Abstract
Mesenchymal stem cells (MSCs) seeded in composite implants formed of hydroxyapatite (HA) and poly (lactide-co-glycolide) (PLG) exhibit increased osteogenesis and enhanced angiogenic potential. Endothelial colony-forming cells (ECFCs) can participate in de novo vessel formation when implanted in vivo. The aim of this study was to determine the capacity of HA–PLG composites to cotransplant MSCs and ECFCs, with the goal of accelerating vascularization and resultant bone formation. The incorporation of HA into PLG scaffolds improved the efficiency of cell seeding and ECFC survival in vitro. We observed increases in mRNA expression and secretion of potent angiogenic factors by MSCs when cultured on HA–PLG scaffolds compared to PLG controls. Upon implantation into an orthotopic calvarial defect, ECFC survival on composite scaffolds was not increased in the presence of MSCs, nor did the addition of ECFCs enhance vascularization beyond increases observed with MSCs alone. Microcomputed tomography (micro-CT) performed on explanted calvarial tissues after 12 weeks revealed no significant differences between treatment groups for bone volume fraction (BVF) or bone mineral density (BMD). Taken together, these results provide evidence that HA-containing composite scaffolds seeded with MSCs can enhance neovascularization, yet MSC-secreted trophic factors do not consistently increase the persistence of co-transplanted ECFCs.
Introduction
The efficient vascularization of developing tissues represents a significant challenge for the success of tissue engineered bone. Current approaches to rapidly vascularize developing bone suffer from limitations including a dependence on locally available responsive cells, cost, immunogenicity, and safety concerns.1–5 Cell transplantation provides bioactive populations of cells that are immediately available to participate in tissue repair and vascularization and may not require stimulation by exogenous inductive cues. Endothelial colony-forming cells (ECFCs) are a subpopulation of endothelial progenitor cells that can be isolated from both adult peripheral and umbilical cord blood.6 ECFCs exhibit tremendous proliferative potential and survival in oxygen microenvironments characteristic of bone and ischemic tissue defects due to high telomerase activity,6,7 making these cells clinically superior to microvascular endothelial cells from the dermis (HMVECs) and umbilical cord (HUVECs). Moreover, short-term functional vessels result from the transplantation of HMVECs and HUVECs,8–10 yet ECFCs exhibit the potential to form long-lasting de novo vessels that spontaneously anastomose with the host vasculature.11
Progenitor cells are a promising cell population for transplantation given the challenges associated with isolating and expanding sufficient numbers of autologous osteoblasts for bone repair. Mesenchymal stem cells (MSCs) can differentiate in response to lineage-specific inductive cues while also functioning as trophic mediators, secreting growth factors that promote angiogenesis and local cell and tissue survival.12–14 The cotransplantation of MSCs with endothelial cell populations has consistently increased vessel density and perfusion when examined in numerous models, suggesting that MSCs can support cell-based vascularization approaches to tissue repair.15–17
Composite implants containing bioceramics such as hydroxyapatite (HA), β-tricalcium phosphate, or bioactive glasses enhance the osteoconductivity and mechanical properties of biodegradable materials designed to bridge bone defects.18 Additionally, MSCs responded to HA-containing composite scaffolds with increased secretion of vascular endothelial growth factor (VEGF) and enhanced vessel formation.19 In this study, we investigated the effect of cotransplanting trophic factor-secreting MSCs with vessel-forming ECFCs when delivered via HA-containing composite implants. We explored the potential for ECFC survival on bioceramic-containing substrates when cotransplanted with MSCs and probed potential mechanisms. Finally, we evaluated the potential of cellular cotransplantation on bioceramic composite implants to improve vessel density and resultant bone formation in a rodent orthotopic defect.
Materials and Methods
Scaffold preparation
HA–poly (lactide-co-glycolide) (PLG) composite scaffolds (2.5:1 mass ratio of HA to PLG) and control scaffolds lacking HA (0:1) were prepared using a gas foaming/particulate-leaching method as previously described.19 Briefly, microspheres composed of PLG (85:15 DLG 7E; Lakeshore Biomaterials, Birmingham, AL) were prepared using a double-emulsion process. Lyophilized PLG microspheres were mixed with NaCl particles (250–425 μm in diameter) and HA nanocrystals (100-nm diameter, Berkeley Advanced Biomaterials, Berkeley, CA) and compressed to a solid disk (final dimensions: 8.5-mm diameter and 1.5-mm thickness; approximately 85-μL total volume) in a custom-made stainless steel die using a Carver press (Fred S. Carver, Wabash, IN) at 10 MPa for 1 min. The compressed disks were exposed to high pressure carbon dioxide (CO2) gas (5.5 MPa) for at least 16 h, after which the pressure was rapidly (<1 min) released to ambient. NaCl particles were leached from the scaffolds by submersion in distilled water (H2O) for 24 h.
The scaffold sterilization protocol was modified to enhance scaffold permeability. Before sterilization, scaffolds were treated with 0.5 N NaOH for 1 min to functionalize scaffold surfaces, a process that increases surface porosity and enhances cell adhesion. Scaffolds were then repeatedly washed in distilled H2O until pH returned to 7.0. Functionalized scaffolds were sterilized by placing them in a 50-mL conical tube with 30 mL 95% ethanol and applying gentle vacuum for 30 min. Scaffolds were rinsed twice in sterile phosphate buffered saline (PBS) and incubated overnight in an alpha-modified Eagle's medium (α-MEM, Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS, JR Scientific, Woodland, CA) and 1% penicillin/streptomycin (P/S, Mediatech, Manassas, VA).
Cell culture
Human bone marrow-derived MSCs (Lonza, Walkersville, MD) were expanded in the α-MEM supplemented with 10% FBS and 1% P/S until use at passages 4–5. Human umbilical cord blood ECFCs were generously provided by Dr. Mervin Yoder (Indiana University) and isolated using a protocol approved by the Institutional Review Board of the Indiana University School of Medicine as previously described.11 Adherent ECFCs were cultured on tissue culture plastic coated with 5 μg/cm2 rat tail collagen I (BD Biosciences, San Jose, CA) in EGM®-2 media with Lonza's SingleQuot supplements (hydrocortisone, gentamycin, human VEGF, human basic fibroblast growth factor [FGF2], human epidermal growth factor [EGF], human insulin-like growth factor [IGF], and heparin) and further supplemented with 5% FBS and 1% P/S. Culture-expanded ECFCs (passages 12–13) were used for all studies.
Cell seeding
MSCs and ECFCs were collected using trypsin (w/0.25% ethylenediaminetetraacetic acid; Mediatech) and resuspended to 2×107 cells/mL in α-MEM (10% FBS, 1% P/S) or EGM-2 (SingleQuots, 5% FBS, 1% P/S), respectively. Scaffolds loaded with only MSCs were statically seeded using a 50 μL mixture containing equal volumes of the MSC suspension (25 μL containing 5×105 MSCs) in α-MEM and EGM-2 media. Likewise, scaffolds loaded with ECFCs alone were statically seeded with a 50 μL mixture containing equal volumes of the ECFC suspension (25 μL containing 5×105 ECFCs) in EGM-2 media and α-MEM. Scaffolds containing both cell types were seeded using 50 μL of cell suspension containing equal volumes of both MSC and ECFC suspensions (1×106 cells total). In all groups, cells were allowed to attach for 4 h before being transferred to 12-well plates containing a 1:1 mixture of α-MEM and EGM-2 deficient of VEGF, FGF, and IGF SingleQuot supplements. These existing angiogenic supplements that promote cell survival were removed from EGM-2 media to interrogate cell-secreted trophic interactions between ECFCs and MSCs on various material substrates. This 1:1 mixture was chosen to equally promote the survival of both MSC and ECFC populations. Scaffolds were maintained in the media mixture in standard cell culture conditions (37°C, 5% CO2) on an XYZ shaker (Stovall Life Sciences, Inc., Greensboro, NC) at 25 rpm with media changes every 3 days. Scaffolds were collected at day 0 (the day after cell seeding following overnight incubation) and day 7.
Cellular assays
At collection, MSC-seeded scaffolds were washed twice in PBS, minced with a razor blade, and cell lysates obtained by collecting scaffolds in a passive lysis buffer (Promega, San Luis Obispo, CA), followed by sonication for 5 s. Scaffold remnants were additionally washed for 10 min with a 300 mM potassium phosphate solution (pH 7.2) to collect bound lysates. Total DNA was determined from cell lysates (n=4) using the Quant-iT PicoGreen dsDNA kit (Invitrogen).
ECFC persistence in response to the scaffolding material was quantified in vitro using green fluorescent protein (GFP)-transduced cells. At each time point, cell-seeded scaffolds were quantified using fluorescence spectrophotometry (excitation 485 nm, emission 528 nm) with a microplate reader (BIO-TEK Synergy HTTR, Wisnooski, VT). ECFC persistence was represented as relative light units (RLU) derived from the sum of total fluorescence from both sides of scaffolds. ECFCs transduced with GFP or luciferin were prepared by the UC Davis Center of Excellence in Translational Human Stem Cell Research using an HIV-1-derived lentiviral vector containing the cytomegalovirus promoter20 at a multiplicity of infection (MOI) of 10. We confirmed that these cells behave similarly to native ECFCs with regards to proliferation, migration, and tubule formation at this MOI (data not shown).
Before scaffold collection, the medium was replaced with a fresh medium for 24 h, and the conditioned medium was collected and assayed for secreted VEGF using a commercially available sandwich enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). We explored the contribution of MSCs to ECFC survival and persistence by inhibiting the effect of VEGF secretion by MSCs with a human VEGF antibody (AB-293-NA, R&D Systems, Minneapolis, MN). Per manufacturer's instructions, 3–6 μg/mL of antibody is necessary to neutralize 10 ng/mL of recombinant VEGF. An antibody concentration of 15 μg/mL was used to neutralize cell-secreted VEGF with antibodies replaced every 3 days with medium changes.
Quantitative polymerase chain reaction
MSC-containing scaffolds were washed with PBS, total RNA was collected using the RNeasy Micro kit (Qiagen, Valencia, CA), and 500 ng of total RNA was reverse-transcribed with the QuantiTect Reverse Transcription kit (Qiagen). Quantitative polymerase chain reaction (qPCR) was performed using the TaqMan® Universal PCR Master Mix (Applied Biosystems, Foster City, CA) on a Mastercycler® realplex2 (Eppendorf, Westbury, NY). Primers and probes for VEGFA (Hs00173626_m1), PDGF (Hs00234042_m1), FGF1 (Hs00265254_m1), and FGF2 (Hs00266645_m1) were purchased from Applied Biosystems. Amplification conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. qPCR results were normalized to the RPL13 (Hs00204173_m1) transcript level to yield ΔCt. Fold change in expression was subsequently calculated using the formula 2−ΔCt.
Critical-sized cranial defect model
Treatment of all experimental animals was in accordance with the UC Davis animal care guidelines and all National Institutes of Health animal handling protocols. MSCs and luciferin-transduced ECFCs (1×106 cell total) were cotransplanted on 2.5:1 HA–PLG scaffolds into a rodent critical-sized calvarial defect, and the capacity of this system to induce angiogenesis and drive bone formation was examined. This composition was selected in light of preliminary data derived from in vitro and in vivo subcutaneous studies (e.g., capacity to increase cell-secreted proangiogenic growth factors and ECFC persistence, Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea), improved mechanical properties of the substrate compared to ceramic-free vehicles, and improved seeding efficiency compared to PLG control scaffolds. Scaffolds were prepared as described above and cut to 8-mm diameter using a biopsy punch before scaffold sterilization. Experimental groups included 2.5:1 HA–PLG acellular control scaffolds, scaffolds seeded with MSCs alone, ECFCs alone, or both MSCs and ECFCs.
Skeletally mature 10-week-old male albino nude rats (n=10 per group) were anesthetized and maintained using 2% isoflurane with O2 flow at 2 L/min delivered through a mask. A rostral to caudal incision, roughly 2 cm in length, was made along the central axis of the head over the calvarium. The flaps of skin were pulled back and the periosteum ablated using a surgical blade. A piece of calvarial bone was removed using an 8-mm outer diameter trephine bur (Bone Grafting Solutions, Hebron, KY). Scaffolds were manually placed above the circular defect and gently pressed into the defect using a metal spatula. The incision was closed, rats were allowed to recover on a heating pad, and animals were allowed access to food and water ad libitum. Buprenorphine (0.05 mg/kg) was given as an analgesic by an intraperitoneal (IP) injection every 12 h over the first 48 h.
ECFC persistence in the defect, indicated by the presence of luciferin-expressing cells, was assessed over 2 weeks by whole-body bioluminescence imaging 30 min following the IP injection of luciferin substrate (0.15 mg/g body weight, Caliper Life Sciences, Hopkinton, MA) using an IVIS-imaging system (100 series, Caliper Life Sciences). A subset of animals from each group (n=5) was euthanized at week 4 by CO2 inhalation and cervical dislocation and their calvaria removed. Recovered bone tissues were fixed in phosphate-buffered formalin for 24 h and then moved to 70% EtOH for storage before processing and analysis. Calvaria were demineralized in Calci-Clear (National Diagnostics, Atlanta, GA), paraffin embedded, and sectioned at 5-μm thickness. Vessel counts were performed using hematoxylin and eosin (H&E)-stained complete calvarial cross-sections at a 10× magnification as previously described.19 The presence of human cells was determined by immunohistochemistry using antibodies for human nuclear antigen (HNA, MAB1281, Millipore, Billerica, MA), human CD31 (ECM590, Millipore), and von Willebrand Factor (vWF) (ECM590, Millipore) that cross-reacts with both human and rat cell-surface antigens.
All remaining animals were euthanized as described above after 12 weeks and their calvaria collected. The bone volume fraction (BVF) and bone mineral density (BMD) were determined using microcomputed tomography (micro-CT). A cylinder 6.0-mm diameter and a 0.45-mm long cylinder centered in the scaffold core was imaged (70 kVp, 114 μA, 300-ms integration time, average of three images) using a high-resolution micro-CT specimen scanner (micro-CT 35; Scanco Medical, Bassersdorf, Switzerland). About 696 contiguous slices of 2048×2048 pixels were imaged with 15 μm resolution and slice thickness (voxels). Serial tomograms were reconstructed from raw data of 1000 projections per 180° using a cone beam-filtered back projection algorithm adapted from Feldkamp et al.21 The tomograms were calibrated to 0.0, 99.6, 200.0, 401.0, and 800.3 mg HA/cc concentrations of HA so that grey-values (X-ray attenuation) of the images were converted to units of density in mg HA/cc. Bone tissue in the reconstructed images was determined by thresholding (191–3000 mg HA/cc) to partition mineralized tissue from fluid and soft-tissues. After thresholding, the image noise was reduced using a low-pass Gaussian filter (σ=0.8, support=1). BVF was determined by dividing the number of pixels representing bone tissue (bone volume) by the number of pixels in the cylindrical segment (total volume). The mean density of the bone material or BMD was the average density (mg HA/cc) of the BVF.
Statistical analysis
Results are expressed as mean±standard error of the mean (SEM), assuming normal distribution of data sets. Statistical analyses were performed between two groups using the Student's t-test or between multiple groups using a Student–Newman–Keuls multiple comparisons test where probability values (p) for significance were calculated; p<0.05 was considered statistically significant.
Results
MSC seeding is improved by HA content
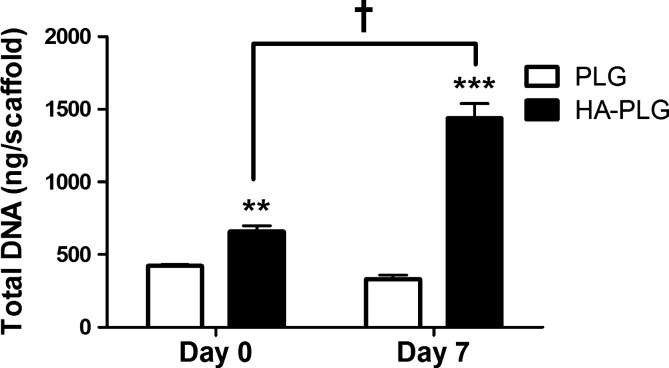
We detected significant increases in DNA content, an indicator of more cells, when examining HA-containing scaffolds (Fig. 1). We observed significantly more MSCs on 2.5:1 HA–PLG scaffolds versus PLG controls at day 0, suggestive of improved binding and increased seeding efficiency. In addition, DNA content increased significantly on HA-containing scaffolds from day 0 to 7, suggesting increased proliferation of MSCs on composite scaffolds. Total DNA mass in scaffolds containing HA more than doubled in 7 days, while total DNA mass remained relatively constant in scaffolds without HA.
FIG. 1.
DNA quantification of MSC-seeded scaffolds was performed at days 0 and 7 on PLG and HA–PLG scaffolds. Values are mean±SEM (n=4); **p<0.01 versus PLG at day 0; ***p<0.001 versus PLG at day 7. †p<0.001 versus HA–PLG at day 0. MSC, mesenchymal stem cell; PLG, poly (lactide-co-glycolide); HA, hydroxyapatite; SEM, standard error of the mean.
Angiogenic genes are upregulated by HA content
qPCR was used to probe the expression of several proangiogenic genes in MSCs, including VEGFA, PDGF, FGF1, and FGF2. VEGF, encoded by VEGFA, is a potent mitogen of endothelial cells and enhances vessel permeability and sprouting.22 At day 0, VEGFA expression was significantly lower in MSCs on HA–PLG compared to PLG scaffolds (Fig. 2A). VEGFA expression dropped significantly between days 0 and 7 for MSCs seeded on PLG scaffolds. By day 7, VEGFA expression was significantly higher for MSCs on HA-containing scaffolds; roughly twice the level of MSCs on PLG scaffolds. Platelet-derived growth factor (PDGF), encoded by PDGF, is involved in vessel maturation, promoting the recruitment and migration of mural cells.23 PDGF mRNA was not expressed at detectable levels by MSCs seeded on PLG scaffolds, even after 7 days (Fig. 2B). Over the same period, MSCs seeded on HA-containing scaffolds began expressing detectable levels of PDGF. FGF1 (acidic FGF) is encoded by FGF1 and stimulates the proliferation and migration of all cell types involved in neovascularization.24 Initial FGF1 expression was low in cells on both scaffold types (Fig. 2C). Similar to trends observed with VEGFA and PDGF, MSCs on HA-containing scaffolds exhibited almost three times greater FGF1 expression after 7 days. FGF1 expression increased significantly for cells on both scaffolds from day 0 to 7, and gene expression on HA–PLG scaffolds at day 7 was significantly greater than all other time points and scaffold compositions. Finally, FGF2 (basic FGF) is encoded by FGF2 and promotes endothelial cell proliferation and tubule formation.24 FGF2 expression decreased over 7 days in MSCs on both scaffold types and was consistent across both scaffold types at each time point, regardless of bioceramic content (Fig. 2D).
FIG. 2.
Quantitative polymerase chain reaction was used to analyze proangiogenic gene expression by MSCs on various scaffolds. (A) VEGFA, (B) PDGF, (C) FGF1, and (D) FGF2 expression were normalized to RPL13 expression and data were represented as ΔCt. Values are mean±SEM (n=4); *p<0.05 versus PLG at the same time point; **p<0.01 versus PLG at day 0; $p<0.05 versus PLG at day 0; ***p<0.001 versus expression at day 0.
ECFCs exhibit improved persistence on HA-containing scaffolds
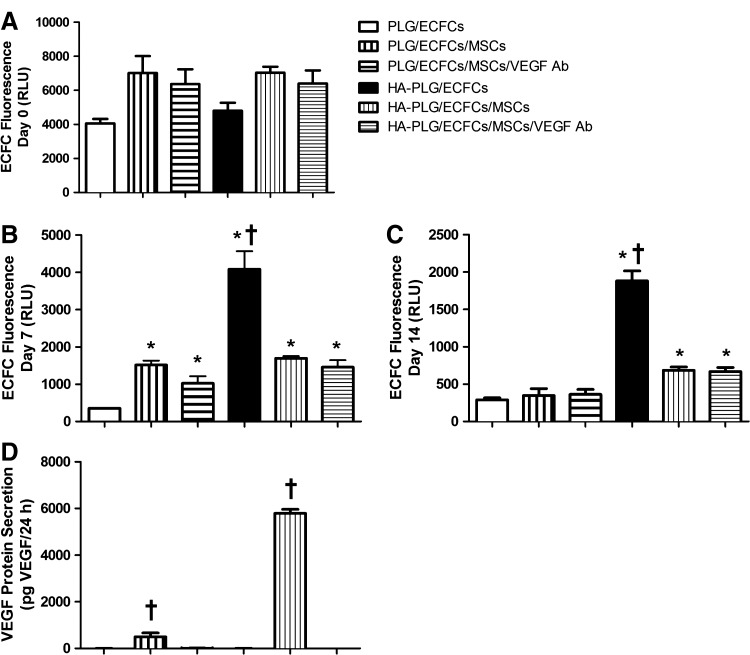
After confirming that HA upregulates multiple proangiogenic genes, we examined the potential synergistic activity of HA-loaded substrates with MSCs to affect ECFC survival in vitro. The number of cells initially seeded to all scaffold types were not significantly different (p>0.05) (Fig. 3A). From day 0 to 7, we observed significant reductions in total fluorescence (i.e., ECFC count) for all groups except for ECFCs on HA–PLG scaffolds. At day 7, we detected significantly more cells on PLG and HA–PLG scaffolds seeded in coculture compared to PLG seeded with ECFCs alone, suggesting that MSCs play an active role in promoting ECFC survival and persistence (Fig. 3B). For scaffolds seeded with ECFCs alone, we observed a significant RLU reduction on PLG scaffolds at day 7 compared to day 0, while the decrease in ECFC fluorescence for cells on HA–PLG scaffolds was much less. These data highlight the differential response of MSCs and ECFCs to this composite substrate, as we observed a significant increase in the MSC number after 7 days on HA–PLG scaffolds (Fig. 1). Furthermore, VEGF secretion by MSCs was significantly increased on HA-loaded scaffolds compared to all other groups (Fig. 3D), and we did not detect appreciable levels of VEGF from constructs lacking MSCs. ECFC fluorescence values continued to fall between day 7 and 14 in all study groups, yet significant reductions were only detected for HA–PLG scaffolds seeded with ECFCs alone (p<0.01 vs. day 7 value). At day 14, ECFC persistence was significantly greater for coculture-seeded HA–PLG scaffolds compared to PLG scaffolds (Fig. 3C), likely a result of enhanced MSC persistence and resulting concentration of trophic factors. The addition of a VEGF antibody did not appreciably reduce ECFC proliferation or persistence as measured by fluorescence, indicating that multiple signaling molecules are active in sustaining ECFCs.
FIG. 3.
(A–C) Fluorescent quantification of ECFCs seeded individually or in coculture on PLG and HA–PLG scaffolds at days 0, 7, and 14. (D) VEGF secretion by cells on scaffolds at day 7. Values are mean±SEM (n=4); *p<0.05 versus PLG, †p<0.05 versus all groups. All groups except HA-PLG/ECFCs demonstrated significant reductions in fluorescence by day 7. ECFCs, endothelial colony-forming cells; VEGF, vascular endothelial growth factor; RLU, relative light units.
Neovascularization is enhanced by MSCs on HA-containing scaffolds
A critical-sized calvarial defect (rat cranium critical-sized defect=8 mm25) was surgically created to explore the contribution of MSCs and ECFCs when transplanted on composite scaffolds, both individually and synergistically, to angiogenesis and bone formation. IVIS imaging revealed continued ECFC persistence in groups containing ECFCs 2 weeks post-transplantation on HA-loaded scaffolds in an orthotopic defect (Fig. 4A, B). Significantly higher optical density was observed in scaffolds containing only ECFCs, hence supporting in vitro observations.
FIG. 4.
In vivo bioluminescent measurements used to measure luciferin-transduced ECFCs on scaffolds transplanted to cranial defects after (A) 7 and (B) 14 days. *p<0.05 versus MSC+ECFC.
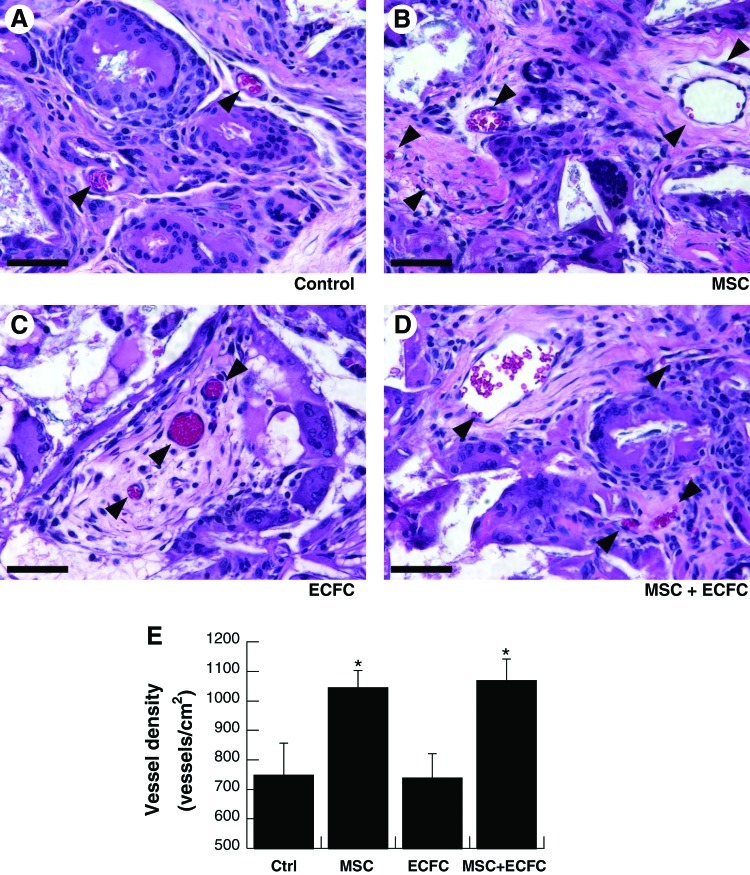
Vessel density was quantified after 4 weeks by enumerating blood vessels in repair tissue from H&E-stained histological sections (Fig. 5A–D). Scaffolds containing MSCs exhibited significantly more vessels (1044±58 vessels/cm2 for scaffolds with MSCs; 1070±72 vessels/cm2 for scaffolds with MSCs and ECFCs) than those without (748±108 vessels/cm2 for acellular scaffolds and 738±83 vessels/cm2 for scaffolds with ECFCs; p<0.05 vs. MSC-containing scaffolds) (Fig. 5E). The transplantation of ECFCs alone did not increase vessel density over acellular control scaffolds. Finally, the lack of positive staining for HNA and human CD31, together with positive staining for vWF, indicated that the cells within vessels were of a host origin (Supplementary Fig. S2).
FIG. 5.
Neovascularization of composite scaffolds after implantation for 4 weeks. Representative hematoxylin and eosin sections near the center of explanted scaffolds imaged at 40×(A–D). (Arrowheads denote vessels; scale bar=50 μm). (E) Vessel density within repair tissue; values are mean±SEM (n=5); *p<0.05 versus acellular control scaffolds. Color images available online at www.liebertpub.com/tea
Osteogenesis on HA-containing scaffolds
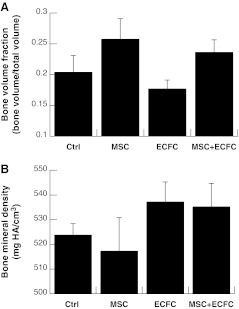
Bone formation was analyzed using micro-CT on calvaria collected after 12 weeks. We did not detect differences in either BVF (Fig. 6A) or BMD (Fig. 6B). However, the mean BVF exhibited a trend consistent with that observed for vessel density, suggesting that BVF may be increased by the rapid establishment of increased vessel density, yet the degree of enhancement was not great enough to discern statistical improvement given the relative HA content within the composite scaffold.
FIG. 6.
micro-CT was used to quantify (A) BVF and (B) BMD after removal of calvarial tissue. No significant differences were detected. Micro-CT, microcomputed tomography; BVF, bone volume fraction; BMD, bone mineral density.
Discussion
In this study, our objective was to determine the capacity of HA–PLG scaffolds, previously confirmed to enhance VEGF secretion by MSCs, to effectively deploy a coculture of ECFCs with MSCs to increase angiogenesis and resultant bone formation. Bioceramic composite materials represent a promising implant for treating bone defects with increased osteoconductivity and substrate stiffness that is lacking from biodegradable polymers. Such implants are also under investigation as delivery vehicles for cell-based therapies of bone regeneration.
Implant composition had a striking effect on the expression of potent proangiogenic genes in MSCs such as VEGFA, FGF1, and PDGF. These genes encode for secreted proteins that work synergistically to recruit and support cells that are directly involved in angiogenesis and result in wound healing and repair.23,26–29 The implantation of human progenitor cells induces the migration and resultant angiogenesis from responsive rodent endothelial cells,30,31 and we detected increased vascularization in defect sites treated with human MSCs in the presence and absence of ECFCs. These data provide evidence that the observed increases in cellular survival or proliferation are due in part to growth factor production, with more than twice the VEGFA, FGF1, and PDGF expression by MSCs on HA-containing scaffolds.
We probed the ability of composite scaffolds of varying compositions to promote ECFC survival when cotransplanted with MSCs into a highly vascularized subcutaneous site (Supplementary Fig. S1) similar to our previous report.19 The rat subcutaneous implant model facilitates the simultaneous study of four treatment groups, and we elected to include the 5:1 HA–PLG scaffolds to observe the effect of ECFC persistence over a range of concentrations above and below our expected optimal 2.5:1 HA–PLG scaffold. We detected statistically similar ECFC persistence at both 7 and 14 days between 2.5:1 and 5:1 HA–PLG scaffolds. We used 2.5:1 HA–PLG scaffolds for the remainder of the study for their ability to enhance ECFC persistence compared to PLG scaffolds and higher porosity than 5:1 HA–PLG scaffolds.19 In previous studies, despite showing enhanced scaffold surface hydrophilicity and protein (fibronectin)-binding affinity on HA–PLG scaffolds, we did not detect increases in cellular seeding efficiency, a potential contributor to differences in the cell response. By modifying our sterilization technique, particularly by increasing media permeation using vacuum pressure, we were able to exploit the available surface area of both internal and external scaffold surfaces for significant improvements in cell-seeding efficiency.
When cells were transplanted on 2.5:1 HA–PLG scaffolds into the orthotopic defect, we observed increased vessel density for scaffolds seeded with MSCs at 4 weeks. However, the transplantation of ECFCs did not further increase vessel density, regardless of whether they were transplanted alone or with MSCs. Kaigler et al. reported similar vessel densities in calvarial defects treated with a monoculture of BMSCs or coculture of BMSCs and HMVECs after 6 weeks when transplanted in PLG scaffolds.2 We detected more than three-fold greater vessel density in bone defects when transplanting cells on HA–PLG scaffolds compared to previous reports using PLG alone. Furthermore, bone defects treated with acellular 2.5:1 HA–PLG scaffolds attained an average vessel density more than twice that previously reported in animals treated with acellular PLG scaffolds after 12 weeks.2 In these studies, we did not detect human cells in the defect, while others have observed a small percentage of human-derived vessels when transplanting similar cocultures. These data suggest that cells associated with the scaffold, whether transplanted or resident host cells migrating into the defect, are responsive to the substrate and stimulate a cascade of events to enhance neovascularization at the defect site. The results of these studies suggest that transplanted cells die off rather quickly upon implantation and do not directly contribute to bone formation by differentiating down an osteogenic lineage or contributing as mural cells to stabilize the nascent vascular network. Endothelial cell-derived vessel formation in vivo11 has been observed using compliant biomaterials that are vulnerable to remodeling. For example, HUVECs and MSCs cotransplanted subcutaneously using a demineralized bone matrix exhibited significantly increased neovessel formation.31 The composition of our scaffold, chosen in part for its mechanical properties, may inhibit ECFC migration and tubule formation due to the inability of cells to remodel the material over short time frames. Organic matrices degrade quickly in the body, facilitated by the actions of serine proteases (tPA, uPA) and matrix metalloproteinases (collagenases), while ceramic materials depend on slower processes of hydrolysis and osteoclast degradation.32 Furthermore, mural cells that would have acted to stabilize newly formed capillaries may not have been able to reach them through our scaffold, resulting in ECFC dissociation, apoptosis, and pruning of the neovasculature. Additional work is necessary to optimize the degradation kinetics and pathway of this construct while maintaining the presentation of HA to accommodate cell-based remodeling.
We observed increased persistence of ECFCs on HA–PLG scaffolds both in vitro and in vivo in the absence of MSCs. ECFCs seeded alone on HA–PLG scaffolds demonstrated the greatest ECFC persistence after 7 and 14 days in vitro, and decreases in fluorescence were less pronounced for cells on HA–PLG compared to PLG scaffolds. However, the addition of MSCs resulted in significantly fewer ECFCs at both time points despite the availability of cell-secreted VEGF, a potent ECFC mitogen. The reduction in the ECFC number may be related to numerous factors. In light of increased MSC proliferation on HA–PLG scaffolds, there may be insufficient nutrients available to support proliferation of both cell populations. MSCs and ECFCs may differentially adhere to HA–PLG scaffolds, and these populations may even compete for binding sites. When ECFCs were seeded in monoculture on HA–PLG scaffolds and examined with H&E staining, we observed aggregates and clusters of cells throughout the material that did not adhere to the substrate (data not shown). Potential differences in binding affinity may represent a confounding parameter and limitation of these studies when seeding cells in a coculture. Additionally, the interaction of ECFCs with HA–PLG scaffolds may differ in the presence or absence of MSCs. In the presence of MSCs, ECFCs may respond to cell-secreted proangiogenic trophic factors by attempting to remodel the surrounding matrix and form capillaries. For stiff HA–PLG scaffolds that are not remodeled enzymatically over the time course of early vasculogenesis, neovessels dissociate and ECFCs undergo apoptosis. However, in the absence of MSCs, it is possible that ECFCs remain viable longer while being inactive, as indicated by a lack of observed vasculature.
Numerous studies have correlated increases in angiogenesis to bone formation using a variety of approaches, including the delivery of recombinant proteins,33,34 proangiogenic materials,5 or stem cells.35 However, despite observing increases in vessel densities in cell-seeded scaffolds, we did not detect significant increases in bone formation. The transplantation of osteogenically induced and endothelial differentiated canine bone marrow mononuclear cells exhibited enhanced mineralization when a lower HA:PLG mass ratio (1:1) and roughly twice the initial cell-seeding density were used.36 These data suggest that scaffolds with reduced ceramic content may enable the realization of cotransplanted endothelial cells for bone formation. We observed that BVF at week 12 exhibited a similar trend to vessel density at week 4, suggesting that higher initial cell-seeding densities may increase bone formation.
The interplay between vessel-forming endothelial cells and osteogenic cells, whether osteoblasts or osteoprogenitor cells such as MSCs, represents an important focus to simultaneously accelerate neovascularization and bone formation. In this study, we aimed to capitalize on the ability of HA–PLG scaffolds to upregulate trophic factor secretion by MSCs to induce proliferation or promote survival of cotransplanted ECFCs. However, the cotransplantation of MSCs with ECFCs on these substrates did not yield improved ECFC survival, increased vascularization in an orthotopic site, or resultant increases in bone formation. Recent studies report that the implantation of prevascularized constructs produced by cocultures of osteoblasts and endothelial cells result in accelerated anastomosis and improved long-term survival in vivo,37,38 and the lifespan of the endothelial cells was enhanced by coculture. Additionally, the culture of endothelial cells in direct contact with osteoblastic cells increases osteogenic differentiation.39,40 In light of these reports, greater efficacy may be achieved with HA–PLG scaffolds by allowing cocultures to interact for a period of time before implantation, potentially accelerating differentiation of the osteogenic cells and enabling the self-arrangement and formation of vascular structures that may anastomose with the existing host vasculature. Together, this may yield an implant with desirable stiffness, osteoconductivity, and vascularization.
These data demonstrate that HA composites can be used to increase endogenous trophic factor secretion by MSCs and under certain conditions, increase persistence of ECFCs. This finding has significant clinical applications for enhancing therapeutic angiogenesis where protein therapies are inappropriate. This strategy may advance existing therapies by using materials-based approaches to replace costly and commonly inefficient delivery of recombinant growth factors. Furthermore, our method can be used as a platform to explore the interplay between angiogenic and osteogenic elements. Finally, despite increased angiogenesis when transplanting MSCs, further investigation is needed to tailor the existing construct to better promote new bone formation.
Supplementary Material
Acknowledgments
We acknowledge David Working and Archana Bhat for surgical assistance with the calvarial defect procedure. This work was supported by the AO Research Fund of the AO Foundation (F-06-98L to J.K.L.). The project described was supported by an Award Number T32EB003827 from the National Institute of Biomedical Imaging and Bioengineering (J.H.) and a pilot grant from the Center of Excellence in Translational Human Stem Cell Research (NIH Grant P50-HL085036). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Patel Z.S. Young S. Tabata Y. Jansen J.A. Wong M.E. Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaigler D. Krebsbach P.H. Wang Z. West E.R. Horger K. Mooney D.J. Transplanted endothelial cells enhance orthotopic bone regeneration. J Dent Res. 2006;85:633. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S. Wan C. Ramaswamy G. Clemens T.L. Ponnazhagan S. Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther. 2010;18:1026. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng H. Usas A. Olshanski A. Ho A.M. Gearhart B. Cooper G.M., et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20:2017. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 5.Leu A. Stieger S.M. Dayton P. Ferrara K.W. Leach J.K. Angiogenic response to bioactive glass promotes bone healing in an irradiated calvarial defect. Tissue Eng Part A. 2009;15:877. doi: 10.1089/ten.tea.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingram D.A. Mead L.E. Tanaka H. Meade V. Fenoglio A. Mortell K., et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 7.Decaris M.L. Lee C.I. Yoder M.C. Tarantal A.F. Leach J.K. Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis. 2009;12:303. doi: 10.1007/s10456-009-9152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koike N. Fukumura D. Gralla O. Au P. Schechner J.S. Jain R.K. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 9.Nor J.E. Peters M.C. Christensen J.B. Sutorik M.M. Linn S. Khan M.K., et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81:453. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd B.R. Jay S.M. Saltzman W.M. Tellides G. Pober J.S. Human aortic smooth muscle cells promote arteriole formation by coengrafted endothelial cells. Tissue Eng Part A. 2009;15:165. doi: 10.1089/ten.tea.2008.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoder M.C. Mead L.E. Prater D. Krier T.R. Mroueh K.N. Li F., et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 13.Caplan A.I. Dennis J.E. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 14.Fan C.G. Zhang Q.J. Zhou J.R. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- 15.Sorrell J.M. Baber M.A. Caplan A.I. Influence of adult mesenchymal stem cells on in vitro vascular formation. Tissue Eng Part A. 2009;15:1751. doi: 10.1089/ten.tea.2008.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffy G.P. Ahsan T. O'Brien T. Barry F. Nerem R.M. Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A. 2009;15:2459. doi: 10.1089/ten.TEA.2008.0341. [DOI] [PubMed] [Google Scholar]
- 17.Melero-Martin J.M. De Obaldia M.E. Kang S.Y. Khan Z.A. Yuan L. Oettgen P., et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeGeros R.Z. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res. 2002;395:81. doi: 10.1097/00003086-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 19.He J. Genetos D.C. Leach J.K. Osteogenesis and trophic factor secretion are influenced by the composition of hydroxyapatite/poly(lactide-co-glycolide) composite scaffolds. Tissue Eng Part A. 2010;16:127. doi: 10.1089/ten.tea.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee C.I. Kohn D.B. Ekert J.E. Tarantal A.F. Morphological analysis and lentiviral transduction of fetal monkey bone marrow-derived mesenchymal stem cells. Mol Ther. 2004;9:112. doi: 10.1016/j.ymthe.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Feldkamp L.A. Davis L.C. Kress J.W. Practical cone-beam algorithm. J Opt Soc Am A. 1984;1:612. [Google Scholar]
- 22.Ferrara N. Gerber H.P. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 23.Hellberg C. Ostman A. Heldin C.H. PDGF and vessel maturation. Recent Results Cancer Res. 2010;180:103. doi: 10.1007/978-3-540-78281-0_7. [DOI] [PubMed] [Google Scholar]
- 24.Stegmann T.J. FGF-1: a human growth factor in the induction of neoangiogenesis. Expert Opin Investig Drugs. 1998;7:2011. doi: 10.1517/13543784.7.12.2011. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz J.P. Hollinger J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin Orthop Relat Res. 1986;205:299. [PubMed] [Google Scholar]
- 26.Byrne A.M. Bouchier-Hayes D.J. Harmey J.H. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) J Cell Mol Med. 2005;9:777. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheema S.K. Chen E. Shea L.D. Mathur A.B. Regulation and guidance of cell behavior for tissue regeneration via the siRNA mechanism. Wound Repair Regen. 2007;15:286. doi: 10.1111/j.1524-475X.2007.00228.x. [DOI] [PubMed] [Google Scholar]
- 28.Friesel R.E. Maciag T. Molecular mechanisms of angiogenesis: fibroblast growth factor signal transduction. FASEB J. 1995;9:919. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- 29.Wang J.S. Basic fibroblast growth factor for stimulation of bone formation in osteoinductive or conductive implants. Acta Orthop Scand Suppl. 1996;269:1. doi: 10.3109/17453679609155229. [DOI] [PubMed] [Google Scholar]
- 30.Capoccia B.J. Robson D.L. Levac K.D. Maxwell D.J. Hohm S.A. Neelamkavil M.J., et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koob S. Torio-Padron N. Stark G.B. Hannig C. Stankovic Z. Finkenzeller G. Bone formation and neovascularization mediated by mesenchymal stem cells and endothelial cells in critical-sized calvarial defects. Tissue Eng Part A. 2011;17:311. doi: 10.1089/ten.TEA.2010.0338. [DOI] [PubMed] [Google Scholar]
- 32.Ghajar C.M. George S.C. Putnam A.J. Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr. 2008;18:251. doi: 10.1615/critreveukargeneexpr.v18.i3.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y.C. Kaigler D. Rice K.G. Krebsbach P.H. Mooney D.J. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 34.Leach J.K. Kaigler D. Wang Z. Krebsbach P.H. Mooney D.J. Coating of VEGF-releasing scaffolds with bioactive glass for angiogenesis and bone regeneration. Biomaterials. 2006;27:3249. doi: 10.1016/j.biomaterials.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Cheung W.K. Working D.M. Galuppo L.D. Leach J.K. Osteogenic comparison of expanded and uncultured adipose stromal cells. Cytotherapy. 2010;12:554. doi: 10.3109/14653241003709694. [DOI] [PubMed] [Google Scholar]
- 36.Kim S.S. Park M.S. Cho S.W. Kang S.W. Ahn K.M. Lee J.H., et al. Enhanced bone formation by marrow-derived endothelial and osteogenic cell transplantation. J Biomed Mater Res A. 2010;92:246. doi: 10.1002/jbm.a.32360. [DOI] [PubMed] [Google Scholar]
- 37.Unger R.E. Ghanaati S. Orth C. Sartoris A. Barbeck M. Halstenberg S., et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31:6959. doi: 10.1016/j.biomaterials.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 38.Unger R.E. Sartoris A. Peters K. Motta A. Migliaresi C. Kunkel M., et al. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Choong C.S.N. Hutmacher D.W. Triffitt J.T. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521. doi: 10.1089/ten.2006.12.2521. [DOI] [PubMed] [Google Scholar]
- 40.Kaigler D. Krebsbach P.H. West E.R. Horger K. Huang Y.C. Mooney D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.