Abstract
Background & Aims
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the United States. The etiology is believed to be multi-factorial with a substantial genetic component; however, the heritability of NAFLD is undetermined. Therefore, a familial aggregation study was performed to test the hypothesis that NAFLD is highly heritable.
Methods
Overweight children with biopsy-proven NAFLD and overweight children without NAFLD served as probands. Family members were studied including magnetic resonance imaging to quantify liver fat fraction. Fatty liver was defined as a liver fat fraction ≥ 5%. Etiologies for fatty liver other than NAFLD were excluded. Narrow-sense heritability estimates for fatty liver (dichotomous) and fat fraction (continuous) were calculated using variance components analysis adjusted for covariate effects.
Results
Fatty liver was present in 17% of siblings and 37% of parents of overweight children without NAFLD. Fatty liver was significantly more common in siblings (59%) and parents (78%) of children with NAFLD. Liver fat fraction was correlated with body mass index (BMI), although the correlation was significantly stronger for families of children with NAFLD than those without NAFLD. Adjusted for age, sex, race, and BMI, heritability of fatty liver was 1.000 and of liver fat fraction 0.386.
Conclusion
Family members of children with NAFLD should be considered at high risk for NAFLD. These data suggest that familial factors are a major determinant of whether an individual has NAFLD. Studies examining the complex relations between genes and environment in the development and progression of NAFLD are warranted.
Keywords: magnetic resonance, genetics, family, obesity, fatty liver
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in adults and children in the developed world. Although obesity is a strong risk factor, the condition of obesity alone is not sufficient to produce NAFLD. There are examples of obese, even morbidly obese, individuals who do not have NAFLD as well as examples of NAFLD occurring in individuals of normal weight. 1–5 A major unanswered question is what underlies the difference between people who develop NAFLD and those who do not.
One explanation for variation in the development of NAFLD may be heredity, as supported by two lines of reasoning. First, clinical case series have shown familial clustering of NAFLD. 6, 7 A retrospective case series noted that many patients were from families with multiple subjects demonstrating NAFLD.7 Second, there are racial and ethnic differences in the prevalence of NAFLD. 1, 8 Among patients of comparable body mass index and insulin resistance, NAFLD is much more likely to occur in Hispanic Americans than among African Americans.1, 9–12 Although environmental risk factors are likely to influence the development of NAFLD, aspects of the observed variation in NAFLD phenotypic expression in persons with similar metabolic risk factors strongly implicates a genetic contribution.
An important step in deciding whether or not to pursue a genetic linkage or association study for identifying specific genetic variations that might influence NAFLD susceptibility is to determine the heritability of the NAFLD phenotype.13 Heritability estimates consider the fraction of the total variation exhibited by a phenotype that can be attributed to genetic factors, and rely on the comparison of the phenotype across related and unrelated individuals. Such estimates can be confounded by shared environmental factors that exist among related individuals and are usually environmentally context-specific. Estimates of heritability among individuals exclusively from one environment might be different from those among individuals from a different environment.14 Despite this caveat, heritability estimates are used routinely to assess the likelihood that a phenotype has identifiable genetic determinants. Estimates of the heritability of NAFLD are lacking in large part due to the methodological challenge of accurately determining the phenotype in terms of the presence or absence of NAFLD in each family member. Advances in magnetic resonance imaging (MRI) make accurate noninvasive evaluation feasible for both children and adults. The aim of this study was to estimate the heritability of NAFLD. A familial aggregation study was performed using MRI to assess hepatic steatosis in families of overweight children with and without NAFLD to test the hypothesis that NAFLD is a heritable condition.
METHODS
Subject Selection
Probands
Because most children with NAFLD are overweight or obese, all probands were required to be overweight or obese.15 Probands were further selected on the basis of the presence or absence of NAFLD. The diagnosis of NAFLD was based upon: 1) liver biopsy with at least 5% or greater of hepatocytes containing macrovesicular fat16, and 2) exclusion of other causes of chronic liver disease including hepatitis B (hepatitis B surface antigen), hepatitis C (hepatitis C antibody), alpha-1 antitrypsin deficiency (serum alpha-1 antitrypsin level and histology), autoimmune hepatitis (antinuclear antibody, anti-smooth muscle antibody, and histology), Wilson’s disease (serum ceruloplasmin), drug toxicity, total parenteral nutrition, chronic alcohol intake (clinical history), and chronic disease (cystic fibrosis, HIV, celiac disease, type 1 diabetes mellitus). The absence of NAFLD was based upon a liver MRI with a hepatic fat fraction < 5%.
Family Members
First, second, and third degree family members of probands were recruited. To better ensure that all subjects could follow the study protocol, the minimum age for family members was set at 8 years, with no upper age limit. Exclusion criteria were: 1) inability to complete a MRI evaluation (claustrophobia, metal implants, or body circumference greater than the imaging chamber); 2) potential reasons for hepatic steatosis other than NAFLD including medication (amiodarone, glucocorticoids, L-asparaginase, valproic acid), chronic disease (cystic fibrosis, HIV, hepatitis C, Wilson’s disease, celiac disease, type 1 diabetes mellitus), and chronic alcohol consumption; and 3) pregnancy.
The protocol was approved by the institutional review board of the University of California, San Diego. All adult subjects provided written informed consent. The parent(s) of all subjects < 18 years of age provided written informed consent for their children. Written assent was obtained from all children age 8 to 17.
Clinical Assessment
Subjects made a fasting visit to the General Clinical Research Center. Age and sex were recorded. Participants self-identified race and ethnicity. Height and weight were measured with the subjects standing and wearing light clothing without shoes to the nearest 0.1cm and 0.1kg, respectively. Body mass index (BMI) was calculated as the weight (kg) divided by the height (m) squared. Children were classified based upon BMI percentile as normal weight (BMI percentile between the 5th and 84th percentile), overweight (BMI percentile between the 85th and 94th percentile) or obese (BMI percentile ≥ the 95th percentile).17 Adults were classified based upon BMI as normal weight (BMI 18.5 to 24.9), overweight (BMI 25.0 to 29.9), or obese (BMI = 30.0). Subjects were instructed to fast overnight for 12 hours prior to phlebotomy. Fasting laboratory assays performed in the clinical chemistry laboratory included glucose, insulin, HDL-cholesterol, triglycerides, alanine (ALT) and aspartate (AST) aminotransferase. ALT > 30 U/L was considered abnormal. Diabetes was defined as a pre-existing clinical diagnosis of type 2 diabetes and/or fasting blood glucose ≥ 126 mg/dL.
Determination of Fatty Liver
Hepatic fat content was measured non-invasively using magnetic resonance (MR) imaging with a modified Dixon technique.18 Subjects were scanned supine with a phased-array coil centered over the liver at 1.5T (Siemens Symphony, Siemens Medical Systems, Erlangen, Germany). Multi-slice two-dimensional spoiled gradient recalled echo images were obtained in the axial plane. Field of view was adjusted to individual body habitus. To minimize T1 effects, a low flip angle (10°) was used with a repetition time of 122 msec.19–21 To assess fat-water signal interference effects and correct for T2* effects,19, 21, 22 six echoes were obtained at serial out-of-phase and in-phase echo times (2.3, 4.6, 6.9, 9.2, 11.5, 13.8 msec) during a single breath-hold. Other imaging parameters were 8-mm slice thickness, 500-Hz/pixel receiver bandwidth, one signal average, and 256×160–256 matrix.
For each subject, images at each of the six echo times were reviewed on a diagnostic-quality picture archiving and communication system digital monitor. A representative axial slice of the liver was selected. Avoiding organ boundaries, imaging artifacts, major vessels and bile ducts, a single elliptical region of interest (ROI), approximately 300–400 mm2, was manually selected within the liver parenchyma and automatically propagated to images at each of the six echo times. Image registration was not necessary as images at each echo time were acquired during the same breath hold and were already co-localized. The average signal intensity within each ROI was measured and recorded.
Imaging ROI values were analyzed using MATLAB (The MathWorks, Natick, MA). As described by Bydder, the measured signal intensity variation was modeled as a function of echo time.21 This method models the interference between fat-water and fat-fat while also correcting for exponential T2* decay. From the model, the fractional fat content in the liver was calculated as the ratio of fat proton density to the sum of the fat and water proton densities, and expressed as a percentage. Liver fat fraction ≥ 5% was defined as fatty liver. A radiologist reviewed all images.
Data Analysis and Heritability Calculation
Data were expressed as means ± standard deviations or frequency and percentage. Categorical variables were compared using Fisher’s exact test and continuous variables with Student’s t-test. Pearson correlation coefficients were used to assess the strength of the relationships between continuous variables. Differences between correlation coefficients were assessed with standard Fisher z-transform based tests. All hypothesis tests were two-tailed. Significance was defined at α = 0.05.
Narrow sense heritability (h2) estimates for continuous traits (liver fat fraction, BMI, glucose, triglycerides) and dichotomous traits (fatty liver, obesity, diabetes) were calculated using the suite of variance component-based statistical models and methods implemented in the Sequential Oligogenic Linkage Analysis Routines (SOLAR, Version 4.1.5, Southwest Foundation for Biomedical Research, San Antonio, TX). The range of possible values for h2 is from 0.0 (not heritable) to 1.0 (complete heritability). The variance component models for estimating heritability in SOLAR are linear models that account for the dependencies in the phenotypes among the related individuals in such a way as to partition variation in the phenotype into that variation attributable to the genetic relationships of the subjects and that attributable to other factors. Heritability was based on residual variance after adjusting for covariates (age, sex, race, ethnicity, BMI). Analyses of dichotomous or otherwise non-normally distributed phenotypes assessed in a traditional variance component modeling framework may inflate type I error rates.23, therefore we used the feature in SOLAR that models dichotomous and non-normally distributed traits on the multivariate t-distribution.24 In addition, we corrected for participant ascertainment by conditioning on proband phenotypes.25
RESULTS
Description of Probands
Details for the probands are shown in Table 1. There were 33 children with biopsy-proven NAFLD and 11 children without NAFLD. Age and BMI did not differ between groups. The distribution of overweight and obesity was also the same for both groups: overweight 18% and obese 82%. The racial and ethnic distribution for children with NAFLD was White-Hispanic 23/33 (70%), White-non-Hispanic 7/33 (21%), and Native American Indian-Hispanic 3/33 (9%). For children without NAFLD, the racial and ethnic distribution was White-Hispanic 6/12 (50%), White-non-Hispanic 2/12 (17%), Native American Indian Hispanic 3/12 (25%), Pacific Islander-non-Hispanic 1/12(8%). Probands with NAFLD had a significantly (p < 0.001) higher mean ALT and AST than overweight children without NAFLD. ALT was elevated in 31 of 33 (94%) children with NAFLD and in 3 of 11 (27%) children without NAFLD (p< 0.001). All children with NAFLD had MR-measured liver fat fraction > 5%. Children with NAFLD had a significantly (p < 0.001) higher mean MR-measured liver fat fraction of 18.1% compared to 1.6% for children without NAFLD.
Table 1.
Subject Characteristics
| Characteristic | Probands | Siblings | Parents | |||
|---|---|---|---|---|---|---|
| Normal | NAFLD | Normal Proband | NAFLD Proband | Normal Proband | NAFLD Proband | |
| (N = 11) | (N = 33) | (N = 12) | (N = 29) | (N = 19) | (N = 55) | |
| Age, mean (SD), y | 13.4 (4.3) | 13.5 (2.7) | 17.4 (6.5) | 18.3 (6.0) | 43.1 (5.2) | 42.9 (10.1) |
| Sex, N (%) | ||||||
| Male | 6 (55) | 22 (67) | 5 (42) | 15 (52) | 8 (42) | 23 (42) |
| Female | 5 (45) | 11 (33) | 7 (58) | 14 (48) | 11 (58) | 32 (58) |
| Weight, mean (SD), Kg | 82.9 (19.0) | 83.2 (24.7) | 65.6 (12.4) | 75.5 (22.4) | 83.7 (16.9) | 87.3 (20.2) |
| Height, mean (SD), cm | 160.4 (17) | 159.3 (15) | 158.9 (10) | 162.1 (14) | 167.5 (11) | 162.7 (10) |
| BMI, mean (SD), Kg/m2 | 32.0 (5.4) | 32.1 (5.8) | 26.0 (4.5) | 28.0 (5.7) | 30.0 (6.3) | 32.9 (6.6) |
| Obese, N (%) | 9 (82) | 27 (82) | 6 (50%) | 13 (45) | 10 (53) | 32 (58) |
| ALT, mean (SD), U/L | 26 (14) | 71 (38)* | 30 (24) | 51 (59) | 39 (37) | 40 (28) |
| AST, mean (SD), U/L | 34 (16) | 41 (21) | 30 (14) | 37 (23) | 28 (13) | 31 (15) |
| HDL-Cholesterol, mean (SD), mg/dL | 48 (20) | 40 (9) | 45 (8) | 42 (10) | 44 (13) | 44 (11) |
| Triglycerides, mean (SD), mg/dL | 122 (95) | 138 (105) | 101 (48) | 114 (79) | 103 (67) | 152 (113)‡ |
| Glucose, mean (SD), mg/dL | 90 (7) | 96 (20) | 93 (12) | 92 (13) | 100 (23) | 102 (37) |
| Liver Fat Fraction, mean (SD), % | 1.6 (1.4) | 18.1 (8.7)* | 2.7 (5.9) | 9.3 (7.6)† | 7 (8.3) | 14.0 (10.0)† |
| Liver Fat Fraction >5%, N (%) | 0 (0) | 33 (100)* | 2 (17) | 17 (59)‡ | 7 (37) | 43 (78)† |
Comparisons are within groups (probands, siblings, parents) for normal vs. NAFLD. Those that are significant are labeled as:
p < 0.001;
p<0.01;
p = <0.05.
Description of Family Members
A total of 152 non-proband family members were studied. The median family size studied was 4 people (range 2 to 12). Family members of children with NAFLD included 29 siblings, 55 parents, and 27 2nd or 3rd degree relatives. Family members of children without NAFLD included 12 siblings, 19 parents, and 10 2nd or 3rd degree relatives. Details for the siblings and parents of probands with and without NAFLD are shown in Table 1.
The mean values of the laboratory parameters tested were not significantly different between siblings of children with NAFLD and siblings of children without NAFLD. Serum triglycerides and fasting insulin were significantly (p < 0.05) higher in parents of children with NAFLD than in parents of children without NAFLD. ALT was elevated in 30 of 55 (55%) parents of children with NAFLD and in 7 of 19 (37%) parents of children without NAFLD (p = 0.29). Diabetes was present in 12 of 55 (22%) parents of children with NAFLD and in 2 of 12 (17%) parents of children without NAFLD (p =0.99).
Assessment of Fatty Liver
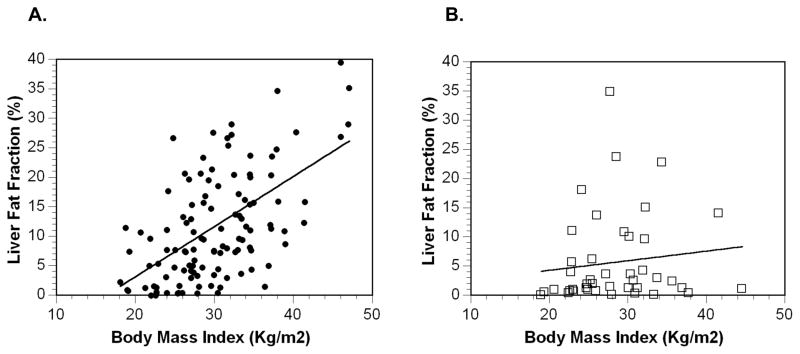
MR-measured liver fat fraction was significantly (p < 0.01) higher in siblings (9.3%) and parents (14.0%) of children with NAFLD than siblings (2.7%) and parents (7%) of children without NAFLD. For all participants combined including probands, liver fat fraction was significantly (p < 0.01) correlated (r = 0.54) with BMI. As shown in Figure 1, the correlation between liver fat fraction and BMI was strong in families of children with NAFLD and mild in families of children without NAFLD. The difference in the strength of correlation was significant (p = 0.029).
Figure 1. Correlation of BMI and Liver Fat Fraction.
Panel A: the correlation between BMI and liver fat fraction was high (r2 = 0.61; p < 0.01) in family members of children with NAFLD (●). Panel B: the correlation (r2 = 0.26; p < 0.05) between BMI and liver fat fraction was low in family members of children without NAFLD ( ). The difference in correlation was significant (p =0.029).
Fatty liver was present in 59% (17 of 29) of the siblings and 78% (43 of 55) of the parents of children with NAFLD. The frequency of fatty liver was significantly (p< 0.01) lower in the siblings (17%, 2 of 12) and the parents (37%, 7 of 19) of children without NAFLD. As highlighted by the family pedigree shown in figure 2, fatty liver was present in 20 of 111 (18%) of family members of children with NAFLD despite being non-obese and having a normal ALT. In family members with fatty liver, mean ALT was mildly elevated (45 ± 38 U/L) but significantly (p < 0.01) higher than in family members without fatty liver (27 ± 21 U/L). Among family members with fatty liver, the mean liver fat fraction was 15.4 ± 9%.
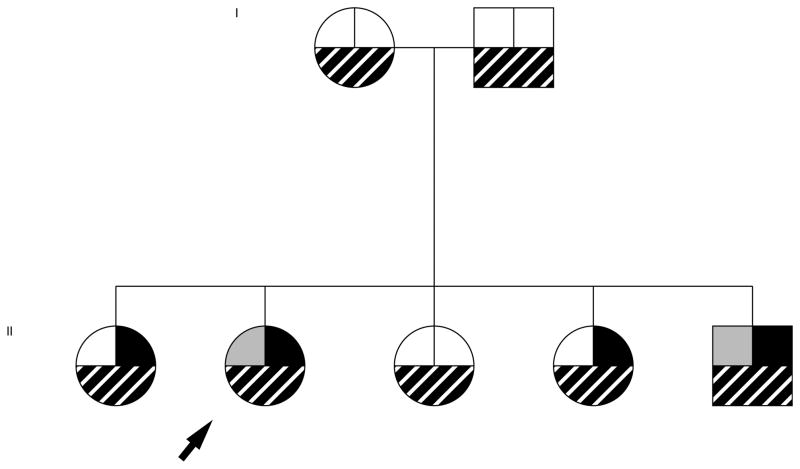
Figure 2. Paradigmatic pedigree of a child with NAFLD.
The arrow indicates the proband, a girl with biopsy-proven NAFLD. She is shown to be obese (left upper box with grey fill), have an elevated ALT (right upper box with black fill) and to have a liver fat fraction > 5% (bottom box with striped fill). Her family can be seen to have only 1 other obese individual, 3 other with an elevated ALT, but fatty liver in all sibliings and parents.
Previously unrecognized cirrhosis was detected by MRI in 2 family members of children with NAFLD. Both cases were subsequently confirmed by clinical evaluation including biopsy. The first case was a 62 year-old grandmother with a history of type 2 diabetes and mild liver chemistry elevation (ALT 47 U/L, AST 42 U/L). She had previously been told that she had mild fatty liver based upon her serum aminotransferase values. MRI evaluation revealed a liver fat fraction of 7%, a shrunken liver, and the presence of esophageal varices. The second case was a 39 -year-old father with no known medical problems. He had a BMI of 33.8 Kg/m2. Serum ALT was 62 U/L and AST was 70 U/L. Liver MRI revealed a hepatic fat fraction of 24% along with an enlarged caudate lobe and lobulated, nodular liver margins consistent with cirrhosis.
Estimate of heritability
As shown in Table 2, in the unadjusted model, NAFLD was significantly (p < 0.001) and highly heritable (h2 = 0.850 (SE 0.325)). Using regression-like adjustments for age, gender, race and BMI, the estimated heritability of NAFLD increased to the boundary or maximum value of 1.0 (p < 0.0001). When assessed as a continuous measure, the heritability for liver fat fraction was 0.581 (p=0.0001). After adjustment for age, sex, race and BMI, the heritability estimate for liver fat fraction was attenuated but retained significance (h2 = 0.386, p < 0.05). Both the continuous measure of BMI and the dichotomous measure of obesity also had high heritability. Serum triglycerides were significantly heritable but at a lower level. Neither the diagnosis of diabetes nor serum glucose as a continuous variable demonstrated significant heritability in this study sample.
Table 2.
Heritability Estimates
| Unadjusted | Adjusted | |||||
|---|---|---|---|---|---|---|
| Trait | h2 | SE | P value | h2 | SE | P value |
| Fatty Liver | 0.850 | 0.325 | < 0.0001 | 1.000 | N/A | <0.0001 |
| Liver Fat Fraction | 0.581 | 0.141 | < 0.0001 | 0.386 | 0.181 | 0.013 |
| Obesity | 0.716 | 0.194 | <0.001 | 0.718 | 0.201 | <0.001 |
| Body mass index | 0.602 | 0.140 | <0.0001 | 0.706 | 0.143 | <0.0001 |
| Diabetes | 0.00 | n/a | 0.500 | 0.483 | 0.588 | 0.211 |
| Glucose | 0.00 | n/a | 0.500 | 0.150 | 0.186 | 0.191 |
| Triglycerides | 0.272 | 0.160 | 0.034 | 0.275 | 0.175 | 0.047 |
Adjusted estimates are adjusted for age, sex, race, ethnicity, and body mass index.
h2 = narrow sense heritability. SE = standard error.
DISCUSSION
We performed a familial aggregation study of fatty liver in overweight children with and without NAFLD. In family members, the presence or absence of fatty liver was documented using MRI. Clinical history and laboratory testing were applied to exclude participants with alternate explanations for fatty liver other than NAFLD. The main finding was that liver fat fraction and the condition of fatty liver are heritable traits. The estimate for the heritability of the fatty liver as a dichotomous trait reached the boundary value of 1.0, thus we were not able to determine the exact value for the heritability of fatty liver, but can confidently conclude that the heritability is high.
Earlier evidence for the familial nature of NAFLD came from retrospective case series. Struben et. al. reviewed a clinical database of 124 patients with NASH and 174 with cryptogenic cirrhosis and identified 32 patients who reported a family history of fatty liver and/or cryptogenic cirrhosis. They reviewed the medical records of a total of 10 family members from 8 of the 32 patients with a positive family history and noted the co-existence of NASH and/or cryptogenic cirrhosis in seven of eight kindreds studied.6 Willner and colleagues reviewed the charts of 90 patients with a diagnosis of NASH at the University of Tennessee and the Medical University of South Carolina.7 The investigators noted that 16 of the 90 patients came from families with 2 or more patients with NASH. Notably cirrhosis was observed in 7 of these 9 families. A case series from Japan described 3 families each with 2 people with biopsy-proven NASH.26 In addition to familial aggregation of NAFLD, the phenotypic features associated with NAFLD may also be more prevalent in families with NAFLD. A study of 20 adults with NAFLD and 20 controls without NAFLD, demonstrated that insulin resistance was more prevalent in first degree relatives of patients with NAFLD than those without NAFLD.27
The frequency of fatty liver seen in the siblings and parents of overweight children without NAFLD was consistent with population-based estimates. The prevalence of fatty liver in children in the County of San Diego was estimated to be 9.6% overall and 17.3% for adolescents age 15 to 19. 1 For adults age 30 to 65, the prevalence of hepatic steatosis was estimated to be 34% in Dallas County.8 In sharp contrast, family members of children with NAFLD appear to be at high risk as the majority of both siblings and parents of children with NAFLD were shown to have hepatic steatosis. Targeted screening may be warranted. Moreover, a demonstration of the familial nature of the disease may serve to enhance efforts at modifying the family environment towards increased physical activity and healthier dietary choices. Of additional concern was the detection of 2 cases of unrecognized cirrhosis out of the 33 families with a child with NAFLD studied. Primary care physicians, endocrinologists, gastroenterologists, hepatologists, and pediatricians should consider the possibility of advanced liver disease in the absence of any symptoms.
A novel finding in the current study was the interaction between BMI and familial factors on liver fat fraction. In families of children with NAFLD (i.e., the families at higher risk for fatty liver), the severity of steatosis as determined by the MRI-measured fat fraction was strongly correlated with BMI. In contrast, in families of children without NAFLD (i.e., the families at lower risk for fatty liver), the correlation between BMI and liver fat fraction was weaker. This difference helps to explain why the adjustment for BMI attenuated the heritability estimate for liver fat fraction as a continuous trait but, by reducing noise, increased the heritability estimate for fatty liver as a dichotomous trait. Moreover, these observations suggest that BMI is a strong determinant of steatosis severity in those with a heritable susceptibility to fatty liver but not in those without a heritable susceptibility and helps to strengthen the argument that familial factors contribute to the development of hepatic steatosis.
Establishing the heritability of a trait is considered a first step towards the identification of specific genes that influence that trait. Although the strength of the heritability of a trait does not always translate into the ease with which genes can be identified, it does provide support that genes contribute to the phenotypic expression of that trait. It is likely that fatty liver that begins early in life has a stronger genetic component than fatty liver that does not develop until adulthood. Once heritability has been established, a logical next step is to pursue the identification of specific genes that influence the trait via linkage and association analysis.13
The accumulation of fat, largely composed of triglyceride, in the hepatocyte is the essential first step in the development of NAFLD. The pathogenesis of steatosis is likely multi-factorial, involving both complex genetic and environmental factors that regulate lipid metabolism and the flux of fatty acids to, within and from the hepatocyte.28, 29 Putative candidate genes include those involved in processes that regulate hepatic lipid metabolism and the flux of fatty acids to the liver from the adipocyte. Free fatty acid in the liver is metabolized by one of two pathways: oxidation to generate ATP or esterification to produce triacylglycerides. Triglycerides are incorporated into VLDL particles for export or stored within hepatocytes. Therefore, hepatic steatosis can be caused by any combination of factors that increase the hepatocyte free fatty acid pool or that decrease mitochondrial β-oxidation, peroxisomal γ-oxidation, or VLDL synthesis/secretion. Association studies of single nucleotide polymorphisms as a risk factor for NAFLD offer promising findings for genes related to: (1) the magnitude and pattern of fat deposition, (2) hepatic lipid export, and (3) inflammation and oxidant stress. 30–36 Similarly, studies of gene expression in hepatic steatosis have shown upregulation of genes involved in lipid metabolism.37–39 The studies to date are not definitive because of small sample sizes, the complex issue of appropriate control groups and the lack of replication samples. These data along with the current study support the planning and execution of large scale studies examining genetic factors contributing to NAFLD.
A strength of this study was the use of magnetic resonance imaging, as this allowed for objective and precise determination of hepatic fat fraction. Studies based upon less robust measures for case definition, such as liver chemistry or ultrasonography, are subject to greater error. The use of a diverse study population was notable, especially the inclusion of a large number of Hispanic families because they are at greater risk for NAFLD than non-Hispanics. However, this emphasis may limit the generalizability of these findings to non-Hispanic families. Another limitation was the lack of liver biopsies in the family members studied. A design including biopsy would be required to determine the hereditability of a sub-phenotype of NAFLD such as steatohepatitis or advanced fibrosis, but is not feasible for a family study which requires the use of a method that can be applied to all family members. Heritability estimates are influenced by shared environmental factors. In order to further separate environmental from genetic causes, future studies will need to collect information on factors including nutrition and activity.
The public health burden of NAFLD is considerable and likely to increase over time. Understanding the genetic and environmental factors that contribute to NAFLD prevalence and severity has important implications for clinical care and public health. The current data demonstrate for the first time that NAFLD is a highly heritable condition. Family members of children with NAFLD should be considered at high risk for NAFLD, even in the absence of obesity or elevated serum aminotransferases. The current data suggest that familial factors are a major determinant of whether an individual has NAFLD. Further family studies will help unravel the complex interactions between genes and environment in the development of NAFLD.
Acknowledgments
Financial Support: This work was funded in part by grants from the National Institutes of Health including R21 DK71486 from the National Institute of Diabetes and Digestive and Kidney Diseases, P60 MD00220 for the San Diego EXPORT Center from the National Center of Minority Health and Health Disparities, and M01 RR000827 from the National Center for Research Resources for the General Clinical Research Center at UCSD. RS and NJS are supported in part by Scripps Genomic Medicine and The Scripps Translational Sciences Institute. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- BMI
body mass index
- MRI
magnetic resonance imaging
- FF
fat fraction
Footnotes
Conflict of Interests: no conflicts of interest exist
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of Fatty Liver in Children and Adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Beymer C, Kowdley KV, Larson A, Edmonson P, Dellinger EP, Flum DR. Prevalence and Predictors of Asymptomatic Liver Disease in Patients Undergoing Gastric Bypass Surgery. Arch Surg. 2003;138:1240–1244. doi: 10.1001/archsurg.138.11.1240. [DOI] [PubMed] [Google Scholar]
- 3.Xanthakos S, Miles L, Bucuvalas J, Daniels S, Garcia V, Inge T. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clinical Gastro Hep. 2006;4:226–2332. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 4.Kim HJ, Lee KE, Kim DJ, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. 2004;164:2169–2175. doi: 10.1001/archinte.164.19.2169. [DOI] [PubMed] [Google Scholar]
- 5.Nobili V, Marcellini M, Devito R, et al. NAFLD in children: a prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–465. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 6.Struben VM, Hespenheide EE, Caldwell SH. Nonalcoholic steatohepatitis and cryptogenic cirrhosis within kindreds. Am J Med. 2000;108:9–13. doi: 10.1016/s0002-9343(99)00315-0. [DOI] [PubMed] [Google Scholar]
- 7.Willner IR, Waters B, Patil SR, Reuben A, Morelli J, Riely CA. Ninety patients with nonalcoholic steatohepatitis: insulin resistance, familial tendency, and severity of disease. Am J Gastroenterol. 2001;96:2957–2961. doi: 10.1111/j.1572-0241.2001.04667.x. [DOI] [PubMed] [Google Scholar]
- 8.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 9.Browning JD, Kumar KS, Saboorian MH, Thiele DL. Ethnic differences in the prevalence of cryptogenic cirrhosis. Am J Gastroenterol. 2004;99:292–298. doi: 10.1111/j.1572-0241.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 10.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 11.Schwimmer JB, McGreal N, Deutsch R, Finegold M, Lavine JE. The Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 12.Louthan MV, Theriot JA, Zimmerman E, Stutts JT, McClain CJ. Decreased prevalence of nonalcoholic fatty liver disease in black obese children. J Pediatr Gastroenterol Nutr. 2005;41:426–429. doi: 10.1097/01.mpg.0000177314.65824.4d. [DOI] [PubMed] [Google Scholar]
- 13.Khoury MJ, Beaty TH, Cohen BH. Fundamentals of genetic epidemiology. New York: Oxford University Press; 1993. [Google Scholar]
- 14.Vitzthum VJ. A number no greater than the sum of its parts: the use and abuse of heritability. Hum Biol. 2003;75:539–558. doi: 10.1353/hub.2003.0064. [DOI] [PubMed] [Google Scholar]
- 15.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–648. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 17.Committee on Prevention of Obesity in Children and Youth. Preventing Childhood Obesity Health in the Balance. Washington, D.C: The National Academies Press; 2005. [PubMed] [Google Scholar]
- 18.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 19.Hussain HK, Chenevert TL, Londy FJ, et al. Hepatic fat fraction: MR imaging for quantitative measurement and display--early experience. Radiology. 2005;237:1048–1055. doi: 10.1148/radiol.2373041639. [DOI] [PubMed] [Google Scholar]
- 20.Liu CY, McKenzie CA, Yu H, Brittain JH, Reeder SB. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58:354–364. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 21.Bydder M, Yokoo T, Hamilton G, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26:347–359. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, McKenzie CA, Shimakawa A, et al. Multiecho reconstruction for simultaneous water-fat decomposition and T2* estimation. J Magn Reson Imaging. 2007;26:1153–1161. doi: 10.1002/jmri.21090. [DOI] [PubMed] [Google Scholar]
- 23.Allison DB, Neale MC, Zannolli R, Schork NJ, Amos CI, Blangero J. Testing the robustness of the likelihood-ratio test in a variance-component quantitative-trait loci-mapping procedure. Am J Hum Genet. 1999;65:531–544. doi: 10.1086/302487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange K, Little RJA, Taylor JMG. Robust statistical inference using the t distribution. Journal of the American Statistical Association. 1989;84:881–896. [Google Scholar]
- 25.Boehnke M, Lange K. Ascertainment and goodness of fit of variance component models for pedigree data. Prog Clin Biol Res. 1984;147:173–192. [PubMed] [Google Scholar]
- 26.Tokushige K, Yatsuji S, Hashimoto E, et al. Familial aggregation in patients with non-alcoholic steatohepatitis. Intern Med. 2008;47:405–410. doi: 10.2169/internalmedicine.47.0476. [DOI] [PubMed] [Google Scholar]
- 27.Abdelmalek MF, Liu C, Shuster J, Nelson DR, Asal NR. Familial aggregation of insulin resistance in first-degree relatives of patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:1162–1169. doi: 10.1016/j.cgh.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Chitturi S, Farrell GC. Etiopathogenesis of nonalcoholic steatohepatitis. Semin Liver Dis. 2001;21:27–41. doi: 10.1055/s-2001-12927. [DOI] [PubMed] [Google Scholar]
- 29.Day CP. The potential role of genes in nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:673–691. doi: 10.1016/j.cld.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Shima Y, Tsukada T, Nakanishi K, Ohta H. Association of Trp64Arg mutation of the B3-adrenergic receptor with fatty liver and mild glucose intolerance in Japanese subjects. Clinica Chimica Acta. 1998;274:167–176. doi: 10.1016/s0009-8981(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 31.Iwamoto N, Ogawa Y, Kajihara S, et al. Gln27Glu beta2-adrenergic receptor variant is associated with hypertriglyceridemia and the development of fatty liver. Clin Chim Acta. 2001;314:85–91. doi: 10.1016/s0009-8981(01)00633-7. [DOI] [PubMed] [Google Scholar]
- 32.Valenti L, Fracanzani AL, Dongiovanni P, et al. Tumor necrosis factor alpha promoter polymorphisms and insulin resistance in nonalcoholic fatty liver disease. Gastroenterology. 2002;122:274–280. doi: 10.1053/gast.2002.31065. [DOI] [PubMed] [Google Scholar]
- 33.Namikawa C, Shu-Ping Z, Vyselaar JR, et al. Polymorphisms of microsomal triglyceride transfer protein gene and manganese superoxide dismutase gene in non-alcoholic steatohepatitis. J Hepatol. 2004;40:781–786. doi: 10.1016/j.jhep.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Nozaki Y, Saibara T, Nemoto Y, et al. Polymorphisms of Interleukin-1B and B3-Adrenergic Receptor in Japanese Patients with Nonalcoholic Steatohepatitis. Alcohol Clin Exp Res. 2004;28:106S–110S. doi: 10.1111/j.1530-0277.2004.tb03226.x. [DOI] [PubMed] [Google Scholar]
- 35.Song J, da Costa KA, Fischer LM, et al. Polymorphism of the PEMT gene and susceptibility to nonalcoholic fatty liver disease (NAFLD) FASEB. 2005;19:1266–1271. doi: 10.1096/fj.04-3580com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brun P, Castagliuolo I, Floreani AR, et al. Increased risk of NASH in patients with the C(-159)T polymorphism in the CD14 gene promoter region. Gut. 2006;55:1212. doi: 10.1136/gut.2006.093336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westerbacka J, Kolak M, Kiviluoto T, et al. Genes involved in fatty acid partitioning and binding, lipolysis, monocyte/macrophage recruitment, and inflammation are overexpressed in the human fatty liver of insulin-resistant subjects. Diabetes. 2007;56:2759–2765. doi: 10.2337/db07-0156. [DOI] [PubMed] [Google Scholar]
- 38.Greco D, Kotronen A, Westerbacka J, et al. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–1287. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- 39.Higuchi N, Kato M, Shundo Y, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]