Abstract
Glycyrrhetinic acid (GA) is the active compound in Glycyrrhizae radix, a famous traditional Chinese medicine. Recently the anticancer activity of GA became the focus of scientific interest and many GA derivatives were developed as anti-tumor lead compounds. We previously reported that AEGA, a GA derivative, has proliferation inhibition and apoptosis-inducing activity in various human tumor cells. The present study was undertaken to further investigate the molecular mechanisms involved in AEGA-induced apoptosis in human leukemia K562 cells. AEGA can inhibit the growth of K562 cells in dose- and time-dependent manners determined by the MTT assay. Induction of apoptosis was evidenced by morphological changes and biochemical markers such as cell shrinkage, chromatin condensation and DNA ladder formation. Further mechanistic analysis revealed that AEGA induced apoptosis through the collapse of mitochondrial membrane potential, the accumulation of the cytosolic cytochrome c and the activation of caspase-9 and caspase-3. The apoptosis induction by AEGA was associated with the alteration in the ratio of Bcl-2/Bax protein expression. These results suggest that AEGA may induce apoptosis through a mitochondria-mediated pathway, and might have the therapeutic value against hematological malignancies.
Keywords: AEGA, Glycyrrhetinic acid derivative, Apoptosis, Mitochondrial membrane potential, Bcl-2/Bax, Human leukemic cells
Introduction
Leukemia is a cancer of blood-forming cells in the bone marrow and one of the leading causes of human death. The most widely used anti-leukemia treatments include chemotherapy, radiotherapy, hormonal therapy and bone marrow transplantation, but no treatment is really satisfactory. Although chemotherapeutic agents research has made great progress in the battle against leukemia, it is still a pressing issue to develop new drugs without damaging healthy cells and tissues (Gu and Mason 2010; Liu and Han 2003; Tallman 1996). The chronic myelogenous leukemia derived K562 cells have the abnormal fusion gene BCR-ABL of the b3a2 type and a mutated gene for the p53 protein (Grebenova et al. 2004; Law et al. 1993; McGahon et al. 1994). These combined mutations make K562 cells a suitable model for the ‘in vitro’ studies to evaluate the growth inhibitory and apoptosis-inducing effects of leading compounds in anticancer drug discovery.
It is one of the effective ways to develop low-toxic high-effective medicine by using active ingredients from natural sources as leading compounds. Glycyrrhetinic acid (GA) is the oleanane pentacyclic triterpenoid aglycone of glycyrrhizin in licorice roots (Glycyrrhiza radix) which is a famous traditional Chinese medicine and have been widely used throughout the world for thousands of years. GA exhibits many pharmacological activities, including anti-inflammation, anti-ulcer, anti-allergenic, antivirus and anticancer activities (Fiore et al. 2005; Shibata 2000). Derivatives of GA also show wide pharmacological effects. Carbenoxolone, a derivative of GA, has been used as a medicine in Europe for the treatment of ulcers and inflammation (Davis and Morris 1991). Methyl 2-cyano-3,11-dioxo-18-olean-1,12-dien-30-oate (CDODA-Me), a GA derivative, has shown growth inhibition effect to prostate, pancreatic, and colon tumor cells. Oral administration of CDODA-Me at the dose of 15 mg/kg/day exhibited excellent inhibition effects on tumor growth without causing any obvious toxic side effects (Chintharlapalli et al. 2009, 2007; Jutooru et al. 2009). It has been reported that GA derivatives with an alkoxyimino group at position C3 and a free C29 carboxyl group have greater antiproliferative and apoptosis induction effects in human leukemia HL60 cells (Liu et al. 2007).
We have previously demonstrated that AEGA, a GA derivative, had stronger inhibitory effects against human hepatocellular carcinoma BEL-7404 cells, human breast cancer MDA-MB-231 cells, human leukemia HL60 and K562 cells compared to the parent compound 18β-GA (Gao et al. 2011). However, the cellular and molecular mechanisms involved in AEGA-mediated apoptosis remained unclear. In this study, we investigated the proapoptotic effects of AEGA on K562 cells in terms of the loss of mitochondrial membrane potential, the release of cytochrome c into the cytosol, the altered expression of Bcl-2 family proteins and the activation of caspase-9 and caspase-3.
Materials and methods
Chemicals and antibodies
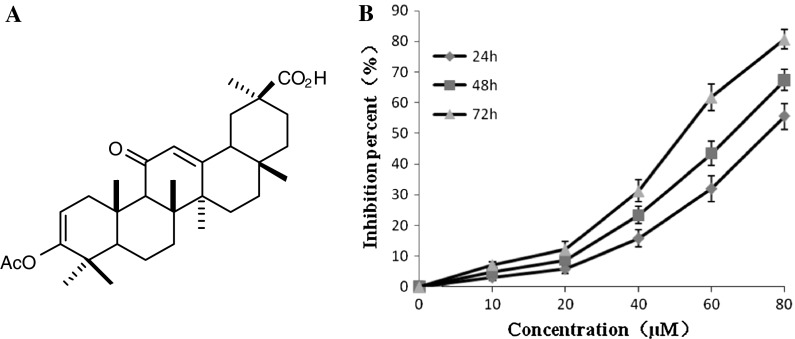
The GA derivative AEGA was synthesized as described previously (Gao et al. 2011). Its chemical structure is given in Fig. 1a. AEGA was weighed and resolved with absolute dimethyl sulfoxide (DMSO) (Sigma, USA) and dilutions were made in DMEM. Primary antibodies against Bcl-2, Bax, caspase-3, caspase-9, cytochrome c, and peroxidase-conjugated goat antimouse or antirabbit secondary antibody were purchased from Beyotime Technology (Beyotime, China).
Fig. 1.
a Chemical structure of AEGA. b Effects of AEGA on the growth of K562 cells in a dose-dependent and time-dependent manner. K562 cells were treated with various doses of AEGA at 24, 48 and 72 h. Each point represents the mean ± SD (n = 3)
Cell culture and in vitro proliferation assay
Human erythroleukemia cells K562 were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. Cells were grown in DMEM medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 2 mM-glutamine, and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin), at 37 °C in a humidified 95% air/5% carbon dioxide atmosphere. Growth inhibition of GA and AEGA on tumor cells was measured by the 3-(4,5-dimethylthiazol-2yl)-2,5-di-phenyl-tetrazolium bromide (MTT) assay with minor modifications (Shao et al. 2005). Cells were seeded in 96-well plates at a density of 1.5 × 104 cells/mL and incubated with the tested compounds at various concentrations in DMEM-10% fetal bovine serum medium for 24, 48 or 72 h and each concentration was tested in triplicate. Control samples were exposed to 0.1% DMSO. At the end of incubation period, 20 μL MTT was added to each well and the plates were incubated for 4 h at 37 °C, then the “triplex solution” (10% SDS, 5% isobutanol, 0.012 M HCl) was added and the plates were incubated for 12 h at 37 °C to solubilize formazon crystals. The Absorbance was read on a scanning multiwell spectrophotometer at the wavelength of 490 nm. The inhibitory rate of cell proliferation was worked out according to the following formula: Growth inhibition (%) = [OD control−OD treated/OD control] × 100%. The TC50 values were calculated by log-probit regression analysis.
Apoptotic induction assays
Propidium iodide (PI) staining assays were done using the PI staining Kit (Keygen, China) according to the manufacturer’s instructions. The cells were stained with PI and observed using fluorescence microscope (Nikon, Japan). For DNA fragmentation assay, cells were harvested by centrifugation and total DNA was extracted with a DNA extraction kit (Beyotime, China) according to the manufacturer’s instructions. The eluants containing DNA pellets were separated by electrophoresis on a 2% agarose gel at 80 V. The gel was visualized with a UV light transilluminator after staining with ethidium bromide.
Western blot analysis
Cells were harvested and washed with PBS. Whole cellular proteins were extracted and cytosolic fractions were prepared following the procedure described by the manufacturer (Beyotime, China). The protein concentration of the extracts was determined using the Bradford method. Equal amounts of sample lysates were subjected to 12% SDS-PAGE and then transferred onto nitrocellulose membranes. The membranes were blocked with 5% non-fat milk in TBST buffer (20 mM Tris pH 7.5, 500 mM NaCl, 0.1% Tween-20) overnight at room temperature. The membranes were incubated with the desired primary antibodies (1:1,000 dilution) for 4 h at 4 °C, and then incubated with enzyme-linked secondary antibodies (1:10,000 dilution) for 1 h at room temperature. Immunoreactive bands were visualized with enhanced chemiluminescence detection regents (Amersham, USA).
Detection of mitochondrial membrane potential (Δψm)
The cells were incubated with or without AEGA (60 μM) for 48 h and the depolarizing agent carbonyl cyanide m-chlorophenyl-hydrazone (CCCP) at 10 μM was used as a positive control. Following treatment, cells were washed with PBS and incubated in DMEM medium containing JC-1 dye for 20 min at 37 °C in the dark. The mitochondrial deopolarization patterns of the cells were observed using a fluorescence microscope (Nikon, Japan) according to the manufacturer’s instructions (Beyotime, China).
Caspase-3 and caspase-9 activities assay
Caspases activities were determined using Caspase Activity Kit (Beyotime, China) according to the manufacturer’s instructions. Briefly, cells were collected,washed with cold PBS and resuspended in lysis buffer for 15 min on ice. Lysates were centrifuged at 16,000 g for 15 min at 4 °C. The protein concentration of the extracts was determined using the Bradford method. Activities of caspase-3, and -9 were measured by cleavage of substrate peptides Ac-DEVD-pNA and Ac-LEHD-pNA to yellow formazan product, p-nitroaniline (pNA), which was quantified on a scanning multiwell spectrophotometer at an absorbance of 405 nm. Results were expressed as the fold change of enzyme activity compared to that of untreated cells.
Statistical analysis
All data were presented as means ± standard deviation (SD) of three independent experiments. Statistical comparisons of the results were done using one-way analysis of variance (ANOVA). A p-value less than 0.05 is considered as statistically significant.
Results
Effects of AEGA on cell proliferation
The antiproliferative activities of AEGA against K562 cells were examined by MTT assay as described in “Materials and methods” section. K562 cells were treated with various doses of AEGA for 24, 48 and 72 h. As shown in Fig. 1b, AEGA inhibited cellular proliferation not only in a dose-dependent manner, but also in a time-dependent way. After K562 cells were exposed to AEGA for 24, 48 and 72 h, the IC50 value was 84.39, 64.45 and 47.39 μM, respectively.
Effect of AEGA on cell apoptosis
As shown in Fig. 2, nuclei with karyopyknosis and conglomeration, a characteristic of apoptosis, were observed under fluorescence microscopy in cells cultured with AEGA, while the control cells appeared with regular contours. Apoptosis was also examined by a DNA fragmentation assay. After treatment of K562 cells with AEGA, typical DNA ladders of 180-bp fragments were clearly visible in agarose gel (Fig. 3).
Fig. 2.

Morphological analysis of AEGA treated K562 cells by Propidium Iodide staining under an inverted fluorescent microscope (200×). Untreated samples were exposed to 0.1% DMSO. Positive samples were exposed to 0.25 μM Paclitaxel for 48 h. Cells treated with 20 μM AEGA or 40 μM AEGA for 48 h both displayed significant morphological characteristic of apoptosis, such as karyopyknosis and conglomeration. Arrows indicate apoptotic cells
Fig. 3.
Assessment of apoptosis in K562 cells by the DNA fragmentation assay. Typical DNA ladders for apoptotic cells were clearly visible in agarose gel in a dose-dependent manner. Lane 1: marker, Lane 2: untreated conditions, Lane 3: with 20 μM AEGA treatment for 48 h, Lane 4: with 40 μM AEGA treatment for 48 h
Effects of AEGA on the mitochondrial membrane potential
The effect of AEGA on Δψm was detected using JC-1 staining. Fluorescent probe JC-1 is a lipophilic cation molecule and mitochondrion specific. In the control cells, JC-1 could aggregate in mitochondria and present high red fluorescence. However, in cells undergoing apoptosis, where the mitochondrial potential has collapsed, JC-1 exists in the cytosol as a monomer which emits green fluorescence. As shown in Fig. 4, the control cells appeared in bright red fluorescence and weak green fluorescence, reflecting hyperpolarized mitochondria. In contrast, cells treated with AEGA displayed enhanced green fluorescence and decreased red fluorescence which indicated the depolarization of mitochondrial potential.
Fig. 4.
Effects of AEGA on the mitochondrial membrane potential. K562 cells were loaded with JC-1 dye in culture medium and incubated for 20 min after different treatments and two fluorescence images (green and red channels, respectively) were captured (200×). The treated cells were exposure to 60 μM AEGA for 48 h. The positive groups were exposure to 10 μM CCCP for 30 min. Red fluorescence represents JC-1 aggregation within the mitochondria, indicating cells with high mitochondrial membrane potential. Green fluorescence represents the JC-1 in the cytosol as a monomer, reflecting dissipation of ΔΨm. Cells treated with AEGA or CCCP show decreased red fluorescence and increased green fluorescence while control cells show bright red fluorescence and decreased green fluorescence (A red channel, B green channel, C merged image)
Effects of AEGA on the expression of apoptosis-related proteins
As shown in Fig. 5, the cytosol cytochrome c increased in a time dependent manner after treatment with AEGA compared to untreated controls. AEGA led to a significant decrease in the level of caspase-3 and caspase-9 proenzymes after K562 cells were treated with 80 μM for 24 h. Treatment of cells with AEGA resulted in the down-regulation of Bcl-2 expression and also significant up-regulation of Bax, as shown in Fig. 5.
Fig. 5.
Effects of AEGA on protein expressions of Bcl-2/Bax (a), procaspase-9 (b), procaspase-3 (c), and cytosolic cytochrome c (Cyto C) (d) in K562 cells. The protein expressions were detected by Western Blotting analysis as described in “Materials and methods” section. Cells were cultured in the presence of 80 μM AEGA for 0, 6, 12, 18, 24 and 36 h, respectively. Total cell proteins or the cytosolic proteins were fractionated through a 12% SDS–polyacrylamide gel, transferred to a nitrocellulose membrane and probed with antibodies directed against various apoptosis-related proteins. Reprobing with β-actin antibody was used as a loading control. The optical density (OD) of the band is normalized with respective β-actin and is expressed as relative optical density (OD). All experiments were carried out at least in triplicates and only one set of gels is presented. Statistical analysis is done using one-way analysis of variance (ANOVA). *p < 0.01, **p < 0.001, compared with untreated groups
Effects of AEGA on the activity of caspase-3 and caspase-9
To explore the activation of caspase-3 and caspase-9, the enzyme activities of caspase-3 and caspase-9 were measured by spectrophotometric method. As shown in Fig. 6, after AEGA stimulation caspase-3 and caspase-9 activities both increase in a time dependent manner from 6 to 36 h.
Fig. 6.
Effects of AEGA on the activity of caspase-3 (a) and caspase-9 (b). Cells were treated with 80 μM AEGA for 0–36 h. Through spectrophotometric method the increase of enzyme activities of caspase-3 and caspase-9 were detected after AEGA stimulation. Relative activity of caspase-3 and caspase-9 were expressed as the fold change of enzyme activity compared to that of untreated cells. Results were the mean ± SD of three independent experiments. *p < 0.05, **p < 0.01, compared with untreated groups
Discussion
There has been a growing interest in developing new chemotherapeutic drugs for cancer therapy from natural sources (Liu et al. 2009; Zhang et al. 1999, 2001). Many GA derivatives were synthesized as anti-tumor agents (Csuk et al. 2010; Gao et al. 2010; Hu et al. 2010; Chadalapaka et al. 2008). We have previously demonstrated that AEGA, a GA derivative, had much stronger inhibitory effects against human leukemia K562 cells compared to the parent compound 18β-GA (Gao et al. 2011). The present study was undertaken to further investigate the molecular mechanisms involved in AEGA-induced apoptosis in K562 cells.
It has been reported that the anti-cancer activity of many chemotherapeutic agents are associated with the apoptosis-inducing effects (Huang et al. 2009; Vahedi et al. 2008; Yu et al. 2009). In Fig. 1b, AEGA inhibited the growth of the K562 cells in a dose- and time-dependent manner. In Fig. 2, after K562 cells were treated with AEGA at 20 μM or 40 μM for 48 h typical morphological characteristic of apoptosis such as karyopyknosis and conglomeration were observed.
The DNA ladder formation is considered the most characteristic feature of conventional apoptosis. As shown in Fig. 3, typical DNA ladders pattern in electrophoresis were visible for K562 cells treated with AEGA of 20 μM or 40 μM for 48 h. This provided an important experimental evidence for that AEGA could induce apoptosis on K562 cells.
In the intrinsic pathway involving mitochondria-dependent process of apoptosis, various pro-apoptotic signals induce the release of cytochrome c and other apoptogenic factors from the mitochondrial intermembrane space into the cytosol. Released cytochrome c binds to Apaf-1 and dATP to form a complex known as apoptosome which activates one of the initiator caspases, caspase-9. Activated caspase-9 in turn leads to the activation of the executioner caspases, such as caspase-3, caspase-6, caspase-7, which catalyzes a series of proteolytic events associated with apoptosis (Olson and Kornbluth 2001). As shown in Fig. 5, after K562 cells exposure to 80 μM AEGA, the cytosol cytochrome c increased in a time dependent manner. This indicated that the apoptosis induced by AEGA in K562 cells may be through the intrinsic pathway.
The dissipation of the Δψm is a manifestation of the functional impairment of mitochondria and is a critical event in mitochondria-dependent apoptosis (Heerdt et al. 1998; Marchetti et al. 1996). As shown in Fig. 4, cells treated with 60 μM AEGA for 48 h presented enhanced green fluorescence and weak red fluorescence which indicated the mitochondrial membrane depolarization.
Members of the Bcl-2 family proteins are composed of both pro- and anti-apoptotic proteins. The balance between these two groups plays a critical role in regulating cell growth and controlling mitochondrial membrane integrity (Basanez et al. 2001; Duan et al. 2010; Luan et al. 2010; Zhang et al. 2009). As shown in Fig. 5, the expression of Bax was up-regulated, but the expression of Bcl-2 was down-regulated after K562 cells exposure to AEGA. As a result of these changes, during AEGA treatment the Bcl-2/Bax ratio decreased significantly. These results indicate that AEGA activated mitochondria-mediated apoptotic pathway by regulating the expression of Bcl-2 family proteins.
Bcl-2 is an upstream substance among the apoptosis-related proteins analysed by western blotting in this paper. Bcl-2 has been validated as the target of some new anticancer agents (Huang 2000). We speculate that bcl-2 may be the reaction point of AEGA in the apoptotic pathway and more experiments are needed to verify this hypothesis.
In Fig. 5, the expression of proenzymes procaspase-3 and procaspase-9 were down-regulated by western blotting assay in a time dependent manner after K562 cells exposure to 80 μM AEGA. As shown in Fig. 6, with spectrophotometric method the increase of enzyme activities of caspase-3 and caspase-9 were detected after 80 μM AEGA stimulation. The activation of caspases is critical for the initiation and execution of apoptosis. These results indicate that AEGA-induced apoptosis may be involved in the regulation of the caspase pathway.
In summary, the results of the present study revealed that AEGA induced apoptosis in K562 cells through mitochondrial dysfunction with the collapse of mitochondrial membrane potential and the alteration in the ratio of Bcl-2/Bax protein expression. Released apoptogenic factors from mitochondrial to cytosol further trigger the apoptotic pathway mediated by the activation of caspase-9 and caspase-3. These results suggest that AEGA may induce apoptosis through a mitochondria-mediated pathway involving the activation of caspase cascade, and might be a promising lead compound suitable for developing new drug as antileukemia therapy.
Acknowledgments
This work is supported in part by Zhejiang Provincial Natural Science Foundation of China (Y4110320), Natural Science Foundation of China (Nos. 20772071, 20972086) and the Opening Foundation of Biomedicine Engineering Key Discipline, Zhejiang, China (SWYX0901).
Conflict of interest
The authors declare no conflict of interest.
Abbreviations
- GA
Glycyrrhetinic acid
- AEGA
3-Acetyl-2-ene-glycyrrhetinic acid
- DMSO
Dimethyl sulphoxide
- FBS
Fetal bovine serum
- MTT
3-(4,5-dimethylthiazoyl)-2,5-diphenyltetrazolium bromide
References
- Basanez G, Zhang J, Chau BN, Maksaev GI, Frolov VA, Brandt TA, Burch J, Hardwick JM, Zimmerberg J. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J Biol Chem. 2001;276:31083–31091. doi: 10.1074/jbc.M103879200. [DOI] [PubMed] [Google Scholar]
- Chadalapaka G, Jutooru I, McAlees A, Stefanac T, Safe S (2008) Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett 18:2633–2639 [DOI] [PMC free article] [PubMed]
- Chintharlapalli S, Papineni S, Jutooru I, McAlees A, Safe S. Structure-dependent activity of glycyrrhetinic acid derivatives as peroxisome proliferator-activated receptor gamma agonists in colon cancer cells. Mol Cancer Ther. 2007;6:1588–1598. doi: 10.1158/1535-7163.MCT-07-0022. [DOI] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Abdelrahim M, Abudayyeh A, Jutooru I, Chadalapaka G, Wu F, Mertens-Talcott S, Vanderlaag K, Cho SD, Smith RR, Safe S. Oncogenic microRNA-27a is a target for anticancer agent methyl 2-cyano-3, 11-dioxo-18beta-olean-1, 12-dien-30-oate in colon cancer cells. Int J Cancer. 2009;125:1965–1974. doi: 10.1002/ijc.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csuk R, Schwarz S, Kluge R, Strohl D. Synthesis and biological activity of some antitumor active derivatives from glycyrrhetinic acid. Eur J Med Chem. 2010;45:5718–5723. doi: 10.1016/j.ejmech.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Davis EA, Morris DJ. Medicinal uses of licorice through the millennia: the good and plenty of it. Mol Cell Endocrinol. 1991;78:1–6. doi: 10.1016/0303-7207(91)90179-V. [DOI] [PubMed] [Google Scholar]
- Duan H, Luan J, Liu Q, Yagasaki K, Zhang G. Suppression of human lung cancer cell growth and migration by berbamine. Cytotechnology. 2010;62:341–348. doi: 10.1007/s10616-009-9240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C, Eisenhut M, Ragazzi E, Zanchin G, Armanini D. A history of the therapeutic use of liquorice in Europe. J Ethnopharmacol. 2005;99:317–324. doi: 10.1016/j.jep.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Guo X, Li X, Liu D, Song D, Xu Y, Sun M, Jing Y, Zhao L. The synthesis of glycyrrhetinic acid derivatives containing a nitrogen heterocycle and their antiproliferative effects in human leukemia cells. Molecules. 2010;15:4439–4449. doi: 10.3390/molecules15064439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hu J, Kang X, Xu C, Ju Y (2011) Synthesis of A-ring functional derivatives of 18β-glycyrrhetinic acid and antiproliferative effect in tumor cells. Chem J Chin Univ, 29 Aug 2011 (Epub ahead of print)
- Grebenova D, Kuzelova K, Fuchs O, Halada P, Havlicek V, Marinov I, Hrkal Z. Interferon-alpha suppresses proliferation of chronic myelogenous leukemia cells K562 by extending cell cycle S-phase without inducing apoptosis. Blood Cells Mol Dis. 2004;32:262–269. doi: 10.1016/j.bcmd.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Gu BW, Mason PJ. A new potential anti-leukemia drug from a tropical plant. Leuk Res. 2010;34:1420–1421. doi: 10.1016/j.leukres.2010.05.032. [DOI] [PubMed] [Google Scholar]
- Heerdt BG, Houston MA, Anthony GM, Augenlicht LH. Mitochondrial membrane potential (delta psi(mt)) in the coordination of p53-independent proliferation and apoptosis pathways in human colonic carcinoma cells. Cancer Res. 1998;58:2869–2875. [PubMed] [Google Scholar]
- Hu J, Wu Y, Zhao C, Ju Y. Synthesis and anti-tumor activity of opened A-ring modified 18β-glycyrrhetinic acid derivatives. Chem J Chin Univ. 2010;31:1762–1768. [Google Scholar]
- Huang Z. Bcl-2 family proteins as targets for anticancer drug design. Oncogene. 2000;19:6627–6631. doi: 10.1038/sj.onc.1204087. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu Q, Liu K, Yagasaki K, Zhang G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59:209. doi: 10.1007/s10616-009-9221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Chintharlapalli S, Papineni S, Safe S. Induction of apoptosis and nonsteroidal anti-inflammatory drug-activated gene 1 in pancreatic cancer cells by a glycyrrhetinic acid derivative. Mol Carcinog. 2009;48:692–702. doi: 10.1002/mc.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JC, Ritke MK, Yalowich JC, Leder GH, Ferrell RE. Mutational inactivation of the p53 gene in the human erythroid leukemic K562 cell line. Leuk Res. 1993;17:1045–1050. doi: 10.1016/0145-2126(93)90161-D. [DOI] [PubMed] [Google Scholar]
- Liu P, Han ZC. Treatment of acute promyelocytic leukemia and other hematologic malignancies with arsenic trioxide: review of clinical and basic studies. Int J Hematol. 2003;78:32–39. doi: 10.1007/BF02983237. [DOI] [PubMed] [Google Scholar]
- Liu D, Song D, Guo G, Wang R, Lv J, Jing Y, Zhao L. The synthesis of 18beta-glycyrrhetinic acid derivatives which have increased antiproliferative and apoptotic effects in leukemia cells. Bioorg Med Chem. 2007;15:5432–5439. doi: 10.1016/j.bmc.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Liu Q, Duan H, Luan J, Yagasaki K, Zhang G. Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology. 2009;59:211–217. doi: 10.1007/s10616-009-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Duan H, Liu Q, Yagasaki K, Zhang G. Inhibitory effects of norcantharidin against human lung cancer cell growth and migration. Cytotechnology. 2010;62:349–355. doi: 10.1007/s10616-009-9250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti P, Castedo M, Susin SA, Zamzami N, Hirsch T, Macho A, Haeffner A, Hirsch F, Geuskens M, Kroemer G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J Exp Med. 1996;184:1155–1160. doi: 10.1084/jem.184.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGahon A, Bissonnette R, Schmitt M, Cotter KM, Green DR, Cotter TG. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood. 1994;83:1179–1187. [PubMed] [Google Scholar]
- Olson M, Kornbluth S. Mitochondria in apoptosis and human disease. Curr Mol Med. 2001;1:91–122. doi: 10.2174/1566524013364239. [DOI] [PubMed] [Google Scholar]
- Shao HJ, Qing C, Wang F, Zhang YL, Luo DQ, Liu JK. A new cytotoxic lanostane triterpenoid from the basidiomycete Hebeloma versipelle. J Antibiot (Tokyo) 2005;58:828–831. doi: 10.1038/ja.2005.111. [DOI] [PubMed] [Google Scholar]
- Shibata S. A drug over the millennia: pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- Tallman MS. Differentiating therapy with all-trans retinoic acid in acute myeloid leukemia. Leukemia. 1996;10:S12–S15. [PubMed] [Google Scholar]
- Vahedi F, Fathi NM, Bozari K. Evaluation of inhibitory effect and apoptosis induction of Zyzyphus Jujube on tumor cell lines, an in vitro preliminary study. Cytotechnology. 2008;56:105–111. doi: 10.1007/s10616-008-9131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology. 2009;59:219–229. doi: 10.1007/s10616-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Effects of green, oolong and black teas and related components on the proliferation and invasion of hepatoma cells in culture. Cytotechnology. 1999;31:37–44. doi: 10.1023/A:1008076306672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Inhibitory effects of theanine and sera from theanine-fed rats on receptor-mediated cancer cell invasion beneath mesothelial-cell monolayers. Cytotechnology. 2001;36:195–200. doi: 10.1023/A:1014005423181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan H, Luan G, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191–200. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]