Abstract
Aims/hypothesis
The pathogenic role of excessive vascular endothelial growth factor (VEGF)-A in diabetic nephropathy has not been defined. We sought to test whether increased podocyte VEGF-A signalling determines the severity of diabetic glomerulopathy.
Methods
Podocyte-specific, doxycycline-inducible Vegf164 (the most abundant Vegfa isoform) overexpressing adult transgenic mice were made diabetic with low doses of streptozotocin and examined 12 weeks after onset of diabetes. We studied diabetic and non-diabetic transgenic mice fed a standard or doxycycline-containing diet. VEGF-A and albuminuria were measured by ELISA, creatinine was measured by HPLC, renal morphology was examined by light and electron microscopy, and gene expression was assessed by quantitative PCR, immunoblotting and immunohistochemistry.
Results
Podocyte Vegf164 overexpression in our mouse model of diabetes resulted in advanced diabetic glomerulopathy, characterised by Kimmelstiel–Wilson-like nodular glomerulosclerosis, microaneurysms, mesangiolysis, glomerular basement membrane thickening, podocyte effacement and massive proteinuria associated with hyperfiltration. It also led to increased VEGF receptor 2 and semaphorin3a levels, as well as nephrin and matrix metalloproteinase-2 downregulation, whereas circulating VEGF-A levels were similar to those in control diabetic mice.
Conclusions/interpretation
Collectively, these data demonstrate that increased podocyte Vegf164 signalling dramatically worsens diabetic nephropathy in a streptozotocin-induced mouse model of diabetes, resulting in nodular glomerulosclerosis and massive proteinuria. This suggests that local rather than systemic VEGF-A levels determine the severity of diabetic nephropathy and that semaphorin3a signalling and matrix metalloproteinase-2 dysregulation are mechanistically involved in severe diabetic glomerulopathy.
Keywords: MMP, Podocytes, Proteinuria, Semaphorin3a, Transgenic mice, Type 1 diabetes mellitus, VEGF-A
Introduction
Diabetic nephropathy is the major cause of end-stage renal disease in the industrialised world. Vascular endothelial growth factor (VEGF)-A and its receptors have been implicated in the pathogenesis of diabetic nephropathy in animals and humans [1–3]. Increased VEGF-A levels have been reported in animal models of diabetes, and VEGF antagonists have renoprotective effects in early diabetic nephropathy [4–6]. Uncoupling of VEGF–nitric oxide pathways has been postulated to be a driving force in the progression of diabetic nephropathy [7–9]. However, the pathogenic mechanism of excessive VEGF-A in diabetic nephropathy has not been established.
We have previously shown in vitro that podocytes possess a functional autocrine VEGF-A system, whereby secreted VEGF-A promotes podocyte survival via VEGF receptor (VEGFR)2 and the interaction between slit-diaphragm proteins [10]. Recently, our group and others reported podocyte production of VEGFR2 in vivo [10, 11]. We also demonstrated an interaction between VEGFR2 and nephrin, as well as VEGF-induced phosphorylation of both proteins in vivo [12]. Moreover, overexpression of podocyte Vegf164 (the most abundant Vegfa isoform) in adult mice induced a reversible early diabetic nephropathy-like phenotype characterised by proteinuria, glomerulomegaly, mesangial expansion, glomerular basal membrane thickening and podocyte effacement, in the absence of diabetic milieu [12]. Collectively, these data suggest that VEGF-A has crucial autocrine effects on podocyte phenotype and behaviour, in addition to its well-established paracrine functions.
Semaphorin3a, a guidance protein that plays an important role in neural, cardiovascular and renal patterning [13, 14], and its receptors neuropilin (NRP) 1 and 2 are produced in podocytes in vitro and in vivo [15, 16]. We recently demonstrated that tight regulation of the Sema3a gene is required for normal podocyte differentiation and glomerular filtration barrier (GFB) development [14]. Semaphorin3a competes with VEGF-A for NRP1 binding and has opposing effects to those of VEGF in several cell types, including endothelial cells and podocytes [10, 15, 17]. Nrp1 mRNA is decreased in glomeruli from diabetic humans and mouse models of diabetes, and is suppressed by advanced glycation end-products in podocytes [18]. The role of semaphorin3a in diabetic nephropathy is unknown.
To test whether induction of podocyte Vegf164 over-expression in diabetic mice causes severe diabetic nephropathy, we examined in the present study the effect of excessive podocyte VEGF-A signalling on diabetic nephropathy and its relationship with semaphorin3a. We determined that podocyte Vegf164 overexpression caused nodular glomerulosclerosis and massive proteinuria in the setting of streptozotocin-induced diabetes, dramatically worsening diabetic nephropathy. Our data suggest that local rather than systemic VEGF-A levels determine the severity of diabetic nephropathy and that increased semaphorin3a signalling and matrix metalloproteinase (MMP)-2 dysregulation are mechanistically involved in severe diabetic glomerulopathy.
Methods
Animal experiments
Mouse protocols were approved by AECOM and Yale Committees for Animal Use and Experimentation. We generated tetracycline-regulated, podocyte-specific inducible transgenic mice (TOPO; podo-cin-rtTA:tet-O-Vegf164), which overexpress Vegf164 in podocytes upon induction with doxycycline on a FVB/N background, as previously described [12]. Male TOPO 5.0±0.6 week old mice (n=15) were made diabetic using the Animal Models of Diabetic Complications Consortium protocol (see www.AMDCC.org). Briefly, mice were fasted for 6 h before receiving streptozotocin (50 mg/kg body weight; Sigma, St Louis, MO, USA); five doses were given i.p. on five consecutive days and 10% sucrose drinking water (wt/vol.) was supplied to prevent hypoglycaemia post-injection. At 1 week after the last streptozotocin injection, all mice were diabetic (random blood glucose >15.5 mmol/l). Blood glucose, urine samples and body weight were obtained every 3 to 4 weeks for 12 weeks (electronic supplementary material [ESM] Fig. 1), when a 24 h urine sample was collected in metabolism cages; mice were killed after removal of kidneys and collection of blood samples. Survival until completion was 100%.
Diabetic TOPO mice were induced with doxycycline (0.625 mg/g chow; Harlan-Teklad, Madison, WI, USA) (diabetes+doxycycline, n=8) following completion of streptozotocin injections. Uninduced diabetic TOPO mice (diabetes−doxycycline, n=7) fed on a standard diet served as diabetic controls. Additional diabetic controls were single transgenic (podocin-rtTA) mice (n=4) induced with doxycycline (single transgenic+doxycycline). Non-diabetic controls were TOPO mice induced with doxycycline (no diabetes+doxycycline, n=10) and TOPO uninduced mice fed on a standard diet (no diabetes−doxycycline, n=10).
Urinary and plasma creatinine were measured by HPLC/mass spectrometry/mass spectrometry [19]; albuminuria was assessed by ELISA kit (Albuwell-M-Elisa; Exocell, Philadelphia, PA, USA) in 24 h urine and expressed as albumin:creatinine ratio (μg/μmol). Random blood glucose (mmol/l) was measured by glucose oxidase biosensor blood glucose meter (One-Touch-Ultra-2; LifeScan, Milpitas, CA, USA). Serum and urine VEGF-A were measured by ELISA (mVEGF-A; R&D, Minneapolis, MN, USA), following the manufacturer’s protocol. Arterial pressure was measured under anaesthesia (1.5–2% isoflurane/O2), through a carotid PE-50 catheter connected to a pressure transducer, and analysed using PowerLab/8SP system (Chart; AD Instruments, Colorado Springs, CO, USA).
Histology and transmission electron microscopy
Kidneys were frozen in isopentane and mounted in optimal cutting temperature medium (OCT) (Sakura Finetek USA, Torrance, CA, USA) or processed for light and transmission electron microscopy (TEM). Histology was evaluated by periodic acid–Schiff’s reagent (PAS) stain. TEM was performed as reported using standard techniques [12]. For morphometric analysis, glomerular diameters were measured in 116±6 glomeruli per section, as previously described [14]. Slit-diaphragm and occluding junctions were counted and foot process width measured as previously reported [12]. Glomerulosclerosis, mesangial expansion, microaneurysms and mesangiolysis were assessed in blinded fashion; mesangial expansion was measured using the following semi-quantitative score [20]: 0=none; 1=1–25%; 2=26–50%; 3 =51–75%; 4=76–100%. Glomerular nodules were quantified as percentage glomeruli/section containing nodules.
Laser-capture microdissection and quantitative PCR
Glomeruli from three mice per experimental group were isolated by laser capture microdissection (AS-LMD System; Leica, Bannockburn, IL, USA) from OCT frozen kidney sections and total RNA was purified as previously described [21]. Glomerular mRNA was assessed by quantitative PCR (Ssofast Eva-Green and CFX96-Real-time system; Bio-Rad, Hercules, CA, USA) and previously reported primers for VEGF164 and hypoxanthineguanine phosphoribosyltransferase (HPRT) [21]. Gene expression level was determined by the 2−ΔΔCt method [22].
Immunohistochemistry
Kidney sections were deparaffinised, blocked and incubated with anti-nephrin (20R-NP002; Fitzgerald, Concord, MA, USA) and anti-Wilms’ tumour antigen 1 (M3561; Dako, Carpinteria, CA, USA). Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) was used to visualise nuclei. Deparaffinised sections were also incubated with anti-VEGF antibody (M7273; Dako). Signals were visualised by immunoperoxidase and haematoxylin counterstain. Cryosections were fixed in acetone and incubated with the following primary antibodies: anti-MMP9 (ab38898; Abcam, Cambridge, MA, USA), anti-collagen IV (Southern Biotech, Birmingham, AL, USA), anti-MMP2 (MAB13134; Chem-icon, Rosemont, IL, USA), anti-laminin (L9393; Sigma), anti-semaphorin 3A (AF1250; R&D) and anti-podocin (P0372; Sigma). Appropriate Cy2- or Cy3-labelled secondary antibodies were used (Jackson Immunoresearch, West Grove, PA, USA). Sections were examined by light microscopy (Eclipse50b; Nikon, Melville, NY, USA) or by confocal microscopy (FV300; Olympus, Center Valley, PA, USA). To avoid bleed-through between signals, each laser was used individually and separate images were merged later.
Western blot analysis
Kidneys were lysed in radioimmunoprecipitation assay buffer plus protease inhibitors (Roche, Indianapolis, IN, USA) with 1 mmol/l NaO3 and 1 mmol/ l NaF. Equal amounts of protein lysate from individual kidneys were combined into pools (diabetes+doxycycline, diabetes−doxycycline, no diabetes+doxycycline, no diabetes−doxycycline), and 200 μg protein per lane was resolved and immunoblotted. Primary antibodies were: anti-nephrin (Fitzgerald), anti-MMP-9 (AB19016; Chemicon), anti-MMP-2 (Chemicon), anti-tissue inhibitor of matrix metalloproteinase (TIMP)-2 (sc21735; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-VEGFR2 (55B11; Cell Signaling, Danvers, MA, USA), anti-semaphorin 3A (sc-28867; Santa Cruz) and anti-NRP1 (gift from A. Kolodkin, Department of Neuroscience, The John Hopkins University School of Medicine, Baltimore, MD, USA) [13]. Anti-actin (A2066; Sigma) or anti-tubulin (sc-58667; Santa Cruz) were used as controls for protein loading. Appropriate horseradish peroxidase-IgG secondary antibodies were used (Amersham, Fitzgerald, Acton, MA, USA). Signals were visualised by chemoluminescence (ECL; Amersham) and quantified by densitometry using ImageJ software (NIH; http://rsb.info.nih.gov/ij/).
Zymogram
Kidney lysate pools (100 μg) were denatured and resolved in zymogram gel, and then stained with colloidal blue, following the manufacturer’s instructions (Invitrogen). Recombinant mouse MMP-9 (909-MM, 2.5 ng; R&D) was used as positive control. Gels from four independent experiments were analysed.
Quantitative PCR
Whole kidney total RNA was isolated, reverse transcribed and individual reverse transcription products combined into pools, as described above for proteins [12]. Quantitative PCR amplifications were performed using a mix (SYBR-Green-PCR-Master-Mix; Applied Biosystems, Foster City, CA, USA) and mastercycler (Realplex2; Eppendorf, Hamburg, Germany), and appropriate primers (ESM Table 1), as described [12], run in triplicate. Experiments were repeated three or more times. Relative quantification was determined using the 2−ΔΔCt method [22]. Data were normalised to GAPDH and expressed as fold change relative to diabetic controls (diabetes+doxycycline/diabetes−doxycycline).
Statistical analysis
All values are expressed as mean ± SEM. To determine statistical significance, we used unpaired Student’s t test and two-way ANOVA; Bonferroni correction was used for multiple comparisons. Kruskal–Wallis test was used for data with non-Gaussian distribution (laser-capture quantitative PCR). We used χ2 test for a categorical variable (glomerular nodules). A p value of <0.05 was deemed statistically significant.
Results
Podocyte Vegf164 overexpression exacerbates glomerular Vegf164 expression in diabetic mice
Podocyte Vegf164 over-expression in diabetic mice caused an increase in glomerular Vegfa mRNA and protein levels, compared with control diabetic and non-diabetic mice (Fig. 1a–c) when determined by quantitative PCR in laser-microdissected glomeruli and by immunohistochemistry. Plasma VEGF-A was twofold higher in diabetic than in non-diabetic mice (Fig. 1d), indicating that the diabetic milieu induces elevated systemic VEGF-A, as described in mice and humans [23, 24]. Podocyte Vegf164 overexpression did not alter plasma VEGF-A in diabetic mice (Fig. 1d), as previously reported in non-diabetic mice [12]. Urine VEGF-A excretion (Fig. 1e) was 18-fold higher in diabetic mice (231±33 pg/day) than in non-diabetic control mice (13±1.2 pg/day), suggesting that the source of urinary VEGF-A in diabetic mice is systemic and local. In non-diabetic mice, podocyte Vegf164 overexpression increased urinary VEGF-A excretion 18-fold (224±73 pg/day), similarly to diabetic mice (Fig. 1e), suggesting that in non-diabetic mice, urinary VEGF-A reflects its production in podocytes.
Fig. 1.
Podocyte Vegf164 overexpression exacerbates glomerular Vegf164 expression in diabetic mice. a Laser microdissection of glomeruli from diabetic and non-diabetic kidney sections, before and after laser capture (magnification ×400). b In glomeruli isolated by laser capture, Vegf mRNA measured by quantitative PCR was significantly higher in diabetic +doxycycline mice overexpressing Vegf164 (black bar) than in diabetic − doxycycline (dark grey bar) and non-diabetic−doxycycline controls (white bar). Induced non-diabetic+doxycycline mice (light grey bar) also overexpressed glomerular Vegf164 mRNA as compared with controls (−doxycycline, white bar). c Immunohistochemistry shows increased VEGF-A in diabetic glomeruli (left) compared with non-diabetic (right). Podocyte Vegf164 overexpression (+doxycycline [+dox]) resulted in a further increase of glomerular VEGF-A expression in diabetic mice. Scale bars 30 μm. −dox, −doxycycline d Plasma VEGF-A was about twofold higher in diabetic mice (black bar, dark grey bar) than in non-diabetic mice (light grey bar, white bar). e Urine VEGF-A increased in diabetic+doxycycline (black bar) and diabetic–doxycycline mice (dark grey bar), as well in non-diabetic+doxycycline mice overexpressing Vegf164 (light grey bar). *p<0.05 vs non-diabetic−doxycycline control; †p <0.05 vs diabetic−doxycycline control
Podocyte Vegf164 overexpression in diabetic mice induces advanced diabetic glomerulopathy
By 12 weeks after the onset of hyperglycaemia, diabetic mice overexpressing podocyte Vegf164 developed a severe glomerular phenotype characterised by nodular glomerulosclerosis, mesangiolysis and microaneurysms (Fig. 2a–f). In addition, arteriolar and vascular pole hyalinosis (Fig. 2c, d), protein casts (Fig. 2b, c) and glomerular adhesions to Bowman’s capsule (Fig. 2a, d, e) were observed. Interstitial lesions were minimal, with mild focal lymphocytic infiltrate (ESM Fig. 2). In contrast, uninduced diabetic controls showed diffuse mesangial expansion (Fig. 2g). Semiquantitative pathological score revealed significantly increased mesangial sclerosis in diabetic mice overexpressing podocyte Vegf164 (2.2±0.2, n=6), compared with uninduced diabetic (1±0, n=5) and non-diabetic mice overexpressing podocyte Vegf164 (1±0, n=3; p<0.05). Notably, glomerular nodules, endothelial injury, mesangiolysis and lymphocytic infiltrates were detected only in diabetic mice overexpressing podocyte Vegf164 (Fig. 2a–f, ESM Fig. 2). PAS-positive nodules were observed in 6% to 36% of glomeruli from diabetic mice overexpressing podocyte Vegf164, compared with 0% in uninduced diabetic and non-diabetic control mice (p<0.0001). Glomerulomegaly was detected in all diabetic mice, but was significantly enhanced by podocyte Vegf164 overexpression (Fig. 2j). Similarly, non-diabetic controls overexpressing podocyte Vegf164 developed milder glomerulomegaly (Fig. 2h, j).
Fig. 2.
Podocyte Vegf164 overexpression in diabetic mice induces advanced diabetic glomerulopathy. In diabetic+doxycycline mice overexpressing podocyte Vegf164 PAS staining shows (a, d–f) nodular glomerulosclerosis (blue arrows), (c, e, f) mesangiolysis (black asterisks) and (b, d–f) microaneurysms (black arrows); (c, d) arteriolar and vascular pole hyalinosis (white asterisks), (b, c) protein casts (green asterisks) and (a, c–e) glomerular adhesions to Bowman’s capsule are also shown. g Diabetic−doxycycline control and (h) non-diabetic+doxycycline mice overexpressing Vegf164 showed moderate mesangial expansion. i Non-diabetic−doxycycline control mice showed normal glomerular structure. Scale bars (a–i), 40 μm. j Glomerular volumes show that glomerulomegaly was present in all diabetic mice, but was significantly increased by podocyte Vegf164 overexpression (+doxycycline, black bar). Non-diabetic+doxycycline mice overexpressing podocyte Vegf164, (light grey bar) had milder glomerulomegaly. White bar, non-diabetic control (−doxycycline); dark grey bar, diabetic control (−doxycycline). *p<0.05 vs non-diabetic −doxycycline control; †p<0.05 vs diabetic−doxycycline control
TEM revealed significant mesangial proliferation, sclerosis, extracellular matrix expansion, focal endothelial cell swelling and glomerular basement membrane (GBM) thickening in diabetic mice overexpressing Vegf164 (Fig. 3a), and milder mesangial expansion and GBM thickening in diabetic control kidneys (Fig. 3b) and in non-diabetic mice overexpressing podocyte Vegf164 (Fig. 3c). Normal ultrastructure in non-diabetic control kidneys is shown in Fig. 3d. TEM morphometry showed significantly increased GBM thickening and density of occluding junctions in diabetic mice overexpressing podocyte Vegf164, compared with uninduced diabetic controls (Fig. 3e, f). Slit-diaphragm density and foot process width were similar in all diabetic mice, but different from non-diabetic controls (Fig. 3g, h). Interestingly, non-diabetic mice overexpressing podocyte Vegf164 had similar GBM thickness, slit-diaphragm density and foot process width to diabetic mice, all of which were significantly different from uninduced controls (Fig. 3e–h).
Fig. 3.
Ultrastructural features of VEGF-induced advanced diabetic glomerulopathy. a TEM shows severe mesangial proliferation, sclerosis, extracellular matrix expansion, focal endothelial cell swelling, GBM thickening, foot process effacement and fusion in diabetic+doxycycline mice overexpressing Vegf164. M, mesangium; CL, capillary lumen; P, podocyte; asterisk, GBM. b Milder mesangial proliferation, GBM thickening and foot process effacement in diabetic−doxycycline control mice. c In non-diabetic+doxycycline mice overexpressing podocyte Vegf164 GBM was thick and podocytes were effaced. d Non-diabetic−doxycycline control mice had normal ultrastructure. Scale bars (a, b) 2 μm, (c, d) 1 μm. Insets (a–d) show foot processes and GBM at higher magnification (scale bars 200 nm). e TEM morphometry shows significantly increased GBM thickness and (f) increased density of occluding junctions in diabetic+doxycycline mice overexpressing podocyte Vegf164 (black bars), compared with uninduced diabetic−doxycycline controls (dark grey bars). g Slit-diaphragm density and (h) foot process width were similar in all diabetic mice, but different from non-diabetic−doxycycline controls (white bars). e Non-diabetic +doxycycline mice overexpressing podocyte Vegf164 (light grey bars) had similar GBM thickness, (g) slit-diaphragm density and (h) foot process width to diabetic mice. *p<0.05 vs non-diabetic −doxycycline control; †p<0.05 vs diabetic−doxycycline control
Podocyte Vegf164 overexpression in diabetic mice induces hyperfiltration and massive proteinuria
By 12 weeks after the onset of hyperglycaemia, diabetic mice overexpressing podocyte Vegf164 had significantly higher creatinine clearance than control diabetic mice (Fig. 4a), but 40% lower than non-diabetic mice overexpressing podocyte Vegf164, suggesting that advanced diabetic glomerulopathy blunted VEGF-induced hyperfiltration. Plasma creatinine was lower in diabetic mice overexpressing podocyte Vegf164 (Fig. 4b), suggesting that increased creatinine clearance reflected hyperfiltration, rather than increased creatinine secretion [25]. In addition, diabetic mice overexpressing podocyte Vegf164 developed massive proteinuria, as reflected by a100-fold increase in the albumin:creatinine ratio as compared with non-diabetic control mice (220±80 vs 1.9± 0.14 μg/μmol) (Fig. 4c); this finding reflects the presence of established diabetic renal disease [26]. Urinary albumin: creatinine ratio was elevated to a lesser degree in diabetic control mice (24±2 μg/μmol) and in non-diabetic mice overexpressing podocyte Vegf164 (6.4±2.3 μg/μmol) as compared with non-diabetic control mice (Fig. 4c). All diabetic mice showed similar levels of hyperglycaemia (ESM Fig. 1a). However, diabetic mice overexpressing podocyte Vegf164 showed significantly increased polyuria, with urinary volume approximately twofold greater than that of diabetic controls and polyuria associated with significantly lower body weight (Table 1, ESM Fig. 1b). Non-diabetic mice overexpressing podocyte Vegf164 showed a milder increase in urinary volume than uninduced controls, as well as normal blood glucose (Table 1). Systolic blood pressure was normal in all diabetic mice: 86±2.6 mmHg in diabetic mice overexpressing podocyte Vegf164 (n=4) and 91±0.3 mmHg in diabetic control mice (n=4; p=NS).
Fig. 4.
Podocyte Vegf164 overexpression in diabetic mice induces hyperfiltration and massive proteinuria. a Creatinine clearance is higher in diabetic+doxycycline mice overexpressing Vegf164 (black bar) than in control diabetic−doxycycline mice (dark grey bar) and lower than in non-diabetic+doxycycline mice overexpressing podocyte Vegf164, (light grey bar). b Plasma creatinine is lower in diabetic+doxycycline mice overexpressing Vegf164 (black bar) than in control diabetic−doxycycline (dark grey bar) and all non-diabetic mice, i.e. +doxycycline (light grey bar) and −doxycycline (white bar). c Diabetic+doxycycline mice over-expressing podocyte Vegf164 (black bar) developed massive proteinuria, which was around 100-fold higher than in non-diabetic−doxycycline controls (white bar). Urinary albumin:creatinine ratio in non-diabetic +doxycycline mice overexpressing podocyte Vegf164 (light grey bar) was higher than in non-diabetic−doxycycline control mice (white bar), but lower than in diabetic mice. *p<0.05 vs non-diabetic−doxycycline control; †p<0.05 vs diabetic−doxycycline control
Table 1.
General variables in diabetic and non-diabetic mice
| Characteristic | Diabetes
|
No diabetes
|
||
|---|---|---|---|---|
| Doxycycline | No doxycycline | Doxycycline | No doxycycline | |
| n | 8 | 7 | 10 | 10 |
| Body weight (g) | 24.89±0.5† | 31.04±0.9 | 27.1±2.2 | 26.3±1.32 |
| Kidney weight (mg) | 249.4±10.1* | 250±12.9* | 205±12.7 | 193.5±10.3 |
| Blood glucose (mmol/l) | 32.1±1.08* | 32.1±0.7 * | 8.1±0.2 | 9.4±0.7 |
| Urine volume (ml/day) | 8.8±1.7†* | 4.6±0.8 * | 1.1±0.2* | 0.5±0.1 |
p<0.05 compared with no diabetes, no doxycycline;
p<0.05 compared with diabetes, no doxycycline
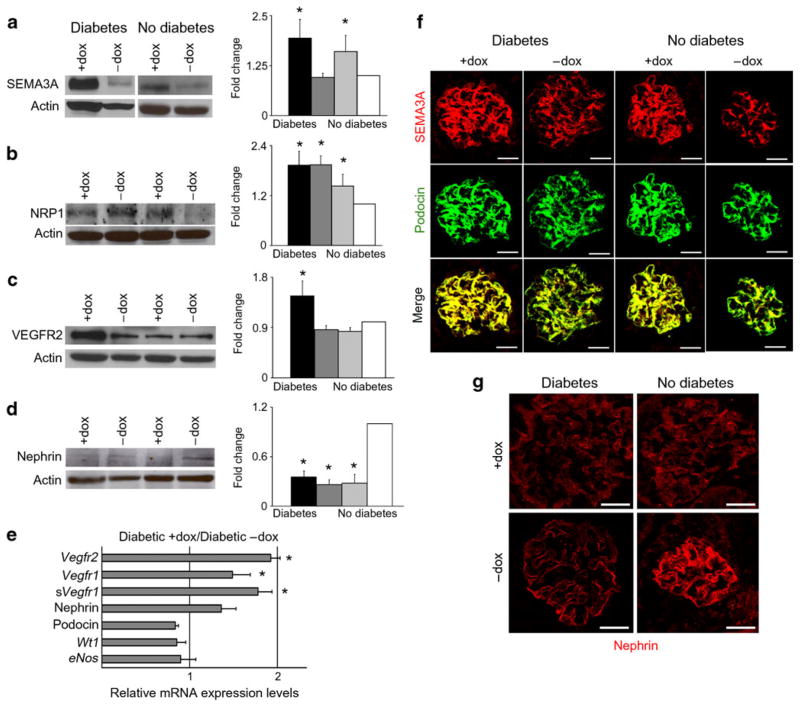
Podocyte Vegf164 overexpression in diabetic mice induces semaphorin3a and VEGFR2 production
Kidney semaphorin3a protein level was increased in diabetic and non-diabetic mice overexpressing podocyte Vegf164 (Fig. 5a) and localised to podocytes, as indicated by co-immunostaining with podocin (Fig. 5f). Expression of semaphorin3a receptor NRP1 was increased in both groups of diabetic mice, as well as in non-diabetic mice over-expressing podocyte Vegf164 (Fig. 5b). Together, these findings suggest that semaphorin3a signalling may be upregulated in kidneys from diabetic mice overexpressing podocyte Vegf164.
Fig. 5.
Podocyte Vegf164 overexpression in diabetic mice induces semaphorin3a and VEGFR2 production. a Western blot and bar graph showing increased kidney semaphorin3a (SEMA3A) levels in diabetic +doxycycline (+dox) (black bar) and non-diabetic+doxycycline (light grey bar) mice overexpressing podocyte Vegf164. b NRP1 levels were increased in both groups of diabetic mice (black bar, dark grey bar) and in non-diabetic+doxycycline mice overexpressing podocyte Vegf164 (light grey bar). c VEGFR2 levels were increased in diabetic +doxycycline mice overexpressing Vegf164 (black bar). d Nephrin levels were decreased in diabetic+doxycycline and diabetic−doxycycline, and in non-diabetic+doxycycline (light grey bar) mice overexpressing podocyte Vegf164 compared with non-diabetic−doxycycline control (white bar). a–d Actin blots shown to document equal loading. Densitometric analysis shows mean±SEM fold change in arbitrary units as compared with controls, n≥4. *p<0.05 compared with control (−doxycycline) e Real-time PCR showing that relative mRNA expression of Vegfr2, Vegfr1 and soluble (s) Vegfr1 increased in diabetic+doxycycline mice overexpressing podocyte Vegf164. *p<0.05 compared with diabetic−doxycycline control. Nephrin, podocin, Wt1 and eNos mRNA levels were not significantly different. f Dual-immunostaining showed that podocyte SEMA3A level was increased in diabetic and non-diabetic glomeruli overexpressing podocyte Vegf164 (+doxycycline), whereas podocin was unchanged. Merge images demonstrate co-localisation. g Nephrin immunostaining shows decreased expression in both diabetic mouse groups (+doxycycline, −doxycycline) and in non-diabetic+doxycycline mice overexpressing podocyte Vegf164. Scale bars, 20 μm
Next, we determined that kidney VEGFR2 production increased in diabetic mice overexpressing Vegf164, as measured by immunoblot and quantitative PCR (Fig. 5c, e), suggesting enhanced local VEGF-A signalling. Vegfr1 (also known as Flt1) and soluble Vegfr1 mRNA levels were also higher in diabetic mice overexpressing podocyte Vegf164 than in uninduced diabetic mice (Fig. 5e).
Nephrin levels decreased in all diabetic mice and in non-diabetic mice overexpressing podocyte Vegf164 compared with uninduced controls, as shown by western blotting and immunofluorescence (Fig. 5d, g), a finding consistent with previous reports in human and experimental models of diabetic nephropathy [27–29]. Podocin and Wt1 mRNA and protein levels were not altered in diabetic mice or in mice overexpressing podocyte Vegf164 (Fig. 5e, f), suggesting that nephrin downregulation was not due to podocyte loss. Moreover, podocyte numbers as determined by Wilms’ tumour antigen 1 immunofluorescence were similar in diabetic and non-diabetic mice, irrespective of overexpression, or not, of podocyte Vegf164 (11.6±0.5 vs 11.0±0.44 podocytes per glomerulus and 12.3±0.5 vs 10.8±0.4 podocytes per glomerulus; p=NS).
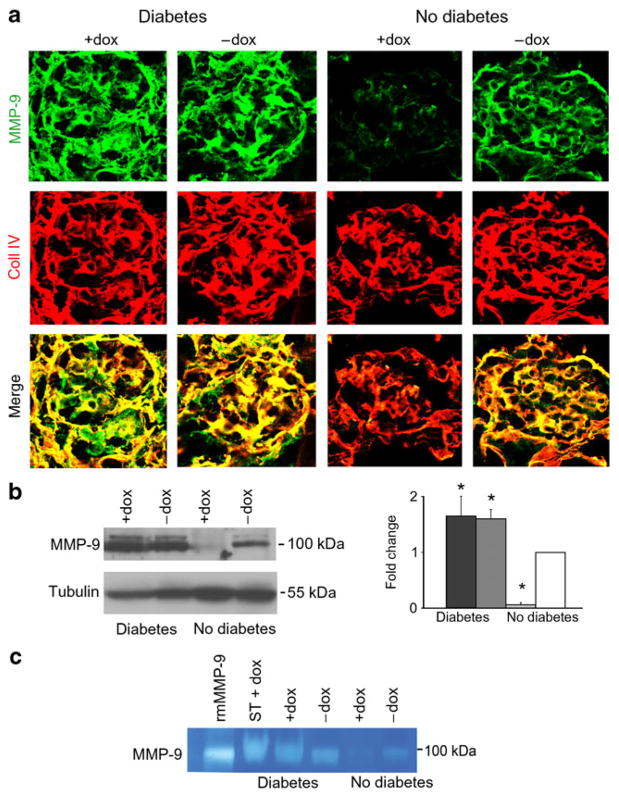
Podocyte Vegf164 overexpression downregulates MMP-2 in diabetic mice
MMP-2 and MMP-9 dysregulation has been implicated in abnormal extracellular matrix turnover, a hallmark of diabetic nephropathy [30]. MMP-9 protein levels and activity were increased in all diabetic mice compared with non-diabetic controls, as assessed by immunohistochemistry, immunoblotting and zymogram (Fig. 6a–c). Since doxycycline is a non-specific inhibitor of MMPs [31], the effects of doxycycline on MMP-9 activity were examined in single transgenic diabetic mice (Fig. 6c), revealing that all diabetic mice had similar MMP-9 activity. Conversely, in non-diabetic mice overexpressing podocyte Vegf164, MMP-9 protein levels and activity were significantly decreased, suggesting that diabetes induces MMP-9 production and activity, overriding the inhibitory effect of podocyte Vegf164 overexpression.
Fig. 6.
MMP-9 protein levels and activity were increased in diabetic mice. a MMP-9 and collagen IV (coll IV) dual-immunostaining showing increased MMP-9 levels in both groups of diabetic mice (+doxycycline [+dox], −doxycycline [−dox]) compared with non-diabetic−doxycycline controls. Non-diabetic+doxycycline mice over-expressing podocyte Vegf164 showed decreased levels of MMP-9; collagen IV was unchanged. b MMP-9 western blot shows increased levels in diabetic mice and decreased levels in non-diabetic +doxycycline mice overexpressing podocyte Vegf164 compared with non-diabetic−doxycycline controls. Tubulin blots document equal loading. Densitometric analysis shows mean±SEM fold change in arbitrary units as compared with controls, n≥4. *p<0.05 compared with control. Black bar, diabetic+doxycycline; dark grey bar, diabetic −doxycycline; light grey bar, non-diabetic+doxycycline; white bar, non-diabetic−doxycycline. c Zymogram showing increased MMP-9 activity in both diabetic mouse groups (+doxycycline, −doxycycline) and decreased activity in non-diabetic+doxycycline mice overexpressing podocyte Vegf164 compared with control non-diabetic−doxycycline mice. Single transgenic diabetic+doxycycline (ST+dox) mice had MMP-9 activity similar to the other diabetic mice. rmMMP-9, recombinant mouse MMP-9
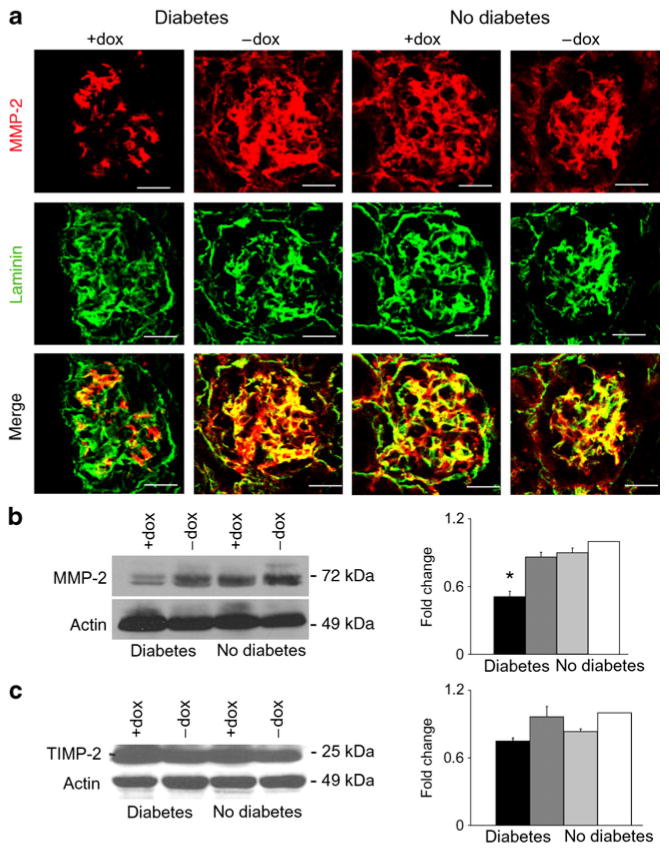
Decreased MMP-2 protein was detected by immuno-fluorescence and western blot in diabetic mice over-expressing podocyte Vegf164 compared with diabetic control mice and non-diabetic mice, including those receiving doxycycline. This ruled out the possibility of unspecific effects (Fig. 7a, b). Given that TIMPs modulate MMPs activity [30], we determined kidney TIMP2 protein levels, which were similar in all groups of mice (Fig. 7c). Collectively, these data suggest that high local VEGF-A signals in a diabetic milieu induce MMP-2 downregulation, resulting in glomerular extracellular matrix accumulation, as shown in Figs 2 and 3.
Fig. 7.
MMP-2 is downregulated by diabetic mice overexpressing podocyte Vegf164. a MMP-2 and laminin dual-immunostaining showing decreased levels of MMP-2 in diabetic+doxycycline (+dox) mice overexpressing podocyte Vegf164. Scale bars, 20 μm. b MMP-2 western blot with bar graph showing decreased expression in diabetic +doxycycline mice overexpressing podocyte Vegf164. c TIMP-2 western blot with bar graph showing similar expression in all experimental groups. b, c Densitometric analysis shows mean±SEM fold change in arbitrary units as compared with controls, n≥4. *p<0.05 compared with control−doxycycline. Black bars, diabetic +doxycycline; dark grey bars, diabetic−doxycycline; light grey bars, non-diabetic+doxycycline; white bars, non-diabetic −doxycycline
Discussion
The role of VEGF-A in kidney disease is complex, as illustrated by the deleterious effects of increased VEGF-A reported in crescentic glomerulonephritis and minimal change disease [21, 32, 33], as well as by renoprotective effects reported in the remnant kidney model [8]. Moreover, decreased VEGF-A availability results in renal thrombotic microangiopathy or hypertensive glomerulosclerosis [34–36]. Diabetic nephropathy involves intricate changes in VEGF-A. Kidney VEGF-A is upregulated in animal models of diabetic nephropathy, mostly explored at the early stage of diabetic nephropathy [2]. These findings are consistent with improvement of renal dysfunction in rodent models of diabetes treated with VEGF-A antibodies [5, 6, 37]. By contrast, human kidney biopsies revealed increased VEGFA mRNA levels at early stages of diabetic nephropathy, but lower VEGFA expression in advanced diabetic nephropathy, with, specifically, sclerotic glomeruli and mesangial nodules expressing less VEGFA mRNA [38, 39]. Circulating VEGF-A levels are elevated in patients with diabetic nephropathy [24]. Thus, elevated glomerular VEGF-A at the onset may decrease as the disease progresses. However, the pathogenic role of VEGF-A in diabetic nephropathy remains unclear.
In this study we examined the role of local VEGF-A signalling in diabetic nephropathy using tetracycline-regulated transgenic mice that overexpress Vegf164 in podocytes upon induction with doxycycline and that were made diabetic with low doses of streptozotocin. The transgene was kept active throughout the study, directly testing the hypothesis that excess podocyte VEGF-A triggers diabetic nephropathy and determines its severity. Podocyte Vegf164 overexpression in diabetic mice led to advanced diabetic glomerulopathy, as evidenced by Kimmelstiel–Wilson-like nodular glomerulosclerosis and massive proteinuria, involving increased VEGF-A and semaphorin3a signalling, as well as MMP-2 dysregulation.
We recently reported that induction of podocyte Vegf164 overexpression in adult mice causes a glomerular disease characterised by glomerulomegaly, mesangial expansion, GBM thickening, podocytes effacement and proteinuria in the absence of hyperglycaemia [12]. This phenotype is similar to incipient diabetic nephropathy or class IIa diabetic nephropathy [40], suggesting that local VEGF-A signalling at the GFB microenvironment plays an important role in the development of diabetic nephropathy.
Podocyte Vegf164 overexpression accelerated the progression of diabetic nephropathy to advanced disease within 12 weeks of exposure to a diabetic milieu. Extensive nodular glomerulosclerosis, mesangiolysis, microaneurysms and arteriolar hyalinosis were observed, in addition to the mesangial expansion and glomerulomegaly seen in diabetic controls and typically reported in mice with streptozotocin-induced diabetes [26, 41]. As described in humans, Kimmel-stiel–Wilson-like nodular glomerulosclerosis (class III) was found in combination with mesangial expansion and was considered an advanced stage of disease [40]. Moreover, glomerular endothelial damage was evidenced by micro-aneurisms and the mesangial matrix was dramatically increased. Massive proteinuria and moderate hyperfiltration were associated with this glomerular phenotype.
Although circulating VEGF-A in diabetic mice was approximately double that of non-diabetic mice, as previously shown in humans and mice [23, 24], its levels were indistinguishable between diabetic mice overexpressing podocyte Vegf164 and those that did not, indicating that the high circulating VEGF-A level depends on the diabetic milieu. Importantly, the former group (i.e. diabetic mice overexpressing podocyte Vegf164) had advanced nephropathy, whereas the latter had incipient nephropathy, suggesting that increased Vegf164 signals at the GFB microenvironment, which do not alter systemic VEGF-A levels, are sufficient to determine the severity of the diabetic glomerular phenotype. In contrast to humans with advanced diabetic nephropathy, glomerular Vegf164 remained elevated in our mouse model throughout the study. Urine VEGF-A excretion was higher in all diabetic mice than in non-diabetic controls, reflecting circulating rather than podocyte Vegf164 expression. Conversely, all non-diabetic mice had normal plasma VEGF-A levels, but urine VEGF-A excretion was dramatically increased in mice overexpressing podocyte Vegf164, reflecting local production. Thus, unfortunately, urine VEGF-A does not appear to be a useful marker for the severity of diabetic nephropathy in this model. Similarly, urinary VEGF did not correlate with albuminuria in patients with type 1 diabetes [42]. The best predictor of advanced glomerular lesions in our model was the magnitude of albuminuria, as severe diabetic glomerulopathy was systematically associated with massive proteinuria (approximately 100-fold higher than controls), in agreement with reports in mice and humans [43, 44].
We established a mouse model of advanced diabetic nephropathy within 12 weeks of sustained hyperglycaemia, featuring two out of three of the modified validation criteria for phenotyping murine models of diabetic nephropathy that were recently proposed by the Animal Models of Diabetic Complications Consortium, i.e. pathological criteria and albuminuria tenfold greater than normal [41]. Moreover, the diabetic nephropathy model reported here does not reveal any morphological and functional signs that are unrelated to diabetic nephropathy and therefore possible confounding factors [41]. Specifically, all diabetic mice reported here were normotensive, irrespective of whether the Vegf164 transgene was induced or not, indicating that hypertension is not a contributing factor to diabetic nephropathy severity in this model. Podocyte Vegf164 overexpression induced semaphorin3a upregulation in diabetic and non-diabetic mice, and immunoreactive semaphorin3a localised to podocytes, which is consistent with our previous report [14]. Kidney NRP1 was upregulated in all diabetic mice and to a lesser extent in non-diabetic mice overexpressing Vegf164, probably due to the high VEGF-A level. Together, these findings suggest that enhanced semaphorin3a signalling is associated with increased podocyte Vegf164 signalling and may contribute to the pathogenesis of the advanced diabetic nephropathy reported herein. Nrp1 mRNA downregulation has been reported in glomeruli from db/db mice and in patients with diabetic nephropathy [18], but NRP1 protein expression and its relationship with VEGF levels were not measured. In addition, differences in diabetes model and species may contribute to the discrepancy between the data. We previously reported that semaphorin3a decreases podocin interaction with nephrin and CD2-associated protein, and induces podocyte apoptosis in vitro [10, 15], and have also shown that semaphorin3a induces podocyte effacement and proteinuria in vivo [14, 45]. Recently we demonstrated that a tight Sema3a gene dosage regulation is required for normal GFB development [14]. In contrast to the previous studies, in which excess semaphorin3a signalling resulted in VEGFR2 downregulation, in the present model of diabetic nephropathy increased VEGFR2 production, VEGF-A and semaphorin3a signalling occurred simultaneously. The mechanistic significance of enhanced semaphorin3a signalling in diabetic nephropathy remains to be established. We speculate that semaphorin3a signalling may play a role in the pathogenesis of microvascular lesions and mesangiolysis in diabetic nephropathy.
Diabetes types 1 and 2 in eNos−/− mice are thought to be the best animal models of diabetic nephropathy, in which mice with a resistant genetic background develop advanced glomerular and interstitial lesions, decreased glomerular filtration rate and hypertension [7, 9]. However, eNos−/− mice develop hypertension, and macro- and microvascular disease unrelated to diabetes [46, 47]. Nakagawa et al. postulated that VEGF is deleterious in diabetic nephropathy because the bioavailability of nitric oxide is reduced [7, 8]. In diabetic mice, podocyte Vegf164 overexpression induced advanced diabetic nephropathy in the absence of changes in endothelial nitric oxide synthase expression. Thus, excessive VEGF-A signalling in the GFB microenvironment may be sufficient to trigger the development of Kimmelstiel–Wilson-like nodular glomerulosclerosis, mesangiolysis and microaneurysms in diabetic mice.
In agreement with previous reports on rodent and human diabetic nephropathy [27–29], nephrin was downregulated in both groups of diabetic mice. Non-diabetic mice over-expressing podocyte Vegf164 also showed decreased nephrin expression, confirming our previous results [12]. Interestingly, the podocyte counts were similar in diabetic and non-diabetic mice, suggesting that nephrin downregulation was not due to podocyte loss, and might be related to VEGF signalling and mediated by VEGFR2–nephrin interaction [12]. Consistent with this, VEGFR2 blockade with SU5416 in db/db mice improved nephrin expression, albuminuria and GBM thickening [37], and podocyte overexpression of soluble VEGFR1, which functions as a dominant negative or sink for VEGF-A, improved albuminuria and GBM thickening [11]. In contrast, increased circulating soluble VEGFR1 decreased albuminuria, but caused loss of function and interstitial damage [48], underscoring the importance of local VEGF-A availability at the GFB as a determinant of severity of diabetic nephropathy.
Extracellular matrix turnover is controlled by protein synthesis and remodelling mediated by MMPs and TIMPs; a balance of TIMP and MMP function determines extra-cellular matrix integrity. However, the reported findings on regulation of extracellular matrix turnover by MMPs and TIMPs in diabetic nephropathy are discordant [30]. Diabetic mice overexpressing podocyte Vegf164 showed significant decreases in whole-kidney and glomerular MMP-2 levels, which were not observed in non-diabetic mice with identical genotypes and Vegf1644 overexpression induced by doxycycline. These data suggest that MMP-2 downregulation is not caused by non-specific MMP inhibition by doxycycline and may contribute to the glomerular extracellular matrix accumulation observed in diabetic mice over-expressing podocyte Vegf164. However, MMP-9 production and activity were increased in diabetic mice overexpressing podocyte Vegf164 and in control diabetic mice, suggesting this change is due to the diabetic milieu rather than to the Vegf transgene or doxycycline. In agreement with our in vivo data, prior in vitro studies demonstrated that high glucose modulates podocyte MMP-9 production and activity [49]. Interestingly, in the absence of a diabetic milieu, podocyte Vegf164 overexpression specifically decreased MMP-9 expression and activity, suggesting that hyperglycaemia overrides Vegf164 regulation of MMP-9 activity in the kidney. TIMP-2 levels were similar in diabetic and non-diabetic mice, in agreement with findings in human diabetes [30], suggesting a weak role in MMP dysregulation at this stage of diabetic nephropathy.
De Vriese et al. and Schrijvers et al. showed that hyperfiltration and proteinuria were reduced by anti-VEGF antibody in mouse models of type 1 and 2 diabetes, demonstrating that VEGF-A plays a crucial role in diabetic nephropathy [5, 6]. Here we showed that podocyte Vegf164 overexpression induced hyperfiltration and massive proteinuria in diabetic mice, in the setting of similarly elevated circulating VEGF-A levels, suggesting that local Vegf164 alters glomerular haemodynamics synergistically with a VEGF-A systemic effect in diabetic mice.
In summary, the present study shows that increased podocyte Vegf164 signalling dramatically worsens diabetic nephropathy in streptozotocin-induced diabetic mice, resulting in diabetic nodular glomerulosclerosis and massive proteinuria. Our data suggest that in addition to VEGF-A, MMP-2 dysregulation and increased semaphorin3a signalling are mechanistically involved in severe diabetic glomerulopathy. This novel model of advanced diabetic nephropathy, which resembles human disease, should prove useful for testing preventive and therapeutic interventions.
Supplementary Material
Acknowledgments
We thank A. Akeson and J. Whittsett (Cincinnati Children’s Hospital) for providing the tet-O-Vegf164 mice, J. Kopp (NIH) for providing the podocin-rtTA mice and A. Kolodkin (John Hopkins University) for the NRP1 antibody. Portions of this work were presented at the 2008 American Society of Nephrology meeting. This study was supported by NIH RO1-DK59333 (to A. Tufro) and by NIH George M. O’Brien Kidney Center P50-DK64236 (to H. Velazquez.). K. Reidy was supported by NIH training grant T32 DK-007110; A. M. Garcia was supported by AHA award 0826014. A. Tufro also received support from the Emerald Foundation.
Abbreviations
- GBM
Glomerular basement membrane
- GFB
Glomerular filtration barrier
- MMP
Matrix metalloproteinase
- NRP
Neuropilin
- OCT
Optimal cutting temperature medium
- PAS
Periodic acid–Schiff’s reagent
- TEM
Transmission electron microscopy
- TIMP
Tissue inhibitor of matrix metalloproteinase
- VEGF
Vascular endothelial growth factor
- VEGFR
VEGF receptor
Footnotes
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Electronic supplementary material The online version of this article (doi:10.1007/s00125-010-2034-z) contains supplementary material, which is available to authorised users.
Contributor Information
D. Veron, Department of Pediatrics, Yale University School of Medicine, 333 Cedar St, P.O. Box 208064, New Haven, CT 06520-8064, USA
C. A. Bertuccio, Department of Pediatrics, Yale University School of Medicine, 333 Cedar St, P.O. Box 208064, New Haven, CT 06520-8064, USA
A. Marlier, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA
K. Reidy, Department of Pediatrics, Albert Einstein College of Medicine, Bronx, NY, USA
A. M. Garcia, Department of Internal Medicine, Albert Einstein College of Medicine, Bronx, NY, USA
J. Jimenez, Analytical Imaging Facility, Albert Einstein College of Medicine, Bronx, NY, USA
H. Velazquez, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT, USA
M. Kashgarian, Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
G. W. Moeckel, Department of Pathology, Yale University School of Medicine, New Haven, CT, USA
A. Tufro, Email: alda.tufro@yale.edu, Department of Pediatrics, Yale University School of Medicine, 333 Cedar St, P.O. Box 208064, New Haven, CT 06520-8064, USA
References
- 1.Wolf G, Ziyadieh F. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 2.Brosius FC, Khoury CC, Buller CL, Chen S. Abnormalities in signaling pathways in diabetic nephropathy. Expert Rev Endocrinol Metab. 2010;5:51–64. doi: 10.1586/eem.09.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gnudi L. Molecular mechanisms of proteinuria in diabetes. Biochem Soc Trans. 2008;36:946–949. doi: 10.1042/BST0360946. [DOI] [PubMed] [Google Scholar]
- 4.Cooper ME, Vranes D, Youssef S, et al. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 5.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- 6.Schrijvers BF, Flyvberg A, de Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa T, Sato W, Glushakova O, et al. Diabetic eNOS knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18:539–550. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am J Physiol Renal Physiol. 2007;292:F1665–F1672. doi: 10.1152/ajprenal.00495.2006. [DOI] [PubMed] [Google Scholar]
- 9.Kanetsuna Y, Takahashi K, Nagata M, et al. Deficiency of endothelial nitric-oxide synthase confers susceptibility to diabetic nephropathy in nephropathy-resistant inbred mice. Am J Pathol. 2007;170:1473–1484. doi: 10.2353/ajpath.2007.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine VEGF-A system in podocytes regulates podocin and its interaction with CD2AP. Am J Physiol Renal Physiol. 2006;291:F422–F428. doi: 10.1152/ajprenal.00448.2005. [DOI] [PubMed] [Google Scholar]
- 11.Ku CH, White KE, Dei Cas A, et al. Inducible over-expression of sFlt-1 in podocytes ameliorates glomerulopathy in diabetic mice. Diabetes. 2008;57:2824–2833. doi: 10.2337/db08-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veron D, Reidy K, Bertuccio C, et al. Induction of podocyte VEGF-A oveexpression in adult mice causes glomerular disease. Kidney Int. 2010;77:989–999. doi: 10.1038/ki.2010.64. [DOI] [PubMed] [Google Scholar]
- 13.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753–762. doi: 10.1016/s0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 14.Reidy KJ, Villegas G, Teichman J, et al. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan F, Villegas G, Teichman J, Mundel P, Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int. 2006;69:1564–1569. doi: 10.1038/sj.ki.5000313. [DOI] [PubMed] [Google Scholar]
- 16.Villegas G, Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev. 2002;119(Suppl 1):S149–S153. doi: 10.1016/s0925-4773(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 17.Miao HQ, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: functional competition of collapsin-1 and vascular endothelial growth factor-165. J Cell Biol. 1999;146:233–242. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bondeva T, Rüster C, Franke S, et al. Advanced glycation end-products suppress neuropilin-1 expression in podocytes. Kidney Int. 2009;75:605–616. doi: 10.1038/ki.2008.603. [DOI] [PubMed] [Google Scholar]
- 19.Yuen PS, Dunn SR, Miyaji T, Yasuda H, Sharma K, Star RA. A simplified method for HPLC determination of creatinine in mouse serum. Am J Physiol Renal Physiol. 2004;286:F1116–F1119. doi: 10.1152/ajprenal.00366.2003. [DOI] [PubMed] [Google Scholar]
- 20.Gross ML, Koch A, Mühlbauer B, et al. Renoprotective effect of a dopamine D3 receptor antagonist in experimental type II diabetes. Lab Invest. 2006;86:262–274. doi: 10.1038/labinvest.3700383. [DOI] [PubMed] [Google Scholar]
- 21.Veron D, Reidy K, Marlier A, et al. Induction of podocyte VEGF164 overexpression at different stages of development causes congenital nephrosis or steroid resistant nephrotic syndrome. Am J Pathol. 2010;177:2225–2233. doi: 10.2353/ajpath.2010.091146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Ichinose K, Maeshima Y, Yamamoto Y, et al. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes. 2005;54:2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- 24.Hovind P, Tarnow L, Oestergaard PB, Parving HH. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int. 2000;75:S56–S61. [PubMed] [Google Scholar]
- 25.Eisner C, Faulhaber-Walter R, Wang Y, et al. Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int. 2010;77:519–526. doi: 10.1038/ki.2009.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breyer MD, Böttinger E, Brosius FC, 3rd, et al. AMDCC. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- 27.Gagliardini E, Corna D, Zoja C, et al. Unlike each drug alone, lisinopril if combined with avosentan promotes regression of renal lesions in experimental diabetes. Am J Physiol Renal Physiol. 2009;297:F1448–F1456. doi: 10.1152/ajprenal.00340.2009. [DOI] [PubMed] [Google Scholar]
- 28.Doublier S, Salvidio G, Lupia E, et al. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 29.Langham RG, Kelly DJ, Cox AJ, et al. Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia. 2002;45:1572–1576. doi: 10.1007/s00125-002-0946-y. [DOI] [PubMed] [Google Scholar]
- 30.Thrailkill KM, Clay Bunn R, Fowlkes JL. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35:1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitta K, Uchida K, Kimata N, et al. Increased serum levels of vascular endothelial growth factor in human crescentic glomerulonephritis. Clin Nephrol. 1999;52:76–82. [PubMed] [Google Scholar]
- 33.Bailey E, Bottomley MJ, Westwell S, et al. Vascular endothelial growth factor mRNA expression in minimal change, membranous, and diabetic nephropathy demonstrated by non-isotopic in situ hybridisation. J Clin Pathol. 1999;52:735–738. doi: 10.1136/jcp.52.10.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in pre-eclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Advani A, Kelly DJ, Advani SL, et al. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A. 2007;104:14448–14453. doi: 10.1073/pnas.0703577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sung SH, Ziyadeh FN, Wang A, Pyagay PE, Kanwar YS, Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17:3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 38.Shulman K, Rosen S, Tognazzi K, Manseau EJ, Brown LF. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996;7:661–666. doi: 10.1681/ASN.V75661. [DOI] [PubMed] [Google Scholar]
- 39.Bortoloso E, del Prete D, Dalla Vestra M, et al. Quantitave and qualitative changes in vascular endothelial growth factor gene expression in glomeruli of patients with type 2 diabetes. Eur J Endocrinol. 2004;150:799–807. doi: 10.1530/eje.0.1500799. [DOI] [PubMed] [Google Scholar]
- 40.Tervaert TW, Mooyaart AL, Amann K, Society RP, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 41.Brosius FC, 3rd, Alpers CE, Bottinger EP, et al. Animal models of diabetic complications consortium. Mouse models of diabetic nephropathy. J Am Soc Nephrol. 2009;20:2503–2512. doi: 10.1681/ASN.2009070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenz T, Haak T, Malek J, Gröne HJ, Geiger H, Gossmann J. Vascular endothelial growth factor in diabetic nephropathy. Kidney Blood Press Res. 2003;26:338–343. doi: 10.1159/000073940. [DOI] [PubMed] [Google Scholar]
- 43.Peterson JC, Adler S, Burkart JM, et al. Blood pressure control, proteinuria, and the progression of renal disease. The modification of diet in renal disease study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 44.Atkins RC, Briganti EM, Lewis JB, et al. Proteinuria reduction and progression to renal failure in patients with type 2 diabetes mellitus and overt nephropathy. Am J Kidney Dis. 2005;45:281–287. doi: 10.1053/j.ajkd.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Tapia R, Guan F, Gershin I, Teichman J, Villegas G, Tufro A. Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int. 2008;73:733–740. doi: 10.1038/sj.ki.5002726. [DOI] [PubMed] [Google Scholar]
- 46.Forbes MS, Thornhill BA, Park MH, Chevalier RL. Lack of endothelial nitric-oxide synthase leads to progressive focal renal injury. Am J Pathol. 2007;170:87–99. doi: 10.2353/ajpath.2007.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arraj M, Lemmer B. Endothelial nitric oxide is not involved in circadian rhythm generation of blood pressure: experiments in wild-type C57 and eNOS knock-out mice under light-dark and free-run conditions. Chronobiol Int. 2007;24:1231–1240. doi: 10.1080/07420520701795357. [DOI] [PubMed] [Google Scholar]
- 48.Kosugi T, Nakayama T, Li Q, et al. Soluble Flt-1 gene therapy ameliorates albuminuria but accelerates tubulointerstitial injury in diabetic mice. Am J Physiol Renal Physiol. 2010;298:F609–F616. doi: 10.1152/ajprenal.00377.2009. [DOI] [PubMed] [Google Scholar]
- 49.Bai Y, Wang L, Li Y, et al. High ambient glucose levels modulates the production of MMP-9 and alpha5(IV) collagen by cultured podocytes. Cell Physiol Biochem. 2006;17:57–68. doi: 10.1159/000091464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.