Abstract
Tissue engineering has recently become available as a treatment procedure for bone augmentation. However, this procedure has several problems, such as high capital investment and expensive cell culture, complicated safety and quality management issues regarding cell handling, and patient problems with the invasive procedure of cell collection. Moreover, it was reported that stem cells secrete many growth factors and chemokines during their cultivation, which could affect cellular characteristics and behavior. This study investigated the effect of stem-cell-cultured conditioned media on bone regeneration. Cultured conditioned media from human bone marrow–derived mesenchymal stem cells (MSC-CM) enhanced the migration, proliferation, and expression of osteogenic marker genes, such as osteocalcin and Runx2, of rat MSCs (rMSCs) in vitro. MSC-CM includes cytokines such as insulin-like growth factor-1 and vascular endothelial growth factor. In vivo, a prepared bone defect of a rat calvarial model was implanted in five different rat groups using one of the following graft materials: human MSCs/agarose (MSCs), MSC-CM/agarose (MSC-CM), Dulbecco's modified Eagle's medium without serum [DMEM(−)]/agarose [DMEM(−)], PBS/agarose (PBS), and defect only (Defect). After 4 and 8 weeks, implant sections were evaluated using microcomputed tomography (micro-CT) and histological analysis. Micro-CT analysis indicated that the MSC-CM group had a greater area of newly regenerated bone compared with the other groups (p<0.05) and histological analysis at 8 weeks indicated that the newly regenerated bone bridge almost covered the defect. Interestingly, the effects of MSC-CM were stronger than those of the MSC group. In vivo imaging and immunohistochemical staining of transgenic rats expressing green fluorescent protein also showed that migration of rMSCs to the bone defect in the MSC-CM group was greater than in the other groups. These results demonstrated that MSC-CM can regenerate bone through mobilization of endogenous stem cells. The use of stem-cell-cultured conditioned media for bone regeneration is a unique concept that utilizes paracrine factors of stem cells without cell transplantation.
Introduction
Reconstruction and replacement of bone loss, atrophy, and injury, including fracture, often need a certain amount of bone or other graft substitutes.
Autogenous bone graft is believed to be an effective method of bone grafting and is still regarded as the “gold standard” for bone augmentation procedures because of the available bone volume, its osteogenic potential, and the fact that numerous studies of this type of graft have been carried out. Although this well-studied technique has a good prognosis,1 it requires significant donor-site morbidity.2 These days, allografts and xenografts are commercially available.3–5 However, in addition to being difficult to shape into the desired form, these materials have a potential risk of infection and of inducing an immune response.4,5 Further, even though biomaterials have the advantage of unlimited availability, these materials also bring a risk of infection and have poor osteoinductivity.
On the other hand, the use of growth factors that regulate cellular chemoattraction, proliferation, and differentiation has begun to be recognized as a new method for bone regeneration. Recent studies confirmed that several growth factors, such as bone morphogenetic proteins (BMPs), insulin-like growth factor (IGF)-1 and −2, transforming growth factor-β1, platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF)-2, could improve cellular ability to undergo osteogenesis by stimulating cellular events.6–8 In particular, BMPs were regarded as effective factors for the processes of bone healing and have been adopted as graft materials in clinical cases of craniofacial bone defects.9,10 However, recent studies in orthopedics indicated unexpected effects of BMPs on clinical bone regeneration. Further, these growth factors are expensive and may exaggerate inflammatory responses.11,12 In addition, application of a single growth factor imposes a limitation on the ability to regenerate bone. It has therefore been considered that a combination of a number of different factors will be better for optimizing bone regeneration, and several studies have investigated mixtures of two or more types of factors for bone regeneration.13,14
Langer and Vacanti first established the concept of tissue engineering15 and regenerative medicine as a tool for a new clinical platform aimed at treatment of a whole spectrum of interactive diseases.16,17 This concept involves the regeneration of tissues using stem cells, scaffolds, and growth factors, on the basis that stem cells play a leading role in tissue regeneration.
Mesenchymal stem cells (MSCs) were first reported as fibroblast-like cells elaborated from bone marrow that attached to tissue culture surfaces.18 MSCs can be easily obtained from bone marrow or other sites and their pluripotent nature allows them to replicate without differentiating, and confers on them the ability to differentiate into lineages of mesenchymal tissue, including bone, cartilage, fat, and muscle.19 Bone marrow MSCs have great potential for bone regeneration and clinical applications of MSCs are under way. We have previously used a mixture of human MSCs (hMSCs) from bone marrow and platelet-rich plasma (PRP) (hMSCs/PRP) for craniofacial reconstruction and dental implants, which are bone graft materials with predictable grafting success.20 Bone regeneration using hMSCs/PRP has achieved a measure of success in clinical cases.21 However, this procedure suffers from some problems, such as high capital investment, expensive cell culture, complicated safety and quality management issues regarding cell handling, and invasiveness of the procedure that is required for the collection of bone marrow MSCs from the patients. Moreover, recent studies of MSCs transplantation in spinal cord injury revealed that the implanted MSCs did not survive for a long time.22 Additionally, it is well established that MSCs secrete a variety of growth factors and cytokines.23 These findings suggest that the paracrine effects of growth factors and cytokines secreted from the implanted MSCs may promote tissue repair and regeneration and therefore transdifferentiation of the implanted MSCs remains controversial. For example, IGF-1, PDGF, and stromal-cell-derived factor (SDF)-1 are known to accelerate the migration of hMSCs in in vitro studies.14,24 The paracrine effects of MSCs have been demonstrated in rodent models of ischemic limb25 and wound healing.23
The paracrine factors secreted by MSCs can accumulate in the conditioned media during cell culture. The conditioned media of MSCs culture (MSC-CM) have been reported to serve multiple positive functions in tissue regeneration.23,25 MSC-CM may induce not only stem cell homing or mobilization into the injured tissues, but also transdifferentiation into several lineages of mesenchymal tissue. If these characteristics of endogenous stem cells can be exploited for bone regenerative medicine, then MSC-CM may provide a substitute for ex vivo culture and implantation of MSCs. A lack of requirement for MSCs will help to reduce several of the existing problems with MSCs culture and implantation.
The purpose of this study was to evaluate the effect of stem-cell-cultured conditioned media on bone regeneration in vitro and in vivo, and to establish a novel bone regenerative medicine that does not require stem cell implantation.
Materials and Methods
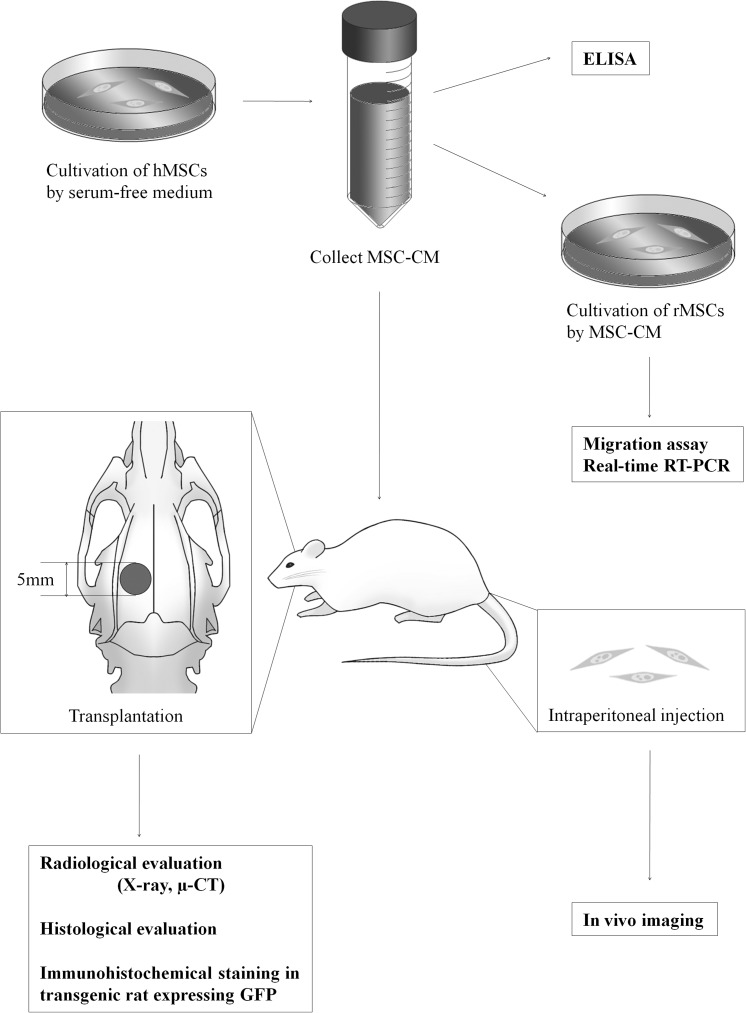
The overall experimental design is summarized in Figure 1.
FIG. 1.
Outline of the experimental protocol. MSC-CM was collected after cultivation of hMSCs for 48 h in serum-free medium. A rat calvarial bone defect of 5 mm in diameter was produced in each parietal bone. The effect of MSC-CM on cell migration, proliferation, and gene expression in vitro, and of implanted MSC-CM on bone regeneration in vivo were then analyzed as shown. hMSCs, human mesenchymal stem cells; CM, conditioned media; rMSCs, rat MSCs; RT-PCR, reverse transcriptase-polymerase chain reaction.
Cell preparation
All animal experiments undertaken in this study were performed in strict accordance with the protocols approved by the Institutional Animal Care Committee.
hMSCs were purchased from Lonza, Inc. (Walkersville, MD) and cultured in MSC basal medium (Lonza, Inc.) containing MSCGM SingleQuots (Lonza, Inc.) at 37°C in 5% CO2/95% air. After primary culture, the cells were subcultured at a density of ∼1×104 cells/cm2. hMSCs at the third to the ninth passages were used for experiments. Subconfluent hMSCs were trypsinized and used for cell implantation.
Rat MSCs (rMSCs) were isolated from 7-week-old Wistar/ST rats (Japan SLC Shizuoka, Japan) as previously reported.26 Briefly, donor rats were sacrificed and the femora were dissected out. Using sterile conditions, the edge of each bone was cut, Dulbecco's modified Eagle's medium (DMEM; Gibco, Rockville, MD) was injected into the bone marrow using a 25-gauge syringe, the bone marrow cells were flushed out to the opposite side, and this maneuver was repeated several times. The marrow was then seeded into each tissue culture flask in DMEM containing antibiotic-antimycotic (100 units/mL penicillin G, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B; Gibco) and the medium was supplemented with 10% fetal bovine serum (FBS). Three days after seeding, floating cells were removed and the medium was replaced with fresh medium. The adherent, spindle-shaped cells were passaged when the cells were approaching confluence. Adherent cells were collected using trypsin/EDTA, resuspended in fresh medium, and transferred to new flasks at a density of 1×104 cells/cm2. Pluripotency of obtained cells for differentiation into classical mesenchymal lineage cells, including osteoblasts, adipocytes, or chondrocytes, was verified by using previously reported methods. These cells were used as rMSCs in this study (data not shown).27 The results indicated that these cells had stem cell characteristics. The rMSCs obtained from cultures at the second to fourth passages were used for experiments.
Preparation of conditioned media
hMSCs that were 70%–80% confluent were re-fed with serum-free DMEM containing antibiotic-antimycotic. The cell-cultured conditioned media were collected after a 48-h incubation. The collected, cultured conditioned media were defined as hMSCs-cultured conditioned media (MSC-CM) and were stored at 4°C or −80°C before being used for the following experiments.
Migration and proliferation of rMSCs
Transwell dishes with 8-μm-pore filters (BD BioCoat Control Inserts; Becton Dickinson and Co., Franklin Lakes, NJ) were used for migration analysis. The second to fourth passages of rMSCs (5×105 cells/cm2) were seeded into the upper chamber, and MSC-CM was added to the lower chamber. To suppress cell proliferation without inhibiting cell survival, 0.25% FBS DMEM was placed into the upper chamber. Cell migration was observed in the presence of 30% FBS or serum-free DMEM, which served as positive and negative controls, respectively. After a 48-h incubation at 37°C in 5% CO2/95% air, the upper side of the filters was carefully rinsed with phosphate-buffered saline (PBS) and remaining cells on the upper surface of the filters were mechanically removed with a cotton wool swab. Transwell filters were stained with hematoxylin, and then cut with a scalpel and mounted onto glass slides, with the lower surface facing upward. The total number of cells that had migrated was counted using a light microscope (CK40; Olympus, Tokyo, Japan) at ×200 magnification.
The proliferation rate of 70%–80% confluent rMSCs was assessed by bromodeoxyuridine (BrdU) incorporation for 24 h using a Zymed BrdU staining kit (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The number of BrdU-positive cells was counted using a light microscope.
Enzyme-linked immunosorbent assay analyses
The levels of IGF-1, vascular endothelial growth factor (VEGF), FGF-2, PDGF-BB, BMP-2, and SDF-1 in MSC-CM were investigated using enzyme-linked immunosorbent assay (ELISA). The concentration of these factors was measured using a Human Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Briefly, 200 μL of MSC-CM, DMEM-0%FBS, or DMEM-30%FBS was added to 96-well microplates that were coated with a monoclonal antibody to the factor of interest and incubated for 2 h. After washing with PBS, a horseradish peroxidase-conjugated cytokine or growth-factor-specific antibody was added to each well, incubated for 2 h, and washed. Substrate solution was added and incubated for 30 min, and the reaction was terminated by addition of the stop solution. Cytokine/growth factor levels were determined by measurement of the optical density at 450 nm using a microplate spectrophotometer (Benchmark Plus; Bio-Rad, Hercules, CA).
Real-time reverse transcriptase–polymerase chain reaction analysis
rMSCs were cultured with MSC-CM or DMEM-10%FBS (expansion medium [EM]) for 48 h, and total RNA was extracted using an RNeasy Mini kit (Qiagen GmbH, Hilden, Germany) according to the manufacturer's protocol. Real-time reverse transcriptase–polymerase chain reaction (RT-PCR) analysis was performed as previously described.28,29 The sequence of the specific primers and probes used for the real-time RT-PCR analysis of Collagen type I alpha 2 (Col I), osteocalcin (OCN), Runx2, and GAPDH is listed in Table 1. RT-PCRs and the resulting relative increase in reporter fluorescent dye emission were monitored in real time using a 7000 Sequence Detector (Perkin-Elmer, Forster City, CA). Signals were analyzed using sequence detector software Ver. 1.1 (Perkin-Elmer). The PCR conditions were as follows: 1 cycle at 50°C for 2 min, 1 cycle at 60°C for 30 min, 1 cycle at 95°C for 5 min, 50 cycles at 95°C for 20 s (denaturation), and then 60°C for 1 min (annealing and extension).
Table 1.
Primer and Probe Sequences Used in the Real-Time Polymerase Chain Reaction
| Gene | Sequence | Accession no. |
|---|---|---|
| Col I | ||
| F | GACAGTCATTGAATACAAAAC | NM_053356 |
| R | ACGGAATTCTTGGTTAGTA | |
| Probe | TAAGCCATCTCGCCTGCCAT | |
| OCN | ||
| F | GACTCTGAGTCTGACAAA | NM_013414 |
| R | AGTCCATTGTTGAGGTAG | |
| Probe | CGGAGTCTATTCACCACCTTACTGC | |
| Runx2 | ||
| F | CCTCTTATCTGAGCCAGA | NM_053470 |
| R | GCAGTGTCATCATCTGAA | |
| Probe | CATCCATCCATTCCACCACGC | |
| GAPDH | ||
| F | GTTCCAGTATGACTCTACC | NM_017008 |
| R | TCACCCCATTTGATGTTA | |
| Probe | TTCAACGGCACAGTCAAGGC | |
Col I, collagen type I alpha 2; OCN, osteocalcin.
The relative amount of each mRNA in one sample was obtained by calculation of the respective standard curves. The standard curves for each mRNA were drawn using different concentrations (2000, 400, 80, 16, and 3.2 ng) of the total RNA of rMSCs. The relative expression levels were normalized to GAPDH expression.
Rat calvarial bone defect model
Ten-week-old male Wistar/ST rats (n=40) were anesthetized by intraperitoneal injection of pentobarbital (Somnopentyl®; Kyoritsu Seiyaku, Tokyo, Japan) (20 mg/kg body weight). After shaving the skin, an L-shaped incision was made in the skull, and the periosteum was opened to expose the surface of the calvarial bones. Two circular bone defects (full-thickness, 5 mm in diameter) were made in calvarial bone using a trephine bur and were irrigated with saline to remove the bone debris. The experimental materials were then implanted into the defects. Agarose powder (Nusieve®; Cambrex Bioscience, Rockland, ME) (0.27 g) was suspended in 6 mL of PBS, serum-free DMEM, or MSC-CM, respectively. The suspension was heated at 37°C to dissolve the agarose, and cooled to room temperature. hMSCs (1×105/defect) were mixed with PBS/agarose gel.
We defined the following groups based on the implanted materials: (1) MSC-CM: MSC-CM/agarose gel; (2) DMEM(−): serum-free DMEM/agarose gel; (3) PBS: PBS/agarose gel; (4) Defect: unfilled defect; (5) MSCs: hMSCs/agarose gel.
The rats were sacrificed at 4 or 8 weeks after transplantation (n=4 per group).
Radiographic and histological analyses
Surgical sites were dissected, fixed in 10% formalin, and subjected to microcomputed tomography (micro-CT) analysis using a laboratory X-ray CT device (LATheta; Aloka Co., Tokyo, Japan). Images were compiled and analyzed to render 3D images using OsiriX® imaging software (Ver.3.9; www.osirix-viewer.com/). We then compared the area (mm2) of newly regenerated bone.
After radiological assessment, explants were decalcified with K-CX solution (Falma Co., Tokyo, Japan) and were then dehydrated using graded ethanol, cleared with xylene, and embedded in paraffin. The specimens were cut in a sagittal direction to make 3-μm-thick histological sections and were stained with hematoxylin and eosin. Histological analysis was performed using a light microscope.
In vivo imaging analysis
rMSCs were harvested and cultured as described previously and were labeled with the lipophilic tracer 1,1-dioctadecyl-3,3,3,3-tetramethylindotricarbocyanine iodide (DiR; Molecular Probes, Eugene, OR) for all imaging experiments.30 This fluorophore is excited at 750 nm and has an emission peak at 782 nm. The cells were incubated with DiR (3×106 cells in 10 mL PBS containing 3.5 μg/mL dye and 0.5% ethanol) for 30 min at 37°C. The cells were then washed twice with PBS and injected intravenously into the caudal vein of Wistar/ST rats with the bone defect described previously, which had been implanted with various implant materials just before the injection of rMSCs. The rats were anesthetized by intraperitoneal injection of Somnopentyl® prior to injection. Xenogen's IVIS® 200 Series Imaging System (Xenogen, Alameda, CA) was used to monitor DiR-labeled rMSC localization within live, as well as sacrificed, animals. Imaging was performed at 1, 24, and 48 h, and at 1 week after injection of DiR-labeled cells.
Immunohistochemical staining of CD44 in transgenic rats expressing green fluorescent protein
Transgenic Sprague–Dawley rats [SD-Tg(CAG-EGFP)Cz-004Osb] carrying the enhanced green fluorescent protein (eGFP) transgene were obtained from Japan SLC, Inc. (Hamamatsu, Japan).31 MSC-CM or PBS with agarose was implanted into calvarial bone defects of these transgenic rats and samples were collected after 4 weeks. Fresh-frozen sections of these samples were made according to the Kawamoto method by using a Multi-Purpose Cryosection Preparation Kit.32 Cryofilm type 2C was applied to the cutting surface of the completely frozen block and the block was cut with a tungsten carbide knife at −25°C in a cryostat chamber (Leica CM3050S; Leica Microsystems, Wetzlar, Germany). The section was fixed with 100% ethanol for 10 min and then washed with PBS for 3 min. A mouse monoclonal anti-CD44 antibody (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) was used to detect MSCs.33 An Alexa Fluor 633-conjugated goat anti-mouse IgG (Molecular Probes, Inc.) was used as the secondary antibody. After DAPI staining, the section was washed with PBS and mounted between a glass slide and the adhesive film. The section was enclosed by the mounting resin SCMM R2 on the glass slide and the resin was hardened with UV ray irradiation for 1 min by using the UV Quick Cryosection Mounter (ATTO Bio-Instrument, Tokyo, Japan). After fixation, the specimen was observed by fluorescence microscopy (BZ8000; Keyence Co., Osaka, Japan).
Statistical analysis
All experiments were conducted in triplicate and repeated at least twice. Group means and standard deviations were calculated for each measured parameter. Statistical differences between two groups were evaluated using the Tukey-HSD test. Differences were considered statistically significant when the p-value was <0.05.
Results
Effect of MSC-CM on rMSC
The effect of MSC-CM on rMSC migration was determined using a transwell assay. The percentage of rMSCs that spontaneously migrated in the presence of the negative control, DMEM(−), was low (2.70%±0.9%), but migration increased in the presence of 30% FBS (47.4%±7.8%). MSC-CM significantly increased the migration rate of rMSCs compared with DMEM(−) (18.3%±4.1%). Thus, MSC-CM increased rMSC migration more than sevenfold compared with that in DMEM(−) (Fig. 2A).
FIG. 2.
Effect of MSC-CM on the migration and proliferation of rMSCs. (A) Transwell migration assay. The migration of rMSCs cultured in MSC-CM was enhanced compared with that of rMSCs cultured in DMEM(−). (B) BrdU cell proliferation assay. Proliferation of rMSCs was determined as the percentage of cells that incorporated BrdU. The proliferation of rMSCs was also enhanced when cultured with MSC-CM compared with culture in DMEM(−). Cells cultured in DMEM-30%FBS were used as a positive control for both (A) and (B). Asterisks indicate a significant difference between the indicated groups (*p<0.05). FBS, fetal bovine serum; BrdU, bromodeoxyuridine; rMSCs, rat MSCs; DMEM, Dulbecco's modified Eagle's medium.
The effect of MSC-CM on the proliferation of rMSCs was evaluated using a BrdU assay. The percentage of BrdU that was incorporated by rMSCs cultured in DMEM(−), 30% FBS, or MSC-CM was 19.94±6.29, 44.05±12.46, and 30.01±5.43 (%), respectively (Fig. 2B). These differences were statistically significant (p<0.05), indicating that MSC-CM also enhanced rMSC proliferation.
Growth factors present in MSC-CM
The concentration of the growth factors IGF-1, VEGF, FGF-2, PDGF-BB, BMP-2, and SDF-1, released by hMSCs into MSC-CM, was quantified using ELISA analysis. Growth factors were not detected in DMEM-0% and DMEM-30%. However, MSC-CM contained IGF-1 and VEGF, at a concentration of 1386±465 and 468.5±109 pg/mL, respectively. The other factors assayed were not detected in MSC-CM (Table 2).
Table 2.
The Levels of Cytokines Present in MSC-CM
| Factors | Concentration (pg/mL) |
|---|---|
| IGF-1 | 1386±465 |
| VEGF | 465.8±109 |
| FGF-2 | ND |
| PDGF-BB | ND |
| BMP-2 | ND |
| SDF-1 | ND |
BMP-2, bone morphogenetic protein-2; IGF-1, insulin-like growth factor-1; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; FGF, fibroblast growth factor; SDF-1, stromal-cell-derived factor-1; ND, not detected.
MSC-CM enhances osteogenic marker gene expression
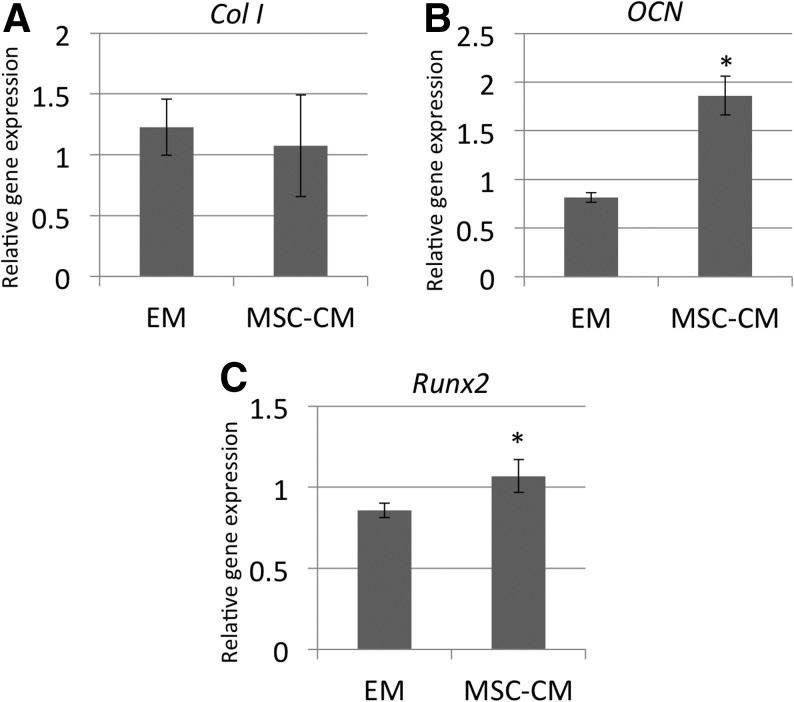
The effect of MSC-CM, or control DMEM-10%FBS (EM), on the relative mRNA expression of the osteogenic markers Col I, OCN, and Runx2 by rMSCs was measured using real-time RT-PCR analysis. The levels of expression of the OCN and the Runx2 genes were significantly upregulated in rMSCs cultured with MSC-CM compared with rMSCs cultured in EM (Fig. 3).
FIG. 3.
Effect of MSC-CM on rMSC gene expression. The mRNA level of (A) Col I, (B) OCN, and (C) Runx2 genes in rMSCs cultured in MSC-CM or DMEM-10%FBS (EM) was assayed using real-time reverse transcriptase–polymerase chain reaction. Cells were lysed for extraction of total RNA on day 7 of culture in MSC-CM or EM, and equal amounts of total RNA (50 ng) were analyzed. The mRNA expression levels of Col I, OCN, and Runx2 were determined relative to the level of GAPDH mRNA in each sample and were quantified by calculation based on their standard curves as described in the Materials and Methods section. To quantitatively compare the levels of gene expression of the different samples, the expression coefficient for each mRNA on the ordinate was calculated by dividing the absolute level of expression of each mRNA (Col I, OCN, and Runx2) with the absolute level of expression of GAPDH mRNA in each sample. Each point represents the mean value calculated from five independent replicates, in which the difference was <10%. An asterisk indicates a significant difference between the EM and MSC-CM groups for the indicated gene (*p<0.05). Col I, collagen type I alpha 2; OCN, osteocalcin; EM, expansion medium.
MSC-CM enhances bone regeneration in vivo
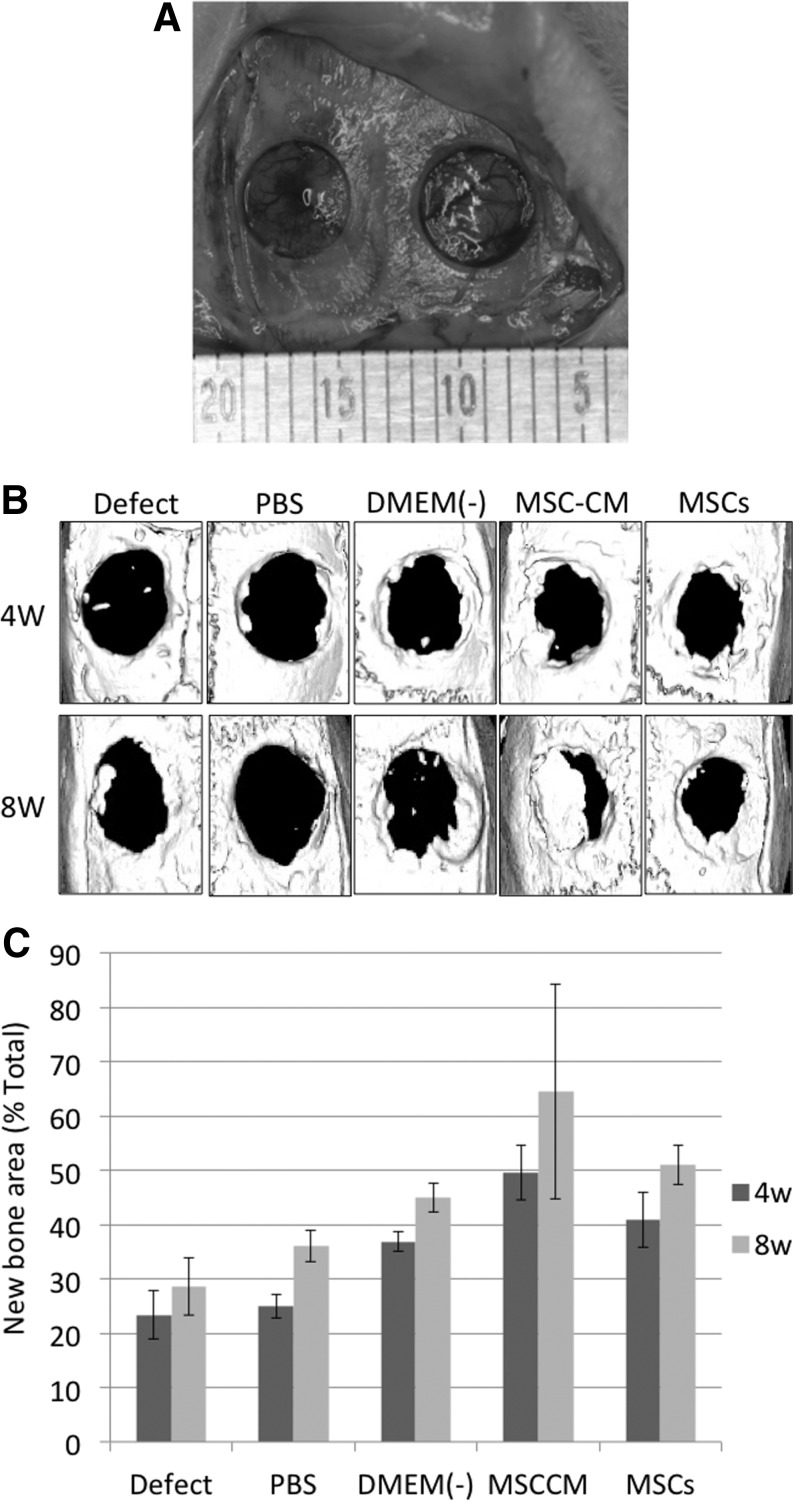
To determine whether MSC-CM could enhance bone regeneration, we implanted an MSC-CM/agarose composite gel, or various controls, into a rat calvarial bone defect (Fig. 4A). We then evaluated the area of newly regenerated bone as a percentage of the total graft area at 4 and 8 weeks after implantation, using micro-CT scanning (Fig. 4B).
FIG. 4.
Micro-CT analysis of bone regeneration following implantation of MSC-CM or controls into a bone defect. (A) Rat calvarial bone defects before implantation of the materials. A bone defect of 5 mm in diameter was prepared in each parietal bone. (B) Micro-CT images of the calvaria 4 and 8 weeks after implantation of the indicated materials. In the PBS, DMEM(−), MSC-CM, and MSCs groups, the materials were implanted as a mixture with an agarose gel, while in the Defect group, the defect was left unfilled. (C) The area of newly regenerated bone in each defect 4 and 8 weeks after implantation of the materials. The area of the newly regenerated bone (mm2) was calculated for each of the experimental groups. The regenerated area is expressed as a percentage of the entire defect area. There were statistical differences between the area of the MSC-CM and that of the other groups both at 4 and 8 weeks except between the MSC-CM and MSCs groups at 8 weeks (p<0.05). Micro-CT, microcomputed tomography; PBS, phosphate-buffered saline.
After 2 weeks, areas of newly regenerated bone in the Defect, PBS, and DMEM(−) groups were <10% of the defect area, whereas the area in the MSC-CM group was over 20% (data not shown). After 4 weeks, bone defect areas were still seen in all groups. However, the area of newly regenerated bone in the MSC-CM group (49.5%±2.7%) was significantly increased compared with that in the Defect (23.4%±4.5%), PBS (24.9%±2.2%), and DMEM(−) (36.9%±1.8%) groups. This relative difference between the MSC-CM and the other groups was even stronger after 8 weeks, at which time the newly regenerated bone area of the MSC-CM group was very obvious and had increased to 64.4%±19.7% of the defect area. Thus, defect areas were almost filled by newly regenerated bone in the MSC-CM group 8 weeks after implantation. In contrast, the area of newly regenerated bone of the other three groups after 8 weeks was <50% [Defect: 28.6%±5.3%, PBS: 36.1%±2.9%, and DMEM(−): 44.9%±2.7%]. Thus, the MSC-CM group showed higher new bone regeneration in the peripheral area surrounding the defect compared with control groups after 2, 4, and 8 weeks. In contrast, bone regeneration of the hMSC-transplanted group (40.9%±5.0% at 4 weeks and 51.0%±3.7% at 8 weeks) was not as good as that of the MSC-CM group (Fig. 4C).
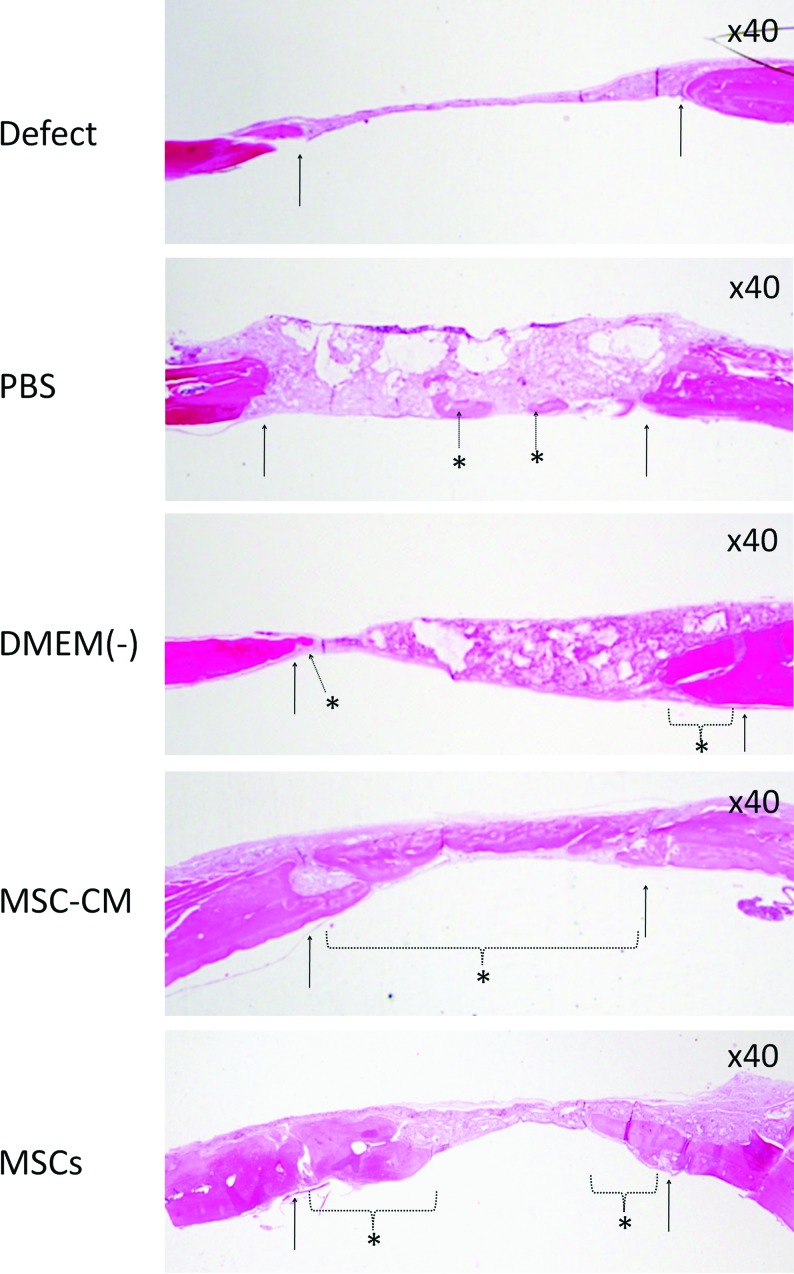
Histological analysis also showed well-regenerated bone in the MSC-CM group compared with the other groups. At 8 weeks, the bone bridge had almost covered the defect in the MSC-CM group and remnants of the agarose gel could not be observed. Calvarial bone regeneration was observed in the MSC group. In the PBS and DMEM(−) groups, some newly regenerated bone, as well as some remnants of the agarose gel, were seen, while in the Defect group most of the defect was filled with connective tissue. Inflammatory responses were not observed in any of the groups (Fig. 5).
FIG. 5.
Histological analysis of newly regenerated bone after implantation. Newly regenerated bone in the defect (Defect), PBS/agarose (PBS), DMEM(−)/agarose [DMEM(−)], MSC-CM/agarose (MSC-CM), and hMSCs/agarose (MSCs) groups was evaluated histologically. Hematoxylin and eosin staining of calvarial histological sections was performed 8 weeks after implantation. The arrows indicate the edges of the host bone and the dotted arrow and dotted line with asterisks indicate the newly regenerated bone. The bone bridge almost covered the defect in the MSC-CM group. In the MSCs group, partial regenerated bone was observed. Agarose gel remnants were not seen in these two groups. In the PBS and DMEM(−) groups, some newly regenerated bone and remnants of the agarose gel were seen, while in the Defect group most of the defect was filled with connective tissue. Inflammatory responses were not observed in any group. Color images available online at www.liebertonline.com/tea
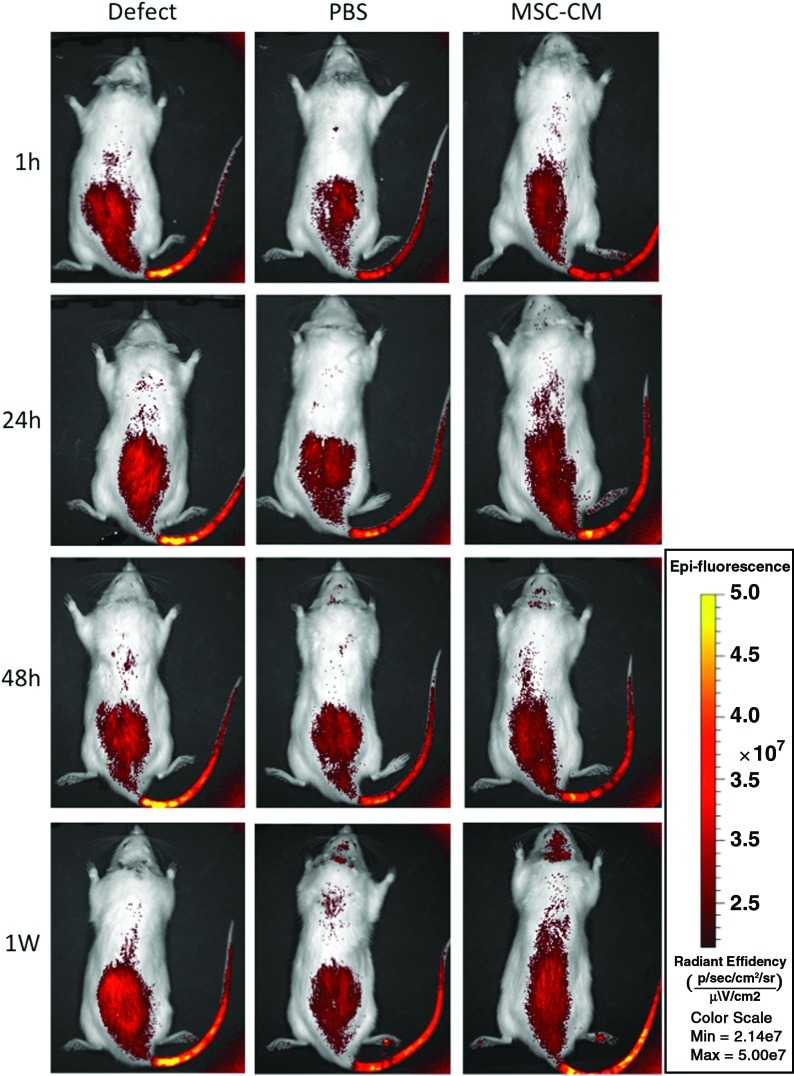
In vivo imaging
Migration of rMSCs to the implants in vivo was analyzed in rats of the different implantation groups in which DiR-labeled rMSCs were injected into the caudal vein. Although it is not possible to detect DiR-labeled cells at a great depth with the imaging system used, this system can detect DiR-labeled cells that accumulate on the calvarial bone. The fluorescent signal in all groups increased immediately after injection. In the control Defect group, the fluorescent signal of the labeled rMSCs was only detected in the tail and abdominal region at 1, 24, and 48 h, and at 1 week after injection. We confirmed that there were no signals at the defect area in the cranium at any time point. In the PBS group, fluorescent signals were observed in the tail and in the abdominal area at 24 and 48 h after injection, and very low signals were observed in the breast and cranial area after 1 week. In the MSC-CM group, a moderate increase in signal intensity was observed in the area of the tail and the abdominal region during the first 24 h after injection. At 48 h after injection, signal intensity in the MSC-CM-implanted area of the parietal bone started to increase. The maximum fluorescent signal in the implanted area was observed 1 week after injection (Fig. 6).
FIG. 6.
In vivo imaging of injected rMSC migration to implants. In vivo imaging analysis shows that DiR-labeled rMSCs that were injected into the caudal vein just after implantation of the materials into the calvarial bone defects started to migrate immediately after injection. At 1 h, 24 h, 48 h, and 1 week after injection, the signal of the fluorescent-labeled rMSCs was only detected in the tail and abdominal region in the control Defect group. We confirmed that there was no signal at the defect area in the cranium at each time point. In the PBS group, fluorescent signals were observed in the tail and the abdominal area at 24 and 48 h after injection, and very low signals were observed in the breast and the cranial area after 1 week. In the MSC-CM group, a moderate increase in signal intensity was observed in the area of the tail and in the abdominal region during the first 24 h after injection. Forty-eight hours after injection, the MSC-CM-implanted area of calvarial bone started to increase in signal intensity, and, 1 week after injection, the MSC-CM implanted area showed the highest fluorescent signal of the experimental groups. Color images available online at www.liebertonline.com/tea
Immunohistochemical staining of cells in regenerated bone in transgenic rats expressing GFP
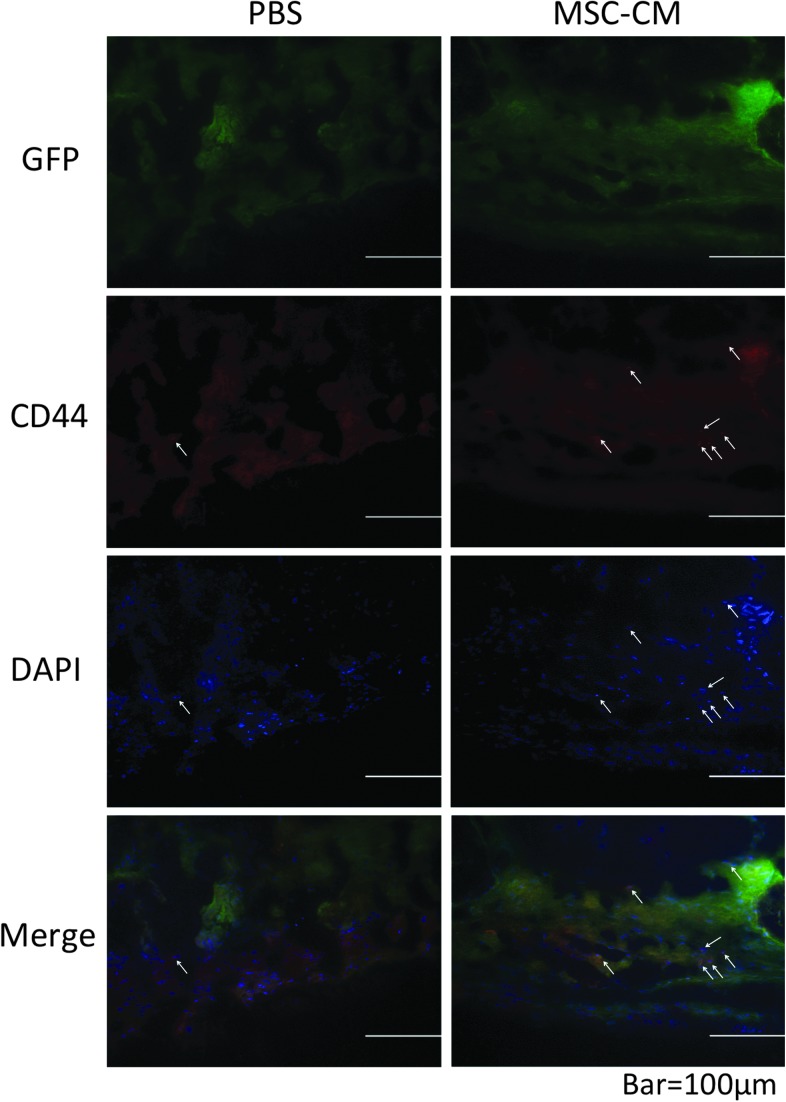
To confirm the mobilization of endogenous MSCs to calvarial defects by the transplanted MSC-CM, we immunohistochemically stained the cells in the regenerated bone of transgenic rats expressing GFP with an anti-CD44 antibody and a fluorescent-labeled second antibody. Cell nuclei were labeled with DAPI (blue). A number of the cells in the newly regenerated bone in the MSC-CM group displayed both CD44 (red) and GFP (green) expression. In contrast, there were fewer CD44/GFP double-positive cells in the PBS group (Fig. 7).
FIG. 7.
Immunohistochemical staining of newly regenerated bone after implantation into transgenic rats expressing GFP. To detect endogenous MSCs in newly regenerated bone in calvarial bone defects, the calvarial bone defects of PBS/agarose (PBS) and MSC-CM/agarose (MSC-CM) groups of transgenic rats that expressed GFP were collected 4 weeks after implantation, and were immunohistochemically stained with anti-CD44 antibodies and Alexa Fluor 633-conjugated secondary antibodies. Nuclei were counterstained with DAPI. The arrows indicate CD44-positive cells (red) and nuclei (blue). In the MSC-CM group, a number of cells in the newly regenerated bone displayed both CD44 and GFP staining, whereas in the PBS group there were fewer CD44-positive cells. GFP, green fluorescent protein.
Discussion
The clinical application of MSCs suffers from a number of problems, including high cost, safety and cell handling issues, and invasive collection procedures. Although bone marrow MSC implantation has beneficial effects on specific diseases, the implanted MSCs do not survive for a long time but disappear 2 to several weeks after transplantation.25 It has been proposed that paracrine mechanisms triggered by growth factors and cytokines secreted by the implanted MSCs may explain the benefit that is observed after MSC implantation.23,25 Since the paracrine factors secreted by MSCs can accumulate in the conditioned media,23 we therefore determined whether MSC-CM contains factors that regulate cell mobilization and osteogenic differentiation. The possibility of enhancing new bone regeneration by implantation of MSC-CM rather than of stem cells is a novel concept in tissue engineering and regenerative medicine. This novel bone regenerative medicine may also reduce the cost and shorten the duration of therapy.
Based on the micro-CT analysis and the histological findings of this study, MSC-CM has dramatic effects on bone regeneration in vivo. Interestingly, bone regeneration following transplantation of hMSCs/agarose (MSCs group) was inferior to that following transplantation of MSC-CM. Further, our in vitro studies suggested that MSC-CM enhanced the migration and osteoinductivity of rMSCs. Based on these results, we hypothesized that MSC-CM stimulated the migration of endogenous rMSCs and accelerated new bone regeneration.
To confirm this hypothesis, and to visualize the dynamics of rMSCs in vivo, we imaged the movement of DiR-labeled rMSCs following injection into rats using an optical whole-body imaging technique. DiR was used to safely and directly label the membrane phospholipids of rMSCs. The proposed method of cellular staining is rapid (30-min incubation) and does not harm the cells. The rMSCs that were labeled with DiR were injected into the caudal vein of rats. The results of in vivo imaging suggested that rMSCs injected into the caudal vein migrated to the calvarial bone defect, where the MSC-CM was implanted. DiR fluorescence at the site at which MSC-CM was implanted continuously increased over time, suggesting that MSC-CM mobilized rMSCs to migrate to the implanted site. Although it is difficult to observe DiR-labeled cells in a deeper position by using the IVIS Imaging System, this method is useful for observing the behavior of cells close to, or on, bone surfaces, such as in calvarial bone. However, this analysis only mimicked endogenous mobilization of MSCs. To confirm that endogenous MSCs migrated to the site where the MSC-CM was implanted, we immunohistochemically stained cells in the bone defects of transgenic rats expressing GFP with anti-CD44 antibodies. CD44 is reported to be a specific marker of MSCs.33 A number of CD44 and GFP double-positive cells were detected in the calvarial bone defects of the MSC-CM group of rats. On the other hand, there were fewer double-positive cells in the PBS group. In addition, tubular formations were seen in the regenerating bone of the MSC-CM group, suggesting the occurrence of angiogenesis in the calvarial bone defects of this group. This experiment indicated that MSC-CM has the potential to mobilize endogenous MSCs and to promote angiogenesis and bone regeneration.
Previous studies23,34 of the factors present in MSC-CM using cytokine array analysis indicated that several cytokines and chemokines are present in MSC-CM and that these factors can influence several different types of cell behavior. In addition, MSC-CM also contains other factors that are present at a low level. We also found, using ELISA analysis, that IGF-1 and VEGF were present at high concentration in MSC-CM.
IGF-1 is known to be present in bone tissue and to be mitogenic for osteoblasts.35 IGF-1 also increases the expression of CXCR4 and enhances SDF-1-induced MSCs migration through the PI-3-kinase (PI3K) pathway.36
VEGF is thought to be the main regulator of angiogenesis and bone marrow stromal cells secrete sufficient quantities of VEGF to enhance survival and differentiation of endothelial cells.37 Further, IGF-1 induces VEGF mRNA in osteoblast-like cells through transcriptional mechanisms involving hypoxia-inducible factor-2α, and these events occur secondary to IGF-1 activation of the PI3K pathway during osteogenesis.38,39 Based on this data, we hypothesized that bone regeneration induced by MSC-CM might be mediated by cooperative effects between IGF-1 and VEGF on angiogenesis and osteogenesis after endogenous cell mobilization.
Growth factors, such as BMP-2, have demonstrated great osteogenic potential9,10 and are already on the market. However, unfortunately, application of these growth factors requires superphysiological doses40 and may induce a severe inflammatory response.11,41 Although Stephan et al. have demonstrated that BMP-2/chitosan gel can enhance bone regeneration in a rat calvarial model, an inflammatory response was also observed in histological findings.42 Thus, although this growth factor can increase bone regeneration, it may also induce some severe complications. In addition, it has been considered that a combination of a number of factors can reduce the dose required and improve therapeutic effects. Some studies have investigated a mixture of two or more types of factors for bone regeneration.13,14
In this study, we used hMSCs and their cultured conditioned media for induction of bone regeneration because our aim was to apply MSC-CM to human patients. Several groups have recently shown that, although MSCs suppress T-lymphocyte proliferation, MSC-CM does not have a T-cell inhibitory effect in vitro.43,44 Le Blanc et al. have reported that MSCs suppress T-cell proliferation that is induced by both allogeneic antigens and by mitogens, suggesting that this suppression is a nonspecific antiproliferative effect.45 There is also evidence for in vivo immunosuppression by MSCs. Using a baboon skin graft model, Bartholomew et al. showed that MSCs appear to suppress alloreactivity in vivo.46 On the other hand, the result of in vivo imaging and immunohistochemical staining of the present study suggest that MSC-CM implantation enhances the early mobilization of endogenous MSCs. Additionally, histological analysis suggested that strong inflammatory responses did not occur in the MSC-CM group. These findings can be explained by the fact that mobilized MSCs suppress T-lymphocyte proliferation, and therefore no obvious immune rejection reaction in the xenografts was observed throughout this study.
In addition, although our aim was bone regeneration, we did not use conditioned media derived from osteoblastic-differentiated hMSCs. Based on our obtained data, we hypothesize that the bone regeneration induced by MSC-CM might be mediated by cooperative effects between IGF-1 and VEGF on angiogenesis and osteogenesis after endogenous cell mobilization. Undifferentiated hMSCs rather than differentiated hMSCs have the potential for such cell behavior. We therefore consider that MSC-CM contains several cytokines and chemokines that regulate several different cell behaviors that are related to bone regeneration, which requires several steps, such as angiogenesis, cell migration, proliferation, and differentiation into osteoblasts. It should also be taken into consideration that not only IGF-1 and VEGF but also other factors that are present in MSC-CM at low concentration may contribute to bone regeneration and that the combination of these several factors can activate a variety of signaling pathways in order to regenerate bone. Further investigation will be necessary to explain how MSC-CM affects bone regeneration.
In this study, we showed that MSC-CM has very high potential for induction of bone regeneration without the necessity for stem cell transplantation. This is the first report of the use of stem-cell-cultured conditioned media for induction of bone regeneration in vitro and in vivo. This novel regenerative medicine is based on a unique concept that utilizes endogenous stem cells without stem cell transplantation. This regenerative medicine approach will also reduce several of the problems that are currently encountered in clinical applications of stem cells, such as problems of expense, time, and safety.
Acknowledgments
The authors wish to thank Drs. Akihito Yamamoto, Kazuhiko Kinoshita, Minoru Inoue, Ms. Mami Naruse, and the members of the Department of Oral and Maxillofacial Surgery as well as Mr. Shinsuke Kimura of Hitachi Aloca Medical, Ltd., for their help and contributions to the completion of this study. This work was supported in part by Grants-in-Aid for Scientific Research (Nos. 21791985 and 23592883) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Disclosure Statement
No competing financial interests exist.
References
- 1.Barome A. Covani U. Maxilary alveolar ridge reconstruction with nonvasculized autogenous block bone: clinical results. J Oral Maxillofac Surg. 2007;65:2039. doi: 10.1016/j.joms.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Kurz L.T. Grafin S.R. Booth R.E. Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine. 1989;14:1324. doi: 10.1097/00007632-198912000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Athanasiou V.T. Papachristou D.J. Panagopoulos A. Saridis A. Scopa C.D. Megas P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: an experimental study in rabbits. Med Sci Monit. 2010;16:BR24. [PubMed] [Google Scholar]
- 4.Eppley B.L. Pietzak W.S. Blanton M.W. Allograft and alloplastic bone substitutes: a review of science and thchnology for the craniomaxillofacial surgeon. J Craniofac Surg. 2005;16:981. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 5.Moore W. Graves S.E. Bain G.I. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354. [PubMed] [Google Scholar]
- 6.Kaigler D. Cirelli J.A. Giannobile W.V. Growth factor delivery for oral and periodontal tissue engineering. Expert Opin Drug Deliv. 2006;3:647. doi: 10.1517/17425247.3.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sykaras N. Opperman L.A. Bone morphogenetic proteins (BMPs): how do they function and what can they offer the clinician? J Oral Sci. 2003;45:57. doi: 10.2334/josnusd.45.57. [DOI] [PubMed] [Google Scholar]
- 8.Linkhart T.A. Mohan S. Baylink D.J. Growth factors for bone regeneration and repair: IGF, TGFβ and BMP. Bone. 1996;19:1S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 9.Shimazu S. Hara T. Kinuta Y. Moriya K. Maruo Y. Hanada S. Minagi S. Enhanced vertical alveolar bone augmentation by recombinant human bone morphogenic protein-2 with carrier in rats. J Oral Rehabil. 2006;33:609. doi: 10.1111/j.1365-2842.2005.01593.x. [DOI] [PubMed] [Google Scholar]
- 10.Herford A.S. Boyne P.J. Reconstruction of mandibular continuity defects with bone morphogenic protein-2 (rhBMP-2) J Oral Maxillofac Surg. 2008;66:616. doi: 10.1016/j.joms.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Perri B. Cooper M. Lauryssen C. Anand N. Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion: a case study. Spine J. 2007;7:235. doi: 10.1016/j.spinee.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Vaidya R. Carp J. Sethi A. Bartol S. Craig J. Les C.M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L. Jiang W. Huang J. He B. Zuo G. Zhang W. Luo Q. Shi Q. Zhang B. Wagner E.R. Luo J. Tang M. Wietholt C. Luo X. Bi Y. Su Y. Liu B. Kim S.H. He C.J. Hu Y. Shen J. Rasteger F. Huang H. Gao Y. Gao J. Zhou J. Reid R.R. Luu H.H. Haydon R.C. He T. Deng Z. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25:2447. doi: 10.1002/jbmr.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozaki Y. Nishimura M. Sekiya K. Suehiro F. Kanawa M. Nikawa H. Hamada T. Kato Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- 15.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 16.Menasché P. Alfieri O. Janssens S. McKenna W. Reichenspurner H. Trinquart L. Vilquin J.T. Marolleau J.P. Seymour B. Larghero J. Lake S. Chatellier G. Solomon S. Desnos M. Hagège A.A. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlloed study of myoblast transplantation. Circulation. 2008;117:1189. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 17.Morigi M. Introna M. Imberti B. Corna D. Abbate M. Rota C. Rottoli D. Benigni A. Perico N. Zoja C. Rambaldi A. Remuzzi A. Remuzzi G. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 18.Colter D.C. Class R. DiGirolamo C.M. Prockop D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Y. Jahagirdar B.N. Reinhardt R.L. Schwartz R.E. Keene C.D. Oritz-Gonzalez X.R. Reyes M. Lenvik T. Lund T. Blackstad M. Du J. Aldrich S. Lisberg A. Low W.C. Largaespada D.A. Verfaillie C.M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 20.Yamada Y. Ueda M. Naiki T. Takahashi M. Hata K. Nagasaka T. Autogenous injectable bone for regeneration with mesenchymal stem cells (MSCs) and platelet-rich plasma (PRP)–tissue-engineered bone regeneration. Tissue Eng. 2004;10:955. doi: 10.1089/1076327041348284. [DOI] [PubMed] [Google Scholar]
- 21.Yamada Y. Nakamura S. Ito K. Kohgo T. Hibi H. Nagasaka T. Ueda M. Injectable tissue-engineered bone using autogenous bone marrow-derived storomal cells for maxillary sinus augmentation: clinical application ewport from 2-6-year follow-up. Tissue Eng Part A. 2008;14:1699. doi: 10.1089/ten.tea.2007.0189. [DOI] [PubMed] [Google Scholar]
- 22.Ide C. Nakai Y. Nakano N. Seo T. Yamada Y. Endo K. Noda T. Saito F. Suzuki Y. Fukushima M. Nakatani T. Bone marrow stromal cell transplantation for treatment of sub-acute spinal cord injury in rat. Brain Res. 2010;1332:32. doi: 10.1016/j.brainres.2010.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Chen L. Tredget E.E. Wu P.Y.G. Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponte L.A. Maris E. Gallay N. Langonne A. Delorme B. Herault O. Charbord P. Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 25.Kinnaird T. Srabile E. Burnett M.S. Shou M. Lee C.W. Barr S. Fuchs S. Epstein S.E. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 26.Azizi S.A. Stokes D. Augelli B.J. DiGirolamo C. Prockop D.J. Engraftment and migration of human bone stromal cells implanted in the brains of albino rats–similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wislet-Gendebien S. Leprince P. Moonen G. Rogister B. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295. doi: 10.1242/jcs.00639. [DOI] [PubMed] [Google Scholar]
- 28.Gibson U.E. Heid C.A. Williams P.M. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 29.Heid C.A. Stevens J. Williams P.M. Real time quantitative PCR. Genome Res. 1996;6:986. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 30.Kalchenko V. Shivtiel S. Malina V. Lapid K. Haramati S. Lapidot T. Brill A. Harmelin A. Use of lipophilic near-infrared dye in whole-body optical imaging of hematopoietic cell homing. J Biomed Opt. 2006;11:50507. doi: 10.1117/1.2364903. [DOI] [PubMed] [Google Scholar]
- 31.Ito T. Suzuki A. Imai E. Okabe M. Hori M. Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001;12:2625. doi: 10.1681/ASN.V12122625. [DOI] [PubMed] [Google Scholar]
- 32.Kawamoto T. Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol. 2003;66:123. doi: 10.1679/aohc.66.123. [DOI] [PubMed] [Google Scholar]
- 33.Sonoda E. Aoki S. Uchihashi K. Soejima H. Kanaji S. Izuhara K. Satoh S. Fujitani N. Sugihara H. Toda S. A new organotypic culture of adopose tissue fragments maintains viable mature adipocytes for a long term, together with development of immature adipocytes and mesenchymal stem cell-like cells. Endocrinology. 2008;149:4794. doi: 10.1210/en.2008-0525. [DOI] [PubMed] [Google Scholar]
- 34.Nakano N. Nakai Y. Seo T.B. Yamada Y. Ohno T. Yamanaka A. Nagai Y. Fukushima M. Suzuki Y. Nakatani T. Ide C. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci Lett. 2010;483:57. doi: 10.1016/j.neulet.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 35.Spencer E.M. Liu C.C. Si E.C.C. Howard G.A. In vivo actions of insulin-like growth factor-I (IGF-I) on bone formation and resorption in rats. Bone. 1991;12:21. doi: 10.1016/8756-3282(91)90050-s. [DOI] [PubMed] [Google Scholar]
- 36.Li Y. Yu X. Lin S. Li X. Zhang S. Song Y-H. Insulin-lile growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 37.Kaigler D. Krebsbach P.H. Polverini P.J. Moony D.J. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng. 2003;9:95. doi: 10.1089/107632703762687573. [DOI] [PubMed] [Google Scholar]
- 38.Akeno N. Robins J. Zhang M. Czyzyk-Krzeska M.F. Clemenc T.L. Induction of vascular endothelial growth factor by IGF-1 in osteoblast-like cell is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2α. Endocrinology. 2002;143:420. doi: 10.1210/endo.143.2.8639. [DOI] [PubMed] [Google Scholar]
- 39.Riddle R.C. Khatri R. Schipani E. Clemens T.L. Role of hypoxia-inducible factor-1α in angiogenic-osteogenic coupling. J Mol Med. 2009;87:583. doi: 10.1007/s00109-009-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawasaki K. Aihara M. Honmo J. Sakurai S. Fujimaki Y. Sakamoto K. Fujimaki E. Wozney J.M. Yamaguchi A. Effects of recombinant human bone morphogenetic protein-2 on differentiation of cells isolated from human bone, muscle, and skin. Bone. 1998;23:223. doi: 10.1016/s8756-3282(98)00105-7. [DOI] [PubMed] [Google Scholar]
- 41.Shields L.B. Raque G.H. Glassman S.D. Campbell M. Vitaz T. Harpring J. Shields C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 42.Stephan S.J. Tholpady S.S. Gross B. Petrie-Aronin C.E. Botchway E.A. Nair L.S. Ogle R.C. Park S.S. Injectable tissue-engineered bone repair of a rat calvarial defect. Laryngoscope. 2010;120:895. doi: 10.1002/lary.20624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maitra B. Szekely E. Gjini K. Laughlin M.J. Dennis J. Haynesworth S.E. Koc O.N. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597. doi: 10.1038/sj.bmt.1704400. [DOI] [PubMed] [Google Scholar]
- 44.Di Nicola M. Carlo-Stella C. Magni M. Milanesi M. Longoni P.D. Matteucci P. Grisanti S. Gianni A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 45.Le Blanc K. Tammik L. Sundberg B. Haynesworth SE. Ringdén O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 46.Bartholomew A. Sturgeon C. Siatskas M. Ferrer K. McIntosh K. Patil S. Hardy W. Devine S. Ucker D. Deans R. Moseley A. Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]