversely affect the paracrine actions of BM-MSCs.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81000018), the Major Program of the Chinese PLA General Hospital Nursery funds (No. 10KMZ04) and the opening foundation of the State Key Laboratory of Space Medicine Fundamentals and Application, Chinese Astroaut Research and Training Center (No. SMFA11K02).
Glossary
- GFP
Green fluorescent protein
- BM-MSCs
Bone marrow-mesenchymal stem cells
- SC
Stem cell
- Ad-GFP
Adenovirus vector carrying GFP
- SD rats
Sprague–Dawley rats
- MI
Myocardial infarction
- MOIs
Multiplicities of infection
- PBS
Phosphate-buffered saline
- LV
Left ventricle
- VEGF
Vascular endothelial growth factor
- FGF
Fibroblast growth factor
- TIMP-1
Tissue inhibitor of metalloproteinase-1
- MMP-2
Matrix metalloproteinases-2
- vWF
von Willebrand factor
References
- Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR. Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transpl. 2006;15:711–721. doi: 10.3727/000000006783981503. [DOI] [PubMed] [Google Scholar]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Phenotypical and functional properties of human bone marrow mesenchymal progenitor cells. J Cell Physiol. 1999;181:67–73. doi: 10.1002/(SICI)1097-4652(199910)181:1<67::AID-JCP7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 8:315–317 [DOI] [PubMed]
- Duan HF, Wu CT, Wu DL, Lu Y, Liu HJ, Ha XQ, Zhang QW, Wang H, Jia XX, Wang LS. Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther. 2003;8:467–474. doi: 10.1016/S1525-0016(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Guo YH, He JG, Wu JL, Yang L, Zhang DS, Tan XY, Qi RD. Hepatocyte growth factor and granulocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. Cytotherapy. 2008;10:857–867. doi: 10.1080/14653240802419278. [DOI] [PubMed] [Google Scholar]
- Hamm A, Krott N, Breibach I, Blindt R, Bosserhoff AK. Efficient transfection method for primary cells. Tissue Eng. 2002;8:235–245. doi: 10.1089/107632702753725003. [DOI] [PubMed] [Google Scholar]
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Zhang Z, Guo J, Ni A, Deb A, Zhang L, Mirotsou M, Pratt RE, Dzau VJ. Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res. 2010;106:1753–1762. doi: 10.1161/CIRCRESAHA.109.196030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser KA, Beagles KE, Kiem HP. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transpl. 2007;16:555–562. doi: 10.3727/000000007783464939. [DOI] [PubMed] [Google Scholar]
- Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines promote in vitro in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–685. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- Kruglyakov PV, Sokolova IB, Zin’kova NN, Viide SK, Aleksandrov GV, Petrov NS, Polyntsev DG. In vitro and in vivo differentiation of mesenchymal stem cells in the cardiomyocyte direction. Bull Exp Biol Med. 2006;142:503–506. doi: 10.1007/s10517-006-0403-x. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy. 2005;7:36–45. doi: 10.1080/14653240510018118. [DOI] [PubMed] [Google Scholar]
- Leiker M, Suzuki G, Iyer VS, Canty JM, Jr, Lee T. Assessment of a nuclear affinity labeling method for tracking implanted mesenchymal stem cells. Cell Transpl. 2008;17:911–922. doi: 10.3727/096368908786576444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwarhz J, Patterson GH. Development and use of fluorescent protein markers in living cells. Science. 2003;300:87–91. doi: 10.1126/science.1082520. [DOI] [PubMed] [Google Scholar]
- Lu ZZ, Ni F, Hu ZB, Wang L, Wang H, Zhang QW, Huang WR, Wu CT, Wang LS. Efficient gene transfer into hematopoietic cells by a retargeting adenoviral vector system with a chimeric fiber of adenovirus serotype 5 and 11p. Exp Hematol. 2006;34:1171–1182. doi: 10.1016/j.exphem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled BOOST (Bone marrow transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- Ohnishi S, Yanagawa B, Tanaka K, Miyahara Y, Obata H, Kataoka M, Kodama M, Ishibashi-Ueda H, Kangawa K, Kitamura S, Nagaya N. Transplantation of mesenchymal stem cells attenuates myocardial injury and dysfunction in a rat model of acute myocarditis. J Mol Cell Cardiol. 2007;42:88–97. doi: 10.1016/j.yjmcc.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Rizzo MA, Davidson MW, Piston DW (2009) Fluorescent protein tracking and detection: fluorescent protein structure and color variants. Cold Spring Harb Protoc 12: pdb.top63 [DOI] [PubMed]
- Stroh A, Boltze J, Sieland K, Hild K, Gutzeit C, Jung T, Kressel J, Hau S, Reich D, Grune T, Zimmer C. Impact of magnetic labeling on human and mouse stem cells and their long-term magnetic resonance tracking in a rat model of Parkinson disease. Mol Imag. 2009;8:166–178. [PubMed] [Google Scholar]
- Suzuki G, Lee T, Fallavollita JA, Canty JM., Jr Adenoviral gene transfer of FGF-5 to hibernating myocardium improves function stimulates myocytes to hypertrophy and reenter the cell cycle. Circ Res. 2005;96:767–775. doi: 10.1161/01.RES.0000162099.01268.d1. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu Q, Mansoor A, Lee J, Wang Z, Lee T, From AH, Zhang J (2006) Bioenergetic and functional consequences of stem cell-based VEGF delivery in pressure-overloaded swine hearts. Am J Physiol Heart Circ Physiol 290: H1393–H1405 [DOI] [PubMed]
- Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Xu X, Yang Z, Liu Q, Wang Y. In vivo fluorescence imaging of muscle cell regeneration by transplanted EGFP-labeled myoblasts. Mol Ther. 2010;18:835–842. doi: 10.1038/mt.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Han Y, He Y, Xie H, Liu J, Zhao L, Wang J, Gao L, Fan D. Cell tracing techniques in stem cell transplantation. Stem Cell Rev. 2007;3:265–269. doi: 10.1007/s12015-007-9004-y. [DOI] [PubMed] [Google Scholar]