Abstract
Calcification and silicification processes of cyanobacterial mats that form stromatolites in two caldera lakes of Niuafo‘ou Island (Vai Lahi and Vai Si‘i) were evaluated, and their importance as analogues for interpreting the early fossil record are discussed. It has been shown that the potential for morphological preservation of Niuafo‘ou cyanobacteria is highly dependent on the timing and type of mineral phase involved in the fossilization process. Four main modes of mineralization of cyanobacteria organic parts have been recognized: (i) primary early postmortem calcification by aragonite nanograins that transform quickly into larger needle-like crystals and almost totally destroy the cellular structures, (ii) primary early postmortem silicification of almost intact cyanobacterial cells that leave a record of spectacularly well-preserved cellular structures, (iii) replacement by silica of primary aragonite that has already recrystallized and obliterated the cellular structures, (iv) occasional replacement of primary aragonite precipitated in the mucopolysaccharide sheaths and extracellular polymeric substances by Al-Mg-Fe silicates. These observations suggest that the extremely scarce earliest fossil record may, in part, be the result of (a) secondary replacement by silica of primary carbonate minerals (aragonite, calcite, siderite), which, due to recrystallization, had already annihilated the cellular morphology of the mineralized microbiota or (b) relatively late primary silicification of already highly degraded and no longer morphologically identifiable microbial remains. Key Words: Stromatolites—Cyanobacteria—Calcification—Silicification—Niuafo‘ou (Tonga)—Archean. Astrobiology 12, 535–548.
1. Introduction
Since the early fossil record is poor and has been modified by long residence times in the crust, competing interpretations of the oldest morphological fossil record are common (Schopf and Packer, 1987; Schopf, 1993; Brasier et al., 2002; Kazmierczak and Kremer, 2002, 2009a; Schopf et al., 2007). For many years, it was authoritatively advanced by some pioneers in the field of Precambrian micropaleontology that the fossilization potential of cellular structures of cyanobacteria was significantly lower in carbonates (i.e., limestones, marls, and dolomites) than in cherts (e.g., Hofmann, 1976). Therefore the search for early cellularly preserved microbiota was focused on siliceous deposits. Experiments with silicification of various microfossils showed in fact that silicified microbiota preserve their structure and maintain their initial dimensions and shapes (Oehler and Schopf, 1971; Oehler, 1976; Walters et al., 1977; Francis et al., 1978; Westall et al., 1995; Bartley, 1996; Toporski et al., 2002). In accordance with this focus, the studies of Proterozoic and Phanerozoic cherts have yielded spectacular discoveries of exceptionally well-preserved cyanobacteria-like forms and other microbial structures. Examples include the Gunflint chert in Canada (Barghoorn and Tyler, 1965; Awramik and Barghoorn, 1977), the Bitter Springs chert in central Australia (Schopf, 1968; Schopf and Blacic, 1971), the Belcher Supergroup (Hofmann, 1976), the McArthur Group (Oehler, 1978), and the Devonian Rhynie chert from Britain (e.g., Trewin, 1996; Krings et al., 2007; Preston and Genge, 2010). Thus, a “taphonomic window” was created that actually restricted the search for fossil microbiota to a particular geochemical setting (Butterfield, 2003). Due to very early mineralization and emplacement of silica, microorganisms preserved in cherts can be reconstructed almost three-dimensionally (e.g., Bitter Springs or Scotia Group—see Knoll, 1992). Along these lines, detailed work on early Silurian black cherts from central and southwestern Poland has shown that they, too, contain spectacularly preserved cyanobacterial and acritarch cells (Kremer, 2006; Kazmierczak and Kremer, 2009b; Kremer et al., 2012). All these discoveries provide a useful test bed for exploring the diversity of cyanobacterial taphonomy (understood as a diversity of diagenetic processes that lead to the formation of different preservational stages of cyanobacterial taxa), giving new insights into the earliest records of biological activity preserved on Earth.
In this context, it appears mysterious why microbial remains, particularly those that resemble cyanobacteria, are so well preserved in Proterozoic and younger cherts but have not been described equally often in Archean cherts, even though these cherts form a sizeable section of the Archean rock column. Studies of Archean fossil records generally have not attempted to explain either this paucity of forms or the inadequate inventory of cyanobacteria-like and other microorganisms in otherwise obviously microbially mediated sedimentary rocks. The paucity of well-preserved Archean sedimentary sequences and the high grade of their metamorphic changes are usually taken as major reasons for the scarcity of microfossils in them. In light of our observations, a key to understanding the rarity of the earliest record of cyanobacteria-like fossils rests also with the physical-chemical processes (early diagenesis) associated with fossilization modes of cyanobacteria in modern mat communities. These form in situ biosedimentary structures commonly referred to as stromatolites or, more generally, microbialites. Here, we describe the modes of fossilization of contemporary stromatolite-forming cyanobacterial mats from two caldera lakes of the volcanic island of Niuafo‘ou (Tonga) (Kazmierczak and Kempe, 2006). We use these detailed studies to highlight the importance of microtaphonomical processes responsible for the presence or absence of morphological traces of microbial life in the mineral matrix. The spectrum of taphonomical phenomena observed in Niuafo‘ou stromatolites on the microscale may, in our opinion, provide a partial explanation of the apparent scarcity of the earliest microbial life.
2. Setting and Material
The stromatolites for this study were sampled by two of the authors (S.K. and J.K.) during a 3-week Niuafo‘ou field campaign in June 1998. Niuafo‘ou is the northernmost island of the Kingdom of Tonga, South Pacific (Fig. 1). The circular volcanic island is 8.1×8.5 km wide and 52.3 km2 in area. It contains a central caldera occupied by 10 lakes, the largest (Vai Lahi: “Water Big”) measuring 5.6×5.4 km (N-S and E-W, respectively) with its deepest point of 121 m in its northern basin, an area of 13.6 km2 and a volume of 1 km3. The next largest lake is Vai Si‘i (“Water Small”), 31 m deep and 1.14 km2 in area, with a volume of 0.0115 km3. The center of the island (the islet Motu Lahi) is at 15°36′00″S, 175°38′30″W. A detailed geological description of Niuafo‘ou is provided in Kempe and Kazmierczak (2012). Table 1 gives the hydrophysical and Table 2 the hydrochemical data of the water samples.
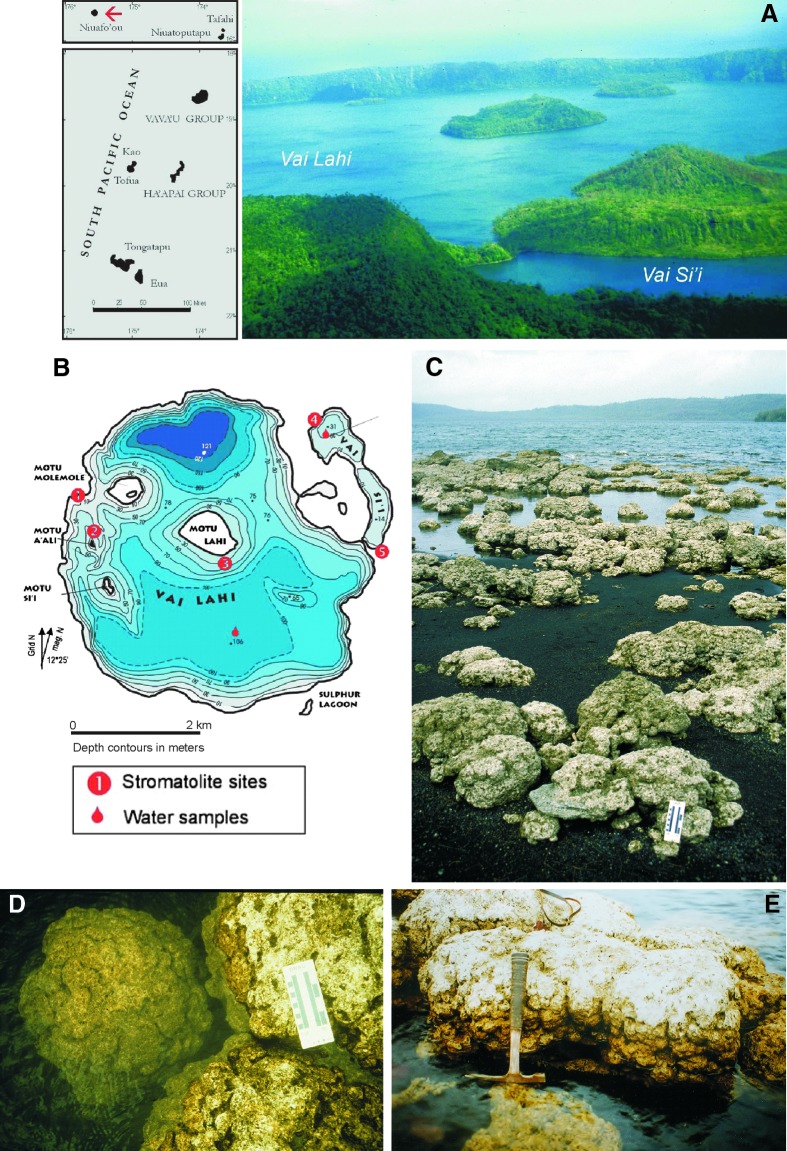
FIG. 1.
(A and B) Geographical location of Niuafo‘ou with aerial view of Vai Lahi and Vai Si‘i crater lakes and simplified bathymetrical map of Niuafo‘ou Island. (C–E) Vai Lahi shore with a stromatolite field and examples of large brainlike Vai Lahi stromatolites extending above water level (C and E) and partly underwater (D).
Table 1.
Hydrophysical Data of Water Samples
| Parameter Number | Depth m | Temp. °C | pCO2 ppmv | pH | Eh mV | Conductivity mS/cm |
|---|---|---|---|---|---|---|
| Vai Lahi | Southern basin | |||||
| W12b | 0 | 26.8 | 8.34 | 278 | 5.65 | |
| W12 | −10 | 26.5 | 870 | 8.35 | 278 | 5.65 |
| W3 | −25 | 27.0 | 788 | 8.39 | 154 | 5.69 |
| W9 | −40 | 26.6 | 1620 | 8.27 | n.d. | 5.63 |
| W11 | −42.5 | 26.4 | 1180 | 8.13 | 138 | 5.67 |
| W10 | −45 | 26.2 | 2330 | 7.67 | 55 | 5.68 |
| W2 | −50 | 26.3 | 2055 | 7.73 | 25 | 5.69 |
| W1 | −100 | 26.5 | 1938 | 7.50 | −4 | 5.69 |
| Vai Lahi | Northern basin | |||||
| W13 | −100 | 26.0 | 2013 | 7.65 | 5.71 | |
| Vai Si‘i | ||||||
| W15 | −10 | 26.9 | 644 | 8.69 | 201 | 3.24 |
| W14 | −30 | 26.5 | >6000 | 7.25 | −206 | 4.18 |
n.d., not determined.
Table 2.
Selected Hydrochemical Data of Niuafo‘ou Lakes (SI=Saturation Index=log(Ion Activity Product/Kmineral))
| Sample | Depth m | Alkalinity meq/L | Si mg/L | Ca meq/L | Mg meq/L | SI Calcite | SI Aragonite | SI Dolomite | δ13C per mill |
|---|---|---|---|---|---|---|---|---|---|
| Vai Lahi | Southern basin | ||||||||
| W12 | −10 | 15.70 | 5.30 | 0.71 | 21.04 | 0.65 | 0.50 | 2.94 | — |
| W3 | −25 | 16.60 | 5.54 | 0.68 | 20.74 | 0.69 | 0.54 | 3.04 | 0.11 |
| W9 | −40 | 15.70 | 5.52 | 0.70 | 21.04 | 0.57 | 0.47 | 2.80 | — |
| W11 | −42.5 | 16.60 | 4.02 | 0.78 | 20.85 | 0.52 | 0.37 | 2.63 | — |
| W10 | −45 | 16.30 | 5.36 | 1.06 | 21.08 | 0.21 | 0.06 | 1.87 | — |
| W2 | −50 | 18.80 | 5.09 | 1.12 | 21.10 | 0.34 | 0.20 | 2.12 | −0.61 |
| W1 | −100 | 18.60 | 5.48 | 1.15 | 21.15 | 0.13 | −0.02 | 1.68 | −0.41 |
| Vai Lahi | Northern basin | ||||||||
| W13 | −100 | 15.50 | 8.95 | 1.09 | 21.01 | 0.18 | 0.03 | 1.80 | — |
| Vai Si‘i | |||||||||
| W15 | −10 | 6.30 | 1.88 | 1.17 | 9.43 | 0.89 | 0.75 | 2.87 | −1.98 |
| W14 | −30 | 10.90 | 3.95 | 2.32 | 11.99 | 0.04 | −0.10 | 0.95 | — |
Vai Lahi shows a distinct stratification in its chemical characteristics. Between 40 and 45 m, pH and Eh change substantially. This layer separates the oxygenated surface layer from an anaerobic bottom layer. The CO2 pressure (pCO2) increases distinctly with depth, which suggests that the lake must have been stagnant for some time since its last overturn. Vai Si‘i has different parameters, which suggests that the two lakes have been separated for some time. The pH is higher but the conductivity much lower than in Vai Lahi. Vai Si‘i is more distinctly stratified than Vai Lahi; pH and Eh are very low in the bottom waters, whereas the pCO2 is much higher than in Vai Lahi. This suggests ongoing volcanic CO2 input to the waters of Vai Si‘i, as well as the influence of hydrothermal activity. The main ion ratios indicate that the water in both lakes is not diluted seawater; rather, it is the product of autonomous systems of rainwater, juvenile water, and ions dissolved from volcanic rocks through silicate weathering (Kazmierczak and Kempe, 2006; Kempe and Kazmierczak, 2012).
Both lakes are alkaline (alkalinity 15.7–18.6 and 6.3–10.9 meq/L in Vai Lahi and Si‘i, respectively). The alkalinity of these lakes (i.e.,
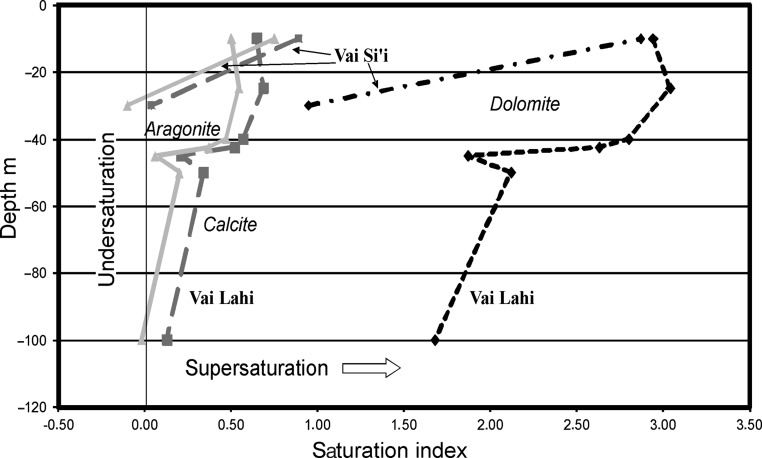
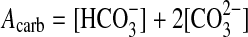 ) is significantly higher than in seawater (2.32 meq/L), which makes bicarbonate the second most concentrated anion after chloride, while the Ca concentration is much lower than in seawater (20.0 meq/L). In detail, Vai Si‘i is lower in alkalinity than Vai Lahi, but the difference in alkalinity between surface and bottom waters is much greater in Vai Si‘i. The supersaturation of all carbonate minerals is very high at the surfaces of both Vai Lahi and Vai Si‘i (Fig. 2, Table 2). The supersaturation decreases with depth, due to an increase in pCO2 (Fig. 3). This pCO2 increase is due in turn to increased respiration at depth as indicated by the lowering of the δ13C values in Vai Lahi. Aragonite and calcite reach saturation at an approximate depth of 100 m. The concentration of dissolved silica in both lakes is very high, roughly 100 times higher than in seawater, a situation typical of alkaline waters.
) is significantly higher than in seawater (2.32 meq/L), which makes bicarbonate the second most concentrated anion after chloride, while the Ca concentration is much lower than in seawater (20.0 meq/L). In detail, Vai Si‘i is lower in alkalinity than Vai Lahi, but the difference in alkalinity between surface and bottom waters is much greater in Vai Si‘i. The supersaturation of all carbonate minerals is very high at the surfaces of both Vai Lahi and Vai Si‘i (Fig. 2, Table 2). The supersaturation decreases with depth, due to an increase in pCO2 (Fig. 3). This pCO2 increase is due in turn to increased respiration at depth as indicated by the lowering of the δ13C values in Vai Lahi. Aragonite and calcite reach saturation at an approximate depth of 100 m. The concentration of dissolved silica in both lakes is very high, roughly 100 times higher than in seawater, a situation typical of alkaline waters.
FIG. 2.
Carbonate mineral saturation in Vai Lahi and Vai Si‘i.
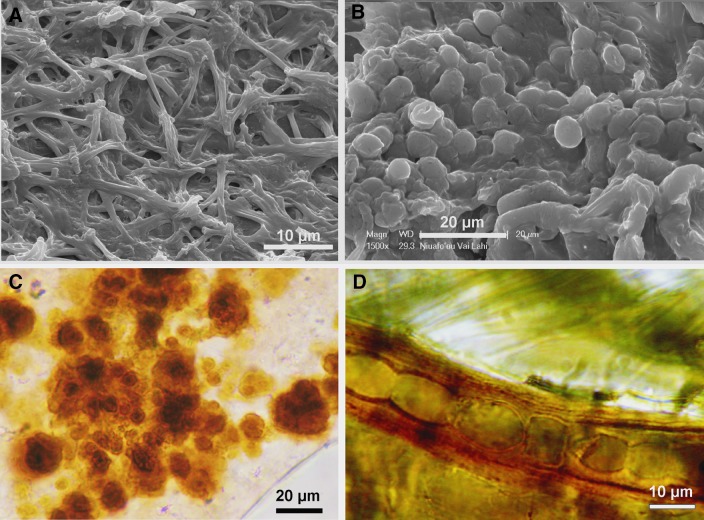
FIG. 3.
Representative cyanobacteria from Niuafo‘ou stromatolites. (A and B) SEM images of a living cyanobacterial mat composed of filaments (A) and coccoids (B) covering the mat surface. Optical images: (C) Myxosarcina-like coccoidal cyanobacteria partly silicified. (D) Almost perfectly preserved silicified filamentous cyanobacterium with thick mucilage sheaths.
Various carbonate deposits, some in the form of stromatolites, constitute most of the sediment of the lake. The largest stromatolites are over 1 m in diameter and up to 0.8 m high, with surfaces displaying patterns reminiscent of tightly convoluted brains. In 1998, when the field study was conducted, some of the stromatolites were aerially exposed along certain sections of the coast and had dried up. At the splash zone, stromatolitic surfaces are covered with a layer of noncalcified filamentous cyanobacteria without growth continuity toward the calcareous stromatolite body. Surfaces of stromatolites permanently occurring underwater are patchily overgrown with only weakly calcifying filamentous and coccoidal cyanobacterial mats. This indicates that at present the cyanobacteria have less potential for actively precipitating calcium carbonate compared to the heavily permineralized stromatolite body formed earlier.
The uncalibrated 14C age measured at the bases of three stromatolites is around 15,000 aBP. Our observations indicate that at present the lake waters cannot sustain vigorous growth (accretion) of the stromatolitic structures. The calcium carbonate supersaturation level (SIAra, SICc) in both of the studied Niuafo‘ou caldera lakes is apparently not high enough to induce cyanobacteria to precipitate and accrete calcium carbonate (Table 2, Fig. 2) (for calculations of SIAra and SICc in other alkaline lakes see Kempe and Kazmierczak, 1990, 2007). This is especially true for Vai Lahi. Therefore, the massive large heads and crusts that occur along Vai Lahi's shores were formed during times of a higher lake level and under conditions in which the supersaturation level was significantly higher than it is today. Vai Si‘i has a lower δ13C value than Vai Lahi. All measured δ13C values from the stromatolite carbonates are positive and heavier than those for marine carbonates. The δ13C values are higher in older samples. A shift toward the present lower values could have been accomplished by the input of light volcanic (juvenile) CO2 into the lake or from anaerobic 12C-rich deep waters during incidental lake destratifications.
3. Methods
Standard microscopic analyses with a Zeiss-Opton light microscope were performed on covered and polished petrographic thin sections. Covered thin sections were used to photograph in transmitted light, because glass cover reduces effect of light scattering. To improve the overall integrity of samples and preserve the original microstructure, we impregnated some pieces of stromatolites with resin. Rock platelets for scanning electron microscopic (SEM) examinations were polished and etched with formic and fluoric acids. These samples were not impregnated with resin. The SEM examination involved a Philips XL 20 instrument, equipped with an EDX dual-window (UTW/Open) microprobe equipped in turn with an ECON detector model Econ-6 (Institute of Paleobiology, PAS, Warsaw). X-ray diffraction analyses of carbonate microsamples were performed with a CGR Inel diffractometer equipped with a cobalt lamp and focusing goniometer with transmission optics for Debye-Scherrer powder preparations. The 14C dating of stromatolites and δ13C analyses of water samples were performed by J. van der Plicht in the Centrum voor Isotopen Onderzoek (Rijksuniversiteit Groningen).
4. Observations and Results
4.1. Modes of biomineralization
Detailed microscopic investigation shows that the studied stromatolites are built by coccoidal and filamentous cyanobacterial mats that today grow actively on top of shallow subaqueous stromatolites (Fig. 1D, 1E, Fig. 3). Although normally a community composed of various coccoidal and filamentous taxa (Myxosarcina, Rivularia, Calothrix, Tolypothrix, Anabaena) forms the living stromatolite surfaces, some of the living mats are composed of coccoidal strains (Pleurocapsa group) only. Diatoms, filamentous green algae (Cladophora spp.), and small ostracods are often associated with the living mats. Surfaces of subaerially exposed stromatolites (Fig. 1D, 1E) are covered by remains of dried microbial mats in which traces of mineralization are barely visible. Within the stromatolite body (below the freshly mineralizing top layer), remnants of coccoidal and filamentous cyanobacteria similar to those from the surface are evident.
Our observations indicate that the Niuafo‘ou stromatolites grow as a result of in vivo and early postmortem calcification of cyanobacterial mats. Vertical sections of Niuafo‘ou stromatolites display several alternating microfabrics (textures) (Fig. 4A–D). The most prominent microfabric is a more or less regular lamination composed in transmitted light of darker and lighter layers. The darker layers are built of micritic carbonate in which remnants of cyanobacterial cells are occasionally visible (Fig. 4B, 4D). These organic-rich layers are usually thin (<300 μm) compared with the much thicker lighter layers. The light layers are composed of fine to coarse crystalline aragonite (Fig. 4B–D). Fragments of stromatolites without lamination have also been observed. These are composed mostly of micritic aragonite that grades into patchily distributed nests of larger aragonite needles (Fig. 4C, 4E–G). The differences in texture and thickness relationships between alternating laminae correlate with certain morphological characteristics of the cyanobacteria that participate in their formation and with the intensity of in vivo or early postmortem biomineralization of the microbial biofilms.
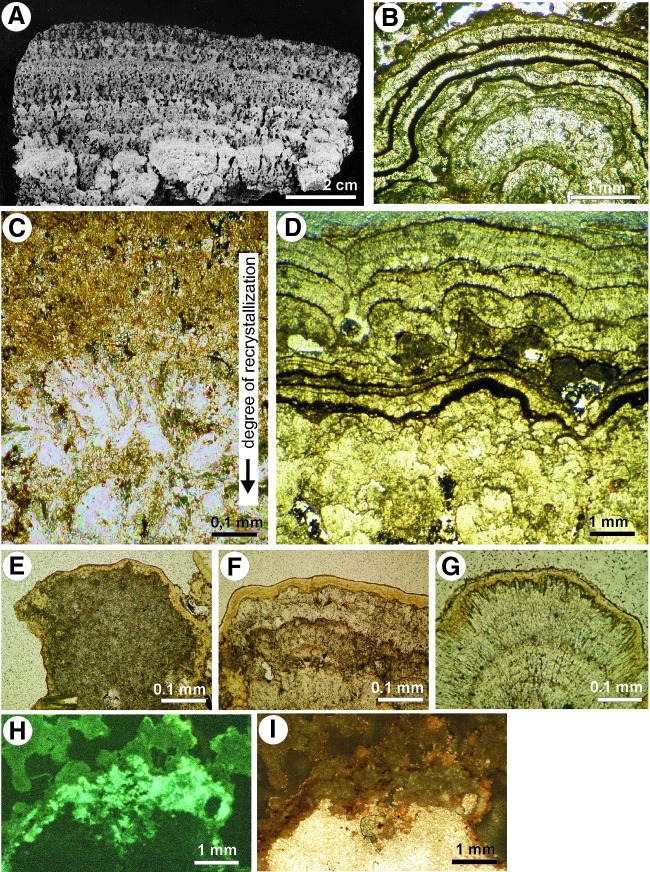
FIG. 4.
(A) Vertical section of a polished slab of Vai Lahi stromatolite showing the internal structure. (B) Vertical section through Vai Lahi stromatolites showing alternation of various microfabrics. (C) Transition from small grains of aragonite into larger crystals (bottom). (D) Laminated and nonlaminated texture of Vai Lahi stromatolite in vertical section. (E–G) Examples of transformation of aragonite nanogranules to needle-like crystals. In the first stage (E) the remains of organic matter are still present, whereas the last stage (G) is practically devoid of organic matter. (H) Part of the aragonite nanoparticles showing fluorescence indicative of the presence of organic matter. (I) The same stromatolite section as in (H), photographed in plain transmitted light.
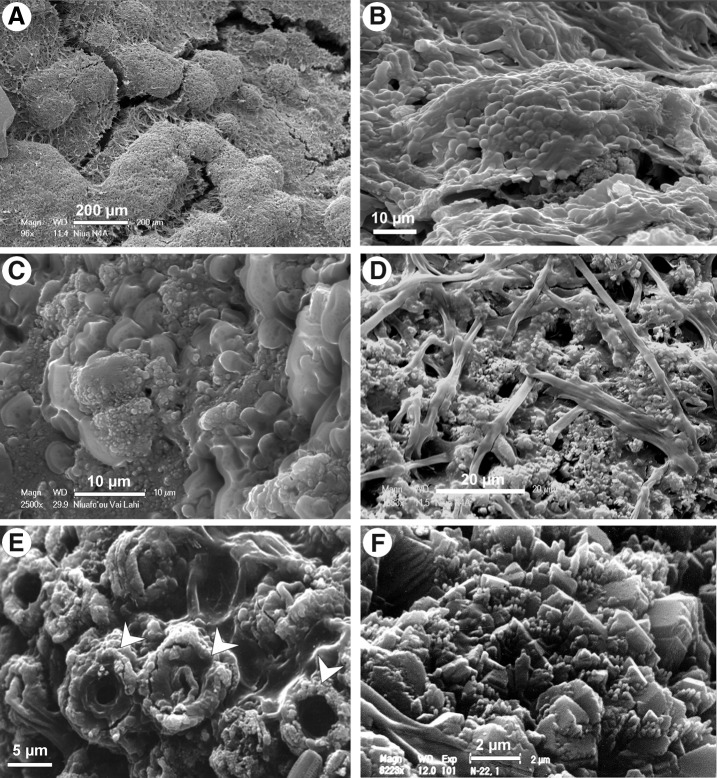
Three mineral phases have been identified in stromatolites from the Niuafo‘ou lakes. The dominant phase is aragonite precipitated by the cyanobacterial mat that covers the surfaces of stromatolites. At present, however, mineralization of cyanobacterial mats is weak, and there are no precipitates visible directly on the mat surface (Fig. 5A, 5B). Today the mineralization is restricted to nanograins of calcium carbonate located within the common mucilage sheaths of permanently water-covered cyanobacterial mats (Fig. 5C–E). The outer mucopolysaccharide sheaths of the coccoid cyanobacteria mineralize with nanogranular aragonite apparently precipitated in vivo (Fig. 5E). The CaCO3 mineralization of the older parts of cyanobacterial mats is much more intense (Fig. 5F).
FIG. 5.
SEM images of filamentous and coccoidal cyanobacteria from surfaces of Vai Lahi and Vai Si‘i stromatolites (air-dried samples). (A and B) Surface of cyanobacterial mats mineralized with aragonite. (C) Permineralized common mucilage sheaths. (D) Aragonite grains precipitated in a filamentous mat. (E) Aragonite nanogranules (white arrows) in the mucus enveloping cells of coccoidal cyanobacteria. (F) An older part of a Vai Si‘i stromatolite with aragonite nanogranules recrystallizing into larger crystals within cyanobacterial mucilage sheaths.
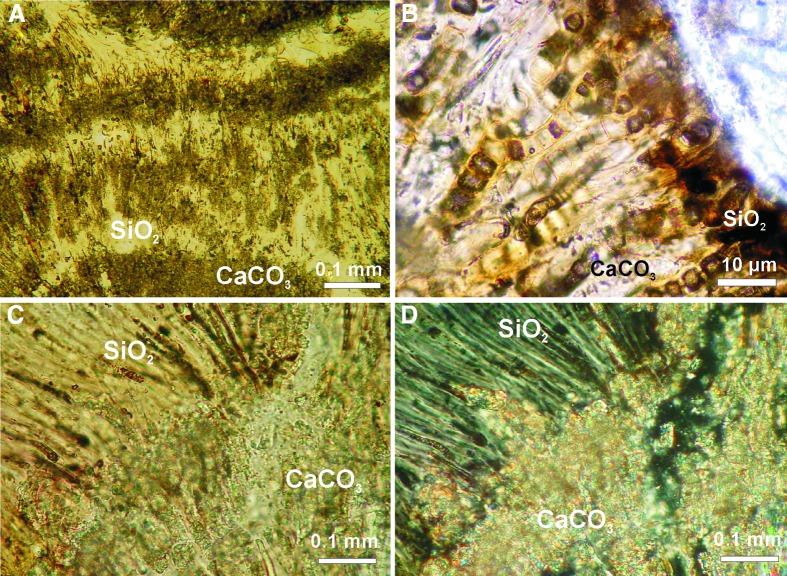
The other mineral phases are amorphous silica and sporadically occurring silicates (clay minerals) (Fig. 6). These phases occur in many different combinations and may occur as alternating microlayers (Figs. 6A, 7A, 7B) from a few to several hundred micrometers thick. Larger parts of stromatolites mineralized only by one (aragonite or silica) mineral phase have also been observed (Fig. 6).
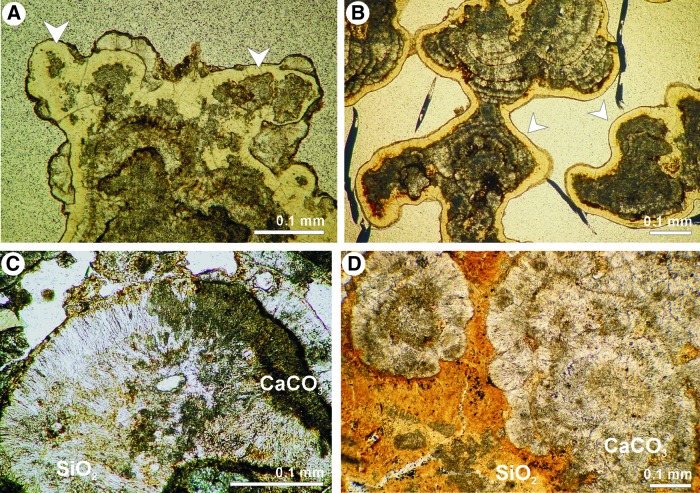
FIG. 6.
Examples of early diagenetic silicification of Niuafo‘ou stromatolites. (A and B) Patchy replacement of primary aragonite by silica (A) and silica coatings (B) in a stromatolite body. (C) Vertical thin section showing patchy silicification of originally aragonite stromatolite structure. (D) Tangential section showing interstitial spaces between aragonite microcolumns filled tightly with silica. All transmitted light photomicrographs.
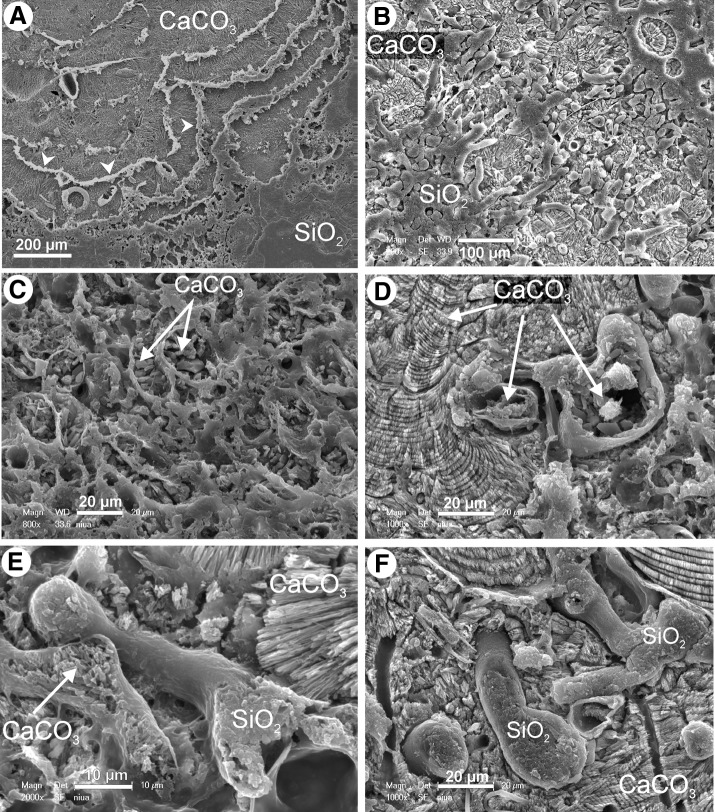
FIG. 7.
SEM images showing modes of early diagenetic mineralization of cyanobacterial mats forming Niuafo‘ou stromatolites. (A) Vertical section of a stromatolite composed of alternating layers of aragonite and silica (white arrows). (B) Section of a filamentous cyanobacterial mat with silicified cyanobacteria and aragonite matrix. (C) Section of a cyanobacterial mat mineralized with aragonite but with cell walls of filamentous taxa permineralized by silicates. (D) Two cyanobacterial filaments mineralized with aragonite in an aragonite matrix; note aragonite fan on the left side. (E) Comparison of two mineralized filaments, in which the larger is mineralized with silica and the smaller, partially degraded, with aragonite; both filaments are entombed in an aragonite matrix. (F) Silicified filament in aragonite matrix; note the perfect silica infilling. All samples etched with formic and hydrofluoric acids.
It has been observed (Knorre and Krumbein, 2000; Kühl et al., 2003; Kazmierczak and Kempe, 2004; Kazmierczak et al., 2004, 2011; Benzerara et al., 2006, 2010; Riding, 2006, 2008; Kremer et al., 2008) that carbonate precipitation in living cyanobacterial mats starts, as a rule, from nucleation of nanograins of aragonite within the capsules surrounding cells and groups of cells and within the mucilage sheaths of colonies (extracellular polymeric substances) (Fig. 6C–E). The same is true for Niuafo‘ou, where, in older stromatolite parts, the aragonitic nanogranules transform into bigger crystals (Fig. 5F). The growth of aragonite nanoparticles on and within the remains of cyanobacteria destroys their original morphological structure (Fig. 7C, 7D). The next stage of mineralization is when the nanoparticles transform into larger crystals: first aragonite platelets and finally into large needle-like aragonite crystals bonded in fan-shaped arrays (Fig. 4G). During these processes the organic parts undergo further breakup. A typical transformation series is shown in Fig. 4E–G. It seems that during aragonite permineralization of cyanobacterial mats the original shapes of the cells are preserved only at the earliest stage of mineralization (Fig. 7C, 7D). Subsequent mineralization that leads to the recrystallization of nanogranules into bigger crystals (Fig. 4C) totally destroys the cell structure, and cell morphology is no longer identifiable. Mineralization usually begins outside the cells, but in some cases crystal nucleation and growth may occur simultaneously outside and inside cells. In such cases, the growth of aragonite destroys their internal structure without a trace.
Silica mineralization is significant in many Niuafo‘ou stromatolites, but it occurs irregularly and is less common than aragonite (Fig. 6). Three modes of silica mineralization can be distinguished: (i) patchy silicification, where only small portions of the stromatolite are silicified (Fig. 6C); (ii) mass-scale silicification, which forms regular silica/aragonite alternating layers (Figs. 6A, 7A, 7B); and (iii) massive silicification, when silica penetrates larger fragments of the stromatolite and forms a kind of siliceous crust (Fig. 6B, 6D). The basic difference between silica and aragonite mineralization lies in the mode of preservation of soft organic material in stromatolites (Fig. 8D, 8E). In the case of silica mineralization, details of the morphology of silicified cyanobacteria remain recognizable. Aragonite permineralization, almost as a rule, negatively affects the quality and quantity of preserved organic matter.
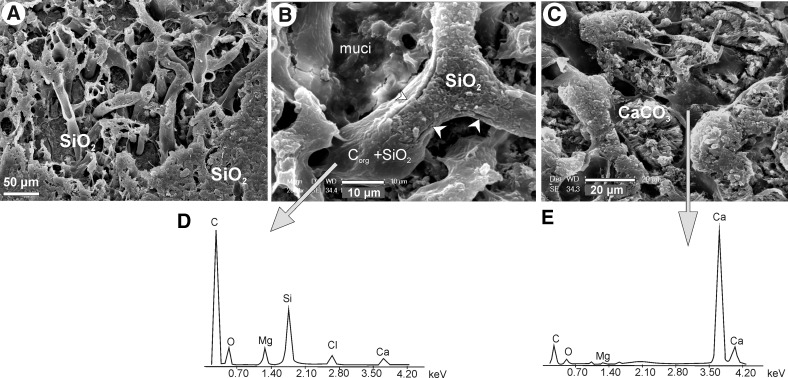
FIG. 8.
SEM images showing modes of early diagenetic mineralization of cyanobacterial mats of Niuafo‘ou Island. (A) Silicified section of a cyanobacterial filament embedded in silica matrix. (B) A tangential section through a silicified cyanobacterial filament with a well-preserved silicified cell wall (arrows); the cell wall still contains a lot of organic matter [see energy-dispersive spectroscopy (EDS) spectrum in D]. (C) A tangential section through several filaments mineralized with aragonite; note the destruction of the cell walls with only traces of organics (see EDS in E). (D and E) EDS spectra from cell walls mineralized with silica (D) and aragonite (E). All samples etched with formic and hydrofluoric acids.
Silicification begins with the precipitation of amorphous nanospheres of silica (opal and chalcedony) that are always associated with organic components of cyanobacteria (cell walls, trichomes, mucilage sheaths). It occurs in pore waters saturated with respect to silica. Our observations indicate that amorphous silica is not transforming into quartz microcrystals during the time of stromatolite formation (i.e., about 15,000 years). Thus, the mineralized microbiota remain intact. Similar preservation can be observed in some geologically older early diagenetic cherts. For instance, observations of early Silurian cherts (Kremer, 2006) show that primary amorphous silica does not necessarily transform during diagenesis into coarse quartz. It is interesting to note that the cherty matrix of Silurian cherts, composed of crypto- and microcrystalline quartz, did not damage organic cyanobacterial or acritarch remains during transformation from the primary amorphous silica to the present chert.
Spectacular examples of the preservation of cyanobacteria in silica are the filaments shown in Fig. 3A, 3D, where the thick multilayered mucilage sheaths that surround individual filaments are preserved. The secret of good silica preservation lies in the nature of the mineralization process. Although the nucleation phase is similar in both cases and starts from amorphous nanograins, the subsequent growth is different. In the case of silica permineralization, the organic material is entirely impregnated by silica (Fig. 7E, 7F), since nucleation from supersaturated solutions of monosilicic acid (Si(OH)4) is considered to be homogenous (Iller, 1979; Benning et al., 2005). Additionally, the rate of silicification must generally be rapid. Depending on the saturation of the monosilicic acid in the solution, the precipitation of silica proceeds in steps that encompass formation of particle nuclei and growth of particles and their subsequent coagulation into gel or sol (Iller, 1979; Benning et al., 2005). This process is similar to the rapid petrifaction of wood by silica (Knoll, 1985). In this way, soft organic parts, such as cells, may undergo petrifaction inside and outside. Compared to silicification, aragonite permineralization is not as complete and leaves many pores and voids in which growth of aragonite crystals can badly damage organic parts (Fig. 7D). Similar spectacularly preserved silicified microbes, including cyanobacteria, are known from modern hot spring environments. In this case, the rapidly silicified microorganisms are preserved with their morphology, diameter, length, and some inner structures, such as remains of cytoplasm and intercellular septation (e.g., Cady and Farmer, 1996; Renaut et al., 1998; Jones et al., 2004). Also, fossil analogues of modern siliceous thermal spring sinters, like the Devonian Rhynie chert (Scotland), enclose cellularly preserved microorganisms (Trewin, 1994, 1996; Cady and Farmer, 1996; Preston and Genge, 2010).
Our observations indicate that the mineralization of Niuafo‘ou cyanobacterial stromatolites was a complex process that involved various modes of mineralization of the stromatolite-forming microbiota, as follows: (i) aragonite-mineralized cyanobacteria embedded in an aragonite matrix (Figs. 7C, 8C), (ii) silica-mineralized cyanobacteria within an aragonite matrix (Figs. 7B, 7E, 7F, 8B), and (iii) silica-mineralized cyanobacteria within a silica matrix (Fig. 8A). Interestingly, silica mineralization is often associated with the formation of Al-Mg-Fe silicates. Two possible explanations can be proposed for such complex mineralization modes. The first implies a very early, almost in vivo, silicification of cyanobacterial cells substituted later by calcification. The second suggests an early diagenetic precipitation of aragonite within the mats, whereas the cellular remains of cyanobacteria underwent later silicification due to an increase in silica concentration in the pore water.
The exceptional mineralization that occurs in Niuafo‘ou cyanobacterial mats allows detailed observation and comparison of silica- and aragonite-mineralized mat sections. Alternating mineralization with silica and aragonite is common within a distance of micrometers. Figures 9 depicts sections of filamentous cyanobacterial mats that have been mineralized with silica and microgranular aragonite. The sections vary strongly with regard to the quality of soft-part preservation. A magnified section of the same part of the mat permineralized with silica and aragonite reveals that silica preserves morphological details of cyanobacterial cells much better (Fig. 8B, 8D). In contrast to the calcified sections, which are almost barren of organics, the silicified parts reveal many details of cell morphology, including often complete filaments (trichomes) or large groups of coccoids, cell walls, shrunken cell content, and internal septa.
FIG. 9.
Images of sections of cyanobacterial mats mineralized with silica and aragonite demonstrating differences in preservation potential of the two mineral phases. (A) Section of filamentous mat showing alternation of silica and aragonite layers photographed in plain light. (B) Magnified section of cyanobacterial mat mineralized with silica and aragonite showing perfectly preserved silicified cyanobacteria co-occurring with poorly preserved filaments permineralized with aragonite. (C and D) Section of the same filamentous mat as in (A) irregularly mineralized with silica and aragonite photographed in plain (C) and polarized (D) light.
Our study of the Niuafo‘ou stromatolites shows that nucleation and growth of silica particles within the mucilage sheaths and cell walls of cyanobacteria started very early postmortem. Microbial cells (cyanobacterial, bacterial, algal, and in few cases fungal) act here as passive surfaces for aragonite and silica nucleation. Observation of the various preservational stages of cyanobacteria from Niuafo‘ou stromatolites revealed that relatively quick silicification preserves almost perfectly morphological details of cyanobacteria and even bacteria. Interestingly, a significant difference has been observed in organic matter preservation between silica and aragonite permineralization. When permineralized by silica, a high percentage of organic matter remains preserved in the mineralized cell walls and mucilage sheaths (Figs. 8D, 9B). Mineralization of aragonite does not preserve organic material nearly as well (Figs. 8E, 9C, 9D). Nucleation and growth of amorphous silica nanogranules on both outer and inner parts of the organic cell wall protect organic material from bacterial degradation and favor preservation of morphological details. Diagenesis of cherts is a process of transformation of the unstable opal-A phase (biogenic or inorganic) into the more stable opal-CT and microcrystalline quartz (Iller, 1979). During such a transformation, the organic matter entombed in silica remains protected. In this way, many Paleozoic, particularly early Silurian, cherts contain spectacularly preserved benthic cyanobacterial mats and other microbiota (Kremer and Kazmierczak, 2005; Kremer, 2006). Such a primary mineralization is, however, rare in the Niuafo‘ou stromatolites. Much more common is secondary silicification. The observation of microstructures indicates that silicification occurs often as a relatively fast replacement of aragonitic nanogranules by silica. Such replacement does not take place throughout the entire stromatolite body but selectively and very often only locally (Fig. 9). As a result of such silicification, some parts of stromatolites are silicified and some remain calcareous. This produces an interesting result on the microscale. In the silicified sections, individual cyanobacteria (filaments) are well preserved, while in the carbonate parts the morphology of cyanobacteria is usually no longer recognizable (Fig. 9).
4.2. The timing of mineralization
Careful studies of the modes of preservation of microbial structures observed in Niuafo‘ou stromatolites lead us to the conclusion that silica and aragonite mineralization occurred almost synchronously (Fig. 7A, 7B, Fig. 9). This is best illustrated by the Niuafo‘ou filamentous mat that has been mineralized alternately with silica and aragonite (Fig. 9A). Since these two mineral phases require different geochemical conditions to precipitate, their precipitation must proceed under particular taphonomic circumstances. This must have been a consequence of the specific geochemical microenvironment created in the mat. Both lakes of Niuafo‘ou are high in dissolved silica. It can be hypothesized that the irregular volcanic activity in the lakes (the last eruption in the caldera occurred in 1886; see Kempe and Kazmierczak, 2012, and citations therein), particularly the settling of fresh and glassy ashes, provides them with this silica. Hydrothermal activities may also play a role in this enrichment process.
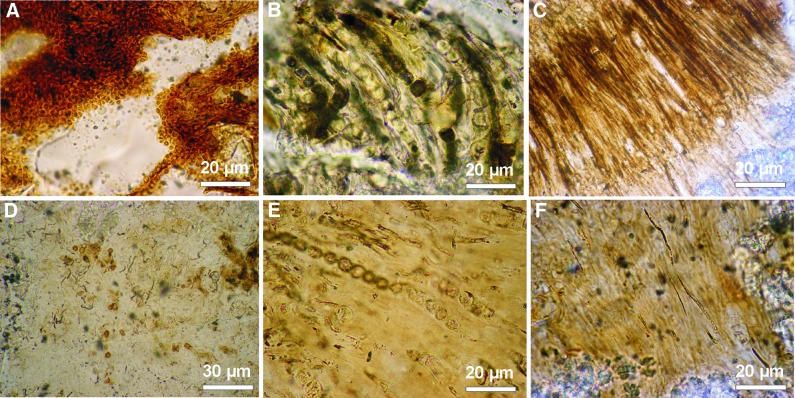
Thus, interstitial pore waters and mucilage sheaths can form restricted geochemical niches where concentrations of SiO2 can be even higher and reach a supersaturation that induces silica precipitation as a primary mineral phase or that replaces the aragonite nanogranules. The various modes of silica mineralization observed seem to support such a model. The silicification occurs usually very fast, prior to bacterial degradation and possibly as an almost in vivo mineralization, which may explain the spectacular preservation of the silicified microbiota (Fig. 10A–C). The local character of such mineralization is indicated by locally observed permineralization of individual filaments half and half by silica and aragonite (Fig. 9).
FIG. 10.
Comparison of variously preserved cyanobacteria in silicified parts of stromatolites from Niuafo‘ou Island. (A–C) Spectacularly well-preserved coccoidal (A) and filamentous cyanobacteria (B, C) due to primary silicification. (D–F) Poorly preserved coccoidal (D) and filamentous (E, F) cyanobacteria due to secondary (i.e., after primary aragonite) or late (i.e., after biodegradation) silicification.
4.3. Implication for the early fossil record
Following the maxim that “the present is the key to the past,” observation of the modern Niuafo‘ou stromatolites may, at least in part, help explain the extremely poor Archean and Proterozoic microbial fossil record. Many of the internal Niuafo‘ou microfabrics resemble those described from Precambrian stromatolites (e.g., Hofmann, 1975; Semikhatov et al., 1979; Buick et al., 1981; Walter, 1983; Knoll et al., 1989; Fairchild, 1991; Knoll and Semikhatov, 1998). The common belief that cherts should preserve morphological traces of life well often leads to the conclusion that the lack of microfossils in Archean cherts is due to the lack or extreme rarity of life itself. The Niuafo‘ou stromatolites provide examples of well-preserved cyanobacterial remains in silica as well as examples of silicified sections that do not preserve recognizable microbial remains. The spectrum of preservations of cyanobacteria observed in Niuafo‘ou stromatolites shows that microfossils are only preserved when silicification occurs relatively early, prior to degradation processes such as hydrolysis, autolysis, or bacterial degradation (Fig. 10A–C). During late silicification, in cases where more time was available for postmortem degradation processes, the cyanobacterial biomass had no chance to be fossilized and usually did not leave a morphologically recognizable fossil record (Fig. 10D–F).
The usual explanation for the poor fossil record of early Earth, especially in the case of the Archean cherts, is that these cherts must have been secondarily silicified (Paris et al., 1985). This would mean that primary calcification destroyed the morphological record of early life and left only unidentifiable carbonaceous traces for subsequent silicification (chertification). Our observations from Niuafo‘ou stromatolites suggest another interpretation: the silicification of Archean cherts could be primary (i.e., without an earlier calcareous stage), but may have occurred at a time when the microbial cellular structures had already undergone significant degradation (Fig. 9C, 9D). On the other hand, if silica, as commonly believed, was the secondary mineral phase, it must have substituted for the primary aragonite (or other carbonate) that, during recrystallization, had almost totally destroyed morphological remains of organic matter. Otherwise, early silicification should have preserved morphological details of microorganisms similar to those observed in Niuafo‘ou stromatolites.
5. Conclusions
It has been suggested many times that modern microbialites, particularly stromatolites, may help us understand the formation of similar structures from the earliest geological record. Contemporary stromatolites growing in the two caldera lakes on Niuafo‘ou island (Vai Lahi and Vai Si‘i) of the Tonga Kingdom appear to be a perfect model for studying mineralization processes in actively growing microbialites. First of all, the stromatolites from the Niuafo‘ou lakes represent a unique site for testing in situ the preservation potential of cyanobacteria permineralized with silica and calcium carbonate. Our study showed that in vivo calcification—typically associated with a transformation of aragonite to calcite—destroys the morphology of calcified cyanobacteria, whereas silicification, when rapid, helps to preserve their morphological details. Our study showed that morphological preservation of cyanobacteria largely depends on two factors: the type of mineral phase and the time of mineralization. We have distinguished four main modes of mineralization of cyanobacterial mats that form these stromatolites: (i) early (primary) postmortem calcification by aragonite nanograins that transform into needle-like crystals and almost totally destroy the cellular structures, (ii) early (primary) postmortem silicification of whole cyanobacterial cells that leave a record of spectacularly preserved cellular structures, (iii) later (secondary) replacement by silica of aragonite which through recrystallization had already destroyed the cellular morphology, (iv) gradual (secondary) replacement by Al-Mg-Fe silicates of primary aragonite precipitated in the mucilage sheaths and extracellular polymeric substances.
Niuafo‘ou is also a unique site for searching for modern analogues of many microfabrics observed in Precambrian microbialites (particularly stromatolites). This wide spectrum of morphological preservation modes encompasses many possible stages of degradation of both filamentous and coccoidal cyanobacteria. Many of the Niuafo‘ou textures resemble microfabrics described from Precambrian stromatolites and can be regarded as their modern analogues.
Acknowledgments
We thank Malcolm Walter and an anonymous reviewer for helpful comments on the manuscript. We would like to thank Cyprian Kulicki and Zbigniew Strak (Warsaw) for technical help. The study was supported by the Homing Plus Programme of the Foundation for Polish Science (B.K.) and National Sciences Centre (grant NN307468938 to B.K.). Field expedition funding was provided by the Deutsche Forschungsgemeinschaft (Ke 287 19/1) and the Polish Academy of Sciences. The paper is an extended version of a talk given during the workshop Geobiology in Space Exploration, Marrakech, Morocco, February 7–9, 2011.
Abbreviation
SEM, scanning electron microscope.
References
- Awramik S. Barghoorn E.S. The Gunflint microbiota. Precambrian Res. 1977;5:121–142. [Google Scholar]
- Barghoorn E.S. Tyler S.A. Microorganisms from the Gunflint chert. Science. 1965;147:563–577. doi: 10.1126/science.147.3658.563. [DOI] [PubMed] [Google Scholar]
- Bartley J.K. Actualistic taphonomy of cyanobacteria: implications for the Precambrian fossil record. Palaios. 1996;11:571–586. [Google Scholar]
- Benning L.G. Phoenix V. Mountain B.W. Biosilicification: the role of cyanobacteria in silica sinter deposition. In: Gadd G.M., editor; Semple K.T., editor; Lapin-Scott H.M., editor. Micro-organisms and Earth Systems—Advances in Geomicrobiology, SGM Symposium 65. Cambridge University Press; Cambridge: 2005. pp. 131–150. [Google Scholar]
- Benzerara S.T. Menguy N. López-Garcia P. Yoon T.-H. Kazmierczak J. Tyliszczak T. Guyot F. Brown E.J., Jr. Nanoscale detection of organic signatures in carbonate microbialites. Proc Natl Acad Sci USA. 2006;103:9440–9445. doi: 10.1073/pnas.0603255103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzerara K. Meibom A. Gautier Q. Kazmierczak J. Stolarski J. Menguy N. Brown G.E., Jr. Nanotextures of aragonite in stromatolites from the quasi-marine Satonda crater lake, Indonesia. Geol Soc Spec Publ. 2010;336:211–224. [Google Scholar]
- Brasier M.D. Green O.R. Jephcoat A.P. Kleppe A.K. Van Kranendonk M.J. Lindsay J.F. Steele A. Grassineau N.V. Questioning the evidence for Earth's oldest fossils. Nature. 2002;247:76–81. doi: 10.1038/416076a. [DOI] [PubMed] [Google Scholar]
- Buick R. Dunlop J.S.R. Groves D.I. Stromatolite recognition in ancient rocks: an appraisal of irregular laminated structures in an early Archean chert–barite unit from North Pole, Western Australia. Alcheringa. 1981;5:161–181. [Google Scholar]
- Butterfield N.J. Exceptional fossil preservation and the Cambrian explosion. Integr Comp Biol. 2003;43:166–177. doi: 10.1093/icb/43.1.166. [DOI] [PubMed] [Google Scholar]
- Cady S.L. Farmer J.D. Fossilization processes in siliceous thermal springs: trends in preservation along thermal gradients. In: Bock G.R., editor; Goode J.A., editor. Evolution of Hydrothermal Ecosystems on Earth (and Mars?), Ciba Foundation Symposium 202. John Wiley and Sons; Chichester, UK: 1996. pp. 150–173. [DOI] [PubMed] [Google Scholar]
- Fairchild I.J. Origins of carbonate in Neoproterozoic stromatolites and identification of modern analogues. Precambrian Res. 1991;53:281–299. [Google Scholar]
- Francis S. Barghoorn E.S. Margulis L. On the experimental silicification of microorganisms. III. Implications of the preservation of the green prokaryotic alga Prochloron and other coccoids for interpretation of the microbial fossil record. Precambrian Res. 1978;7:377–383. [Google Scholar]
- Hofmann H.J. Stratiform Precambrian stromatolites, Belcher Islands, Canada: relations between silicified microfossils and microstructure. Am J Sci. 1975;275:1121–1132. [Google Scholar]
- Hofmann H.J. Precambrian microflora, Belcher Islands, Canada: significance and systematics. J Paleontol. 1976;50:1040–1073. [Google Scholar]
- Iller R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties, and Biochemistry. John Wiley & Sons; New York: 1979. [Google Scholar]
- Jones B. Konhauser K.O. Renaut R.W. Wheeler R.S. Microbial silicification in Iodine Pool, Waimangu geothermal area, North Island, New Zealand: implications for recognition and identification of ancient silicified microbes. J Geol Soc London. 2004;161:983–993. [Google Scholar]
- Kazmierczak J. Kempe S. Microbialite formation in seawater of increased alkalinity, Satonda Crater Lake, Indonesia: discussion. Journal of Sedimentary Research. 2004;74:314–317. [Google Scholar]
- Kazmierczak J. Kempe S. Genuine modern analogues of Precambrian stromatolites from caldera lakes of Niuafo‘ou, Tonga. Naturwissenschaften. 2006;93:119–126. doi: 10.1007/s00114-005-0066-x. [DOI] [PubMed] [Google Scholar]
- Kazmierczak J. Kremer B. Thermal alteration of the Earth's oldest fossils. Nature. 2002;420:477–478. doi: 10.1038/420477b. [DOI] [PubMed] [Google Scholar]
- Kazmierczak J. Kremer B. Thermally altered Silurian cyanobacterial mats: a key to Earth's oldest fossils. Astrobiology. 2009a;9:731–743. doi: 10.1089/ast.2008.0332. [DOI] [PubMed] [Google Scholar]
- Kazmierczak J. Kremer B. Spore-like bodies in early Paleozoic acritarchs: clues to chlorococcalean affinities. Acta Palaeontol Pol. 2009b;54:541–551. [Google Scholar]
- Kazmierczak J. Kempe S. Altermann W. Microbial origin of Precambrian carbonates: lessons from modern analogues. In: Eriksson P.G., editor; Altermann W., editor; Nelson D.R., editor; Mueller W., editor; Catuneanu O., editor. The Precambrian Earth: Tempos and Events Developments in Precambrian Geology. Elsevier; Amsterdam: 2004. pp. 545–563. [Google Scholar]
- Kazmierczak J. Kempe S. Kremer B. López-García P. Moreira D. Tavera R. Hydrochemistry and microbialites of the alkaline crater Lake Alchichica, Mexico. Facies. 2011;57:543–570. [Google Scholar]
- Kempe S. Kazmierczak J. Calcium carbonate supersaturation and the formation of in situ calcified stromatolites. In: Ittekkot V.A., editor; Kempe S., editor; Michaelis W., editor; Spitzy A., editor. Facets of Modern Biogeochemistry. Springer; Berlin: 1990. pp. 255–278. [Google Scholar]
- Kempe S. Kazmierczak J. Hydrochemical key to the genesis of calcareous nonlaminated and laminated cyanobacterial microbialites. In: Seckbach J., editor. Algae and Cyanobacteria in Extreme Environments, COLE Series 11. Springer; Dordrecht: 2007. pp. 241–264. [Google Scholar]
- Kempe S. Kazmierczak J. Terrestrial analogues for early planetary oceans: Niuafo'ou caldera lakes (Tonga) and their geology, water chemistry and stromatolites. In: Hanselmeier A., editor; Kempe S., editor; Seckbach J., editor. Life on Earth and Other Planetary Bodies. Springer; Dordrecht: 2012. in press. [Google Scholar]
- Knoll A.H. Exceptional preservation of photosynthetic organisms in silicified carbonates and silicified peats. Philos Trans R Soc Lond B Biol Sci. 1985;311:111–122. [Google Scholar]
- Knoll A.H. Vendian microfossils in metasedimentary cherts of the Scotia Group, Prins Karls Forland, Svalbard. Palaeontology. 1992;35:751–774. [PubMed] [Google Scholar]
- Knoll A.H. Semikhatov M.A. The genesis and time distribution of two distinctive Proterozoic stromatolite microstructures. Palaios. 1998;13:408–422. [Google Scholar]
- Knoll A.H. Swett K. Burkhardt E. Paleoenvironmental distribution of microfossils and stromatolites in the Upper Proterozoic Backlundtoppen Formation, Spitsbergen. J Paleontol. 1989;63:129–145. [PubMed] [Google Scholar]
- Knorre H.V. Krumbein W.E. Bacterial calcification. In: Riding R.E., editor; Awramik S.M., editor. Microbial Sediments. Springer; Berlin: 2000. pp. 25–31. [Google Scholar]
- Kremer B. Mat-forming coccoid cyanobacteria from early Silurian marine deposits of Sudetes, Poland. Acta Palaeontol Pol. 2006;51:143–154. [Google Scholar]
- Kremer B. Kazmierczak J. Cyanobacterial mats from Silurian Black radiolarian cherts: phototrophic life at the edge of darkness? Journal of Sedimentary Research. 2005;75:897–906. [Google Scholar]
- Kremer B. Kazmierczak J. Stal L.J. Calcium carbonate precipitation in cyanobacterial mats from sandy tidal flats of the North Sea. Geobiology. 2008;6:46–56. doi: 10.1111/j.1472-4669.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- Kremer B. Bauer M. Stark R.W. Gast N. Altermann W. Gursky H.-J. Heckl W.M. Kazmierczak J. Laser-Raman and atomic force microscopy assessment of the chlorococcalean affinity of problematic microfossils. J Raman Spectrosc. 2012;43:32–39. [Google Scholar]
- Krings M. Kerp H. Hass H. Taylor T.N. Dotzler N. A filamentous cyanobacterium showing structured colonial growth from the Early Devonian Rhynie chert. Rev Palaeobot Palynol. 2007;146:265–276. [Google Scholar]
- Kühl M. Fenchel T. Kazmierczak J. Structure, function, and calcification potential of an artificial cyanobacterial mat. In: Krumbein W.E., editor; Paterson D., editor; Zavarzin G.A., editor. Fossil and Recent Biofilms—A Natural History of Life on Earth. Kluwer Academic Publishers; Dordrecht: 2003. pp. 77–102. [Google Scholar]
- Oehler D.Z. Microflora of the middle Proterozoic Balbirini dolomite (McArthur Group) of Australia. Alcheringa. 1978;2:269–309. [Google Scholar]
- Oehler J.H. Experimental studies in Precambrian paleontology: structural and chemical changes in blue-green algae during simulated fossilization in synthetic chert. Geol Soc Am Bull. 1976;87:117–129. [Google Scholar]
- Oehler J.H. Schopf J.W. Artificial microfossils: experimental studies of permineralization of bluegreen algae in silica. Science. 1971;174:1229–1231. doi: 10.1126/science.174.4015.1229. [DOI] [PubMed] [Google Scholar]
- Paris I. Stanistreet I.G. Hughes M.J. Cherts of the Barberton greenstone belt interpreted as products of submarine exhalative activity. J Geol. 1985;93:111–129. [Google Scholar]
- Preston L.J. Genge M.J. The Rhynie Chert Scotland, and the search for life on Mars. Astrobiology. 2010;10:549–560. doi: 10.1089/ast.2008.0321. [DOI] [PubMed] [Google Scholar]
- Renaut R.W. Jones B. Tiercelin J.-J. Rapid in situ silicification of microbes at Loburu hot springs, Lake Bogoria, Kenya Rift Valley. Sedimentology. 1998;45:1083–1103. [Google Scholar]
- Riding R. Cyanobacterial calcification, carbon dioxide concentrating mechanisms, and Proterozoic-Cambrian changes in atmospheric composition. Geobiology. 2006;4:299–316. [Google Scholar]
- Riding R. Abiogenic, microbial and hybrid authigenic carbonate crusts: components of Precambrian stromatolites. Geologia Croatica. 2008;61:73–103. [Google Scholar]
- Schopf J.W. Microflora of the Bitter Springs Formation, late Precambrian, central Australia. J Paleontol. 1968;42:651–688. [Google Scholar]
- Schopf J.W. Microfossils of the Early Archean Apex chert: new evidence of the antiquity of life. Science. 1993;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- Schopf J.W. Blacic J.M. New microorganisms from the Bitter Springs Formation (late Precambrian) of the north-central Amadeus Basin, Australia. J Paleontol. 1971;45:925–960. [Google Scholar]
- Schopf J.W. Packer B.M. Early Archean (3.3-billion to 3.5-billion-year-old) microfossils from Warrawoona Group, Australia. Science. 1987;237:70–73. doi: 10.1126/science.11539686. [DOI] [PubMed] [Google Scholar]
- Schopf J.W. Kudryavtsev A.B. Czaja A.D. Tripathi A.B. Evidence of Archean life: stromatolites and microfossils. Precambrian Res. 2007;158:141–155. [Google Scholar]
- Semikhatov M.A. Gebelein C.D. Cloud P. Awramik S.M. Benmore W.C. Stromatolite morphogenesis—progress and problems. Can J Earth Sci. 1979;16:992–1015. [Google Scholar]
- Toporski J.K.W. Steele A. Westall F. Thomas-Keprta K.L. McKay D.S. The simulated silicification of bacteria—new clues to the modes and timing of bacterial preservation and implications for the search for extraterrestrial microfossils. Astrobiology. 2002;2:1–26. doi: 10.1089/153110702753621312. [DOI] [PubMed] [Google Scholar]
- Trewin N.H. Depositional environment and preservation of biota on the Lower Devonian hot-springs of Rhynie, Aberdeenshire, Scotland. Trans R Soc Edinb Earth Sci. 1994;84:433–442. [Google Scholar]
- Trewin N.H. The Rhynie cherts: an early Devonian ecosystem preserved by hydrothermal activity. In: Bock G.R., editor; Goode J.A., editor. Evolution of Hydrothermal Ecosystems on Earth (and Mars?), Ciba Foundation Symposium 202. John Wiley and Sons; Chichester, UK: 1996. pp. 131–145. discussion 145–149. [DOI] [PubMed] [Google Scholar]
- Walter M.R. Archean stromatolites: evidence of the Earth's earliest benthos. In: Schopf J.W., editor. Earth's Earliest Biosphere. Princeton University Press; Princeton, NJ: 1983. pp. 187–213. [Google Scholar]
- Walters C.C. Margulis L. Barghoorn E.S. On the experimental silicification of microorganisms. I. Microbial growth on organosilicon compounds. Precambrian Res. 1977;5:241–284. [Google Scholar]
- Westall F. Boni L. Guerzoni D. The experimental silicification of microorganisms. Palaeontology. 1995;38:495–528. [Google Scholar]