Abstract
Background
Schizophrenia patients and their relatives show aberrant functional connectivity in default network regions (DRs) such as the medial prefrontal, lateral temporal, cingulate and inferior parietal cortices and executive regions such as the dorsolateral prefrontal cortex (DLPFC). Gray-matter volumetric alterations may be related to these functional connectivity deficits. Also, gray-matter volume inter-regional correlations may reflect altered inter-regional functional connectivity.
Aims
To examine our prediction of alterations of gray-matter volumes and inter-regional volume correlations for DRs and the DLPFC in offspring of schizophrenia patients (OS).
Methods
We assessed 64 adolescent and young adult OS and 80 healthy controls (HC) using T1-MRI. Regional gray-matter volumes and inter-regional volume correlations between the DRs and between the DLPFC and DRs on each side were compared across groups.
Results
Compared to HC, OS had reductions in several DRs and the DLPFC after controlling age, gender, and intra-cranial volume, and correcting for multiple comparisons. OS had stronger (more positive) gray-matter volume inter-correlations between DRs and between DRs and the DLPFC.
Conclusions
Volumetric deficits in the default network and in the DLPFC may be related to familial diathesis to schizophrenia and to functional connectivity abnormalities in those at familial risk. Increased inter-correlations between DRs and between DR and DLPFC gray-matter volumes may serve as surrogate indices of abnormal functional connectivity.
Keywords: Default network, Dorso lateral prefrontal cortex, Inter-regional correlations, Functional connectivity
1. Introduction
Brain regions have been traditionally conceptualized as belonging to either the ‘task-dependent’ or the ‘task-independent’ network(Buckner et al., 2008). While ‘task-dependent’ network regions are primarily activated during specific cognitive tasks like planning, reasoning and problem solving, the ‘task-independent’ network is shown to be active mainly in the absence of task-dependent ideation (resting or ‘default’ state of the brain) (Buckner et al., 2008). The task-dependent network includes the heteromodal cortical regions, thalamus, caudate and superior parietal cortex (Gur et al., 2007; Potkin et al., 2009; Salgado-Pineda et al., 2003; Wang et al., 2009) while the default network may include the anterior cingulate, inferior parietal, posterior cingulate, medial-prefrontal and lateral temporal neocortices and the precuneus (Broyd et al., 2009; Buckner et al., 2008; Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008; Mannell et al., 2009; Whitfield-Gabrieli et al., 2009). The heteromodal association areas are considered to include the dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus, superior temporal cortex, and the inferior parietal lobule (Keshavan et al., 2002; Mesulam, 1998; Pearlson et al., 1996; Ross and Pearlson, 1996; Sabsevitz et al., 2005). The inferior parietal lobule is also thought to belong to the default network (Broyd et al., 2009; Buckner et al., 2008; Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008; Mannell et al., 2009; Whitfield-Gabrieli et al., 2009). Default network regions like the anterior cingulate cortex and medial prefrontal cortex may be involved in attention, working memory and other task-related processes (Buckner et al., 2009; Koski and Paus, 2000; Margulies et al., 2007; Mazoyer et al., 2001; Wang et al., 2005). Recent evidence has also suggested a functional overlap between the ‘task-dependent’ heteromodal and the ‘task-independent’ default networks for the inferior parietal lobule, anterior cingulate cortex and the medial prefrontal cortex (Binder et al., 2009; Broyd et al., 2009; Buckner et al., 2008; Buckner et al., 2009; Fassbender et al., 2009; Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008; Koski and Paus, 2000; Mannell et al., 2009; Margulies et al., 2007; Mazoyer et al., 2001; Pallesen et al., 2009; Wang et al., 2005; Whitfield-Gabrieli et al., 2009). These functionally heterogeneous regions have been posited to comprise of ‘task-related’ and ‘default’ functionally distinct subdivisions (Koski and Paus, 2000; Margulies et al., 2007; Wang et al., 2005; Wu et al., 2009). Alternately, it has been suggested that considering brain regions as ‘compartmentalized’ into task-dependent and default networks may be somewhat simplistic given the complexity of large-scale distributed networks in the brain (Calhoun et al., 2006; Fuster, 2009; Verduzco-Flores et al., 2009).
‘Default’ regions (DRs) may be involved in stimulus-independent ideation including magical thinking, day dreaming, self reflection and theory-of-mind processes (Buckner and Carroll, 2007; Buckner et al., 2008; Mason et al., 2007; Spreng and Grady, 2009). Possible alterations of DR have been implicated in disturbances of these processes in schizophrenia (Broyd et al., 2009; Buckner et al., 2008; Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008; Mannell et al., 2009; Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009; Zhou et al., 2007). DRs are positively functionally connected with each other during the resting state, in healthy subjects (Calhoun et al., 2008a; Mannell et al., 2009). During task performance, the activity in DRs is inversely correlated (anti-correlated) with DLPFC (DLPFC activity coincides with deactivation of DR function during task performance (Calhoun et al., 2009; Calhoun et al., 2008b; Garrity et al., 2007; Whitfield-Gabrieli et al., 2009)) in healthy subjects. Schizophrenia patients (Calhoun et al., 2008a; Calhoun et al., 2009; Calhoun et al., 2008b; Garrity et al., 2007; Jafri and Calhoun, 2006; Jafri et al., 2008; Stevens et al., 2009) and non-psychotic adolescent and young adult relatives of schizophrenia patients show functional abnormalities of the DR and DLPFC. DR in patients (Broyd et al., 2009; Buckner et al., 2008; Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008; Mannell et al., 2009; Pomarol-Clotet et al., 2008; Whitfield-Gabrieli et al., 2009; Zhou et al., 2007) and relatives (Whitfield-Gabrieli et al., 2009), show increased functional connectivity at rest and a lack of anti-correlation with the DLPFC.
Gray-matter volume inter-correlations have been suggested to be possible indices of functional connectivity (DaSilva et al., 2008; Mitelman et al., 2005; New et al., 2007). Functionally connected regions correlate on their blood oxygenation level dependent (BOLD) responses(Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008), and hence on their metabolic activities (Kida and Hyder, 2006). Regional gray-matter volumes may correlate with regional functional activity and metabolism through use-dependent plasticity (DaSilva et al., 2008; Mitelman et al., 2005; New et al., 2007). Inter-regional volume correlations may hence form between two regions, approximately in proportion to their functional connectivity due to use-dependent plasticity (DaSilva et al., 2008; Mitelman et al., 2005; New et al., 2007). Correlated and anti-correlated functional connectivity between regions may hence be mirrored in positive and negative inter-regional volume correlations respectively. We assessed adolescent healthy control (HC) and offspring of schizophrenia (OS) subjects on MRI measures of gray-matter volume of DRs and DLPFC. We predicted DR volumetric alterations in OS, given the previously shown functional DR deficits in OS (Whalley et al., 2007; Whalley et al., 2005; Whalley et al., 2008; Whalley et al., 2004; Whalley et al., 2009; Whalley et al., 2006; Whitfield-Gabrieli et al., 2009; Whyte et al., 2006) and the possible contribution of brain structural deficits to altered brain function (Buckholtz et al., 2007; Lv et al., 2008; Park et al., 2006). We predicted increased inter-regional volume correlations between DRs and between DRs and DLPFC in OS compared to HC due to previous observations of increased resting state functional connectivity between DR and the reduced anti-correlation between DRs and DLPFC in relatives (Whitfield-Gabrieli et al., 2009). Amongst the regions comprising the task dependent network (thalamus, caudate-nucleus, DLPFC and parietal-cortex (Gur et al., 2007; Potkin et al., 2009; Salgado-Pineda et al., 2003; Wager and Smith, 2003; Wang et al., 2009)) we chose the DLPFC, (a) because of its strong implication in the premorbid diathesis for schizophrenia(McIntosh et al., 2006; Meda et al., 2008; Woodward et al., 2009), (b)to reduce multiple comparisons to the extent possible and (c) since altered functional connectivity of DRs in relatives (Whitfield-Gabrieli et al., 2009) has been shown only for the DLPFC.
2. Methods
2.1 Participants
The study was conducted at the Western Psychiatric Institute and Clinic, Pittsburgh. Participants were 80 adolescent and young adult offspring of (OS) of schizophrenia probands and 64 healthy controls (HC). Offspring of parents with schizophrenia or schizoaffective disorder were recruited by approaching patients in the clinic and through advertisements. HC were recruited through advertisements in the same community as OS. Clinical assessments of HC and OS and parental diagnoses of schizophrenia or schizoaffective disorder used the structured clinical interviews for DSM-IV diagnoses (SCID)(First et al., 1995) and were confirmed using consensus meetings led by senior diagnosticians (M.S.K and D.M). Participants with an IQ<80, lifetime evidence of a psychotic disorder, exposure to antipsychotic medications, exposure to anti-depressant medications, current or recent (within the previous month) substance use disorder, neurological or medical condition were excluded. All participants signed informed consent after the study was fully explained to them. For participants <18 years of age, the consent was provided by the parent or guardian, and the subjects provided informed assent. The study was approved by the University of Pittsburgh Institutional Review Board.
2.2. Structural MRI assessments
MRI scans were obtained on subjects using a GE 1.5T whole body scanner (GE Medical Systems, Milwaukee, Wisconsin). The scans were three-dimension spoiled gradient recalled (SPGR), acquired in a steady-state pulse sequence (124 coronal slices, 1.5 mm cortical thickness, TE=5 msec, TR=25 msec, acquisition matrix=256×192, FOV=24 cm, flip angle 40°). The detailed scanning procedure has been described in detail in our previous publication(Gilbert et al., 2001). Images with significant motion artifacts were not included in the study. T1-images were processed using FreeSurfer, an extensively validated automated method, previously used to assess young relatives of schizophrenia patients(Goghari et al., 2007). FreeSurfer has three automated stages (Segonne et al., 2004), each followed by manual image editing by an experienced research assistant(AF). The first stage performs skull stripping and motion correction, while the second performs gray-white segmentation (Fischl et al., 2002). The third automatically parcellates the regions of interest based on gyral anatomical landmarks and performs cortical gray-matter volume measurements (Desikan et al., 2006). Each stage has been shown to be valid and reliable with manual tracing and automated methods (Tae et al., 2008). Processing MRI data was done blind to subject identity and clinical diagnoses. Clinical interviewers were blind to MRI data.
2.3. Statistical Analyses
Regional gray-matter volumes were normally distributed [Shapiro-Wilk’s test (W statistic, p>0.1)]. ANCOVAs controlling for intra cranial volume (ICV), gender and age were used to compare regional volumes of the DLPFC and the DRs (precuneus, inferior parietal, lateral temporal, anterior cingulate, posterior cingulate and medial prefrontal cortices) on each side. The Bonferroni correction threshold of 0.0063 (0.05/8) was used to correct each ANCOVA for experiment wise error to achieve an overall alpha error rate of 0.05 for all tests on each side.
Inter-regional gray-matter volume correlations between DR (between two DRs) and between DLPFC and a DR were computed for each study group using two tailed Pearson partial correlation coefficients controlling for intra cranial volume and age. Fifteen between DR inter-regional correlations resulted on each side by combining the six DR. Alterations reported for the functional connectivity between DLPFC and DRs in offspring, have not been resolved across specific DLPFC-DR pairs (Whitfield-Gabrieli et al., 2009). The DLPFC (seed) was hence correlated with all DRs unilaterally resulting in six correlations between DLPFC and a DR on each side. These fifteen inter-correlations between DRs (DR-DR) and six inter-correlations between DRs and the DLPFC (DLPFC-DR) on each side were compared across study groups using two-tailed difference of r tests. The Bonferroni thresholds of p = 0.008 (0.05/6) for the six DLPFC-DR inter-correlations and of p = 0.003 (0.05/15) for the fifteen between-DR inter-correlations were used on each side to correct the difference of r tests for experiment wise error and achieve an overall alpha error of 0.05 per side.
3. Results
Groups did not differ in age [controls (16.6±4.5 years), offspring (15.4±3.6 years), t = 1.79, p = 0.1], handedness (Chi-square = 0.1, p = 0.7), race (Chi-square = 0.33, p = 0.57) or gender (controls: 42% males, offspring: 53% males, Chi-square = 1.6, p = 0.20).
Homogeneity of variances (Levene’s test p>.1) assumptions were met for all ANCOVA tests. ANCOVAs revealed gray-matter volume reductions in the right DLPFC and in default network regions of lateral temporal cortices, precunei, right inferior parietal lobule, right medial frontal cortex, and left posterior cingulate cortex. All volumetric deficits survived the Bonferroni threshold except for the right DLPFC, left precuneus and right medial frontal cortex (refer table 1).
TABLE 1.
Mean and standard deviations for gray-matter volumes (in cubic mm) of healthy controls (HC) and offspring of schizophrenia patients(OS) and ANCOVA statistics for between-group volumetric differences controlling for ICV, gender and age are mentioned.
| Region | Gray-matter volume in cubic mm [Mean, S.D] for HC | Gray-matter volume in cubic mm [Mean, S.D] for OS | F (1, 145) | p |
|---|---|---|---|---|
| L Lateral Temporal* | 12861, 2237 | 12017, 1804 | 7.81 | 0.006 |
| R Lateral Temporal* | 14019, 2436 | 13246, 1942 | 7.91 | 0.006 |
| L Inferior Parietal lobule | 14405, 2655 | 14283, 2913 | p>0.20 | p>0.20 |
| R Inferior Parietal lobule* | 18334, 2994 | 17202, 2783 | 13.74 | 0.000 |
| L Posterior Cingulate Cortex* | 3771, 593 | 3609, 593 | 9.77 | 0.005 |
| R Posterior Cingulate Cortex | 3822, 635 | 3873, 604 | p>0.20 | p>0.20 |
| L precuneus | 10815, 1994 | 10412, 1652 | 5.46 | 0.021 |
| R precuneus* | 10970, 1828 | 10342, 1533 | 13.80 | 0.000 |
| L Medial Prefrontal Cortex | 26582, 2461 | 26036, 3406 | p>0.20 | p>0.20 |
| R Medial Prefrontal Cortex | 25501, 3869 | 24848, 3732 | 2.81 | 0.096 |
| L Anterior Cingulate Cortex | 4402, 739 | 4276, 877 | p>0.20 | p>0.20 |
| R Anterior Cingulate Cortex | 4501, 425 | 4283, 426 | p>0.20 | p>0.20 |
| L DLPFC | 24686, 5861 | 23988, 5558 | p>0.20 | p>0.20 |
| R DLPFC | 27282, 4088 | 26107, 3834 | 5.61 | 0.019 |
= between group differences surviving the Bonferroni threshold of 0.0063 (0.05/8) to achieve an overall alpha-error-rate of 0.05 for regions on each side.
DLPFC = Dorso lateral Prefrontal Cortex
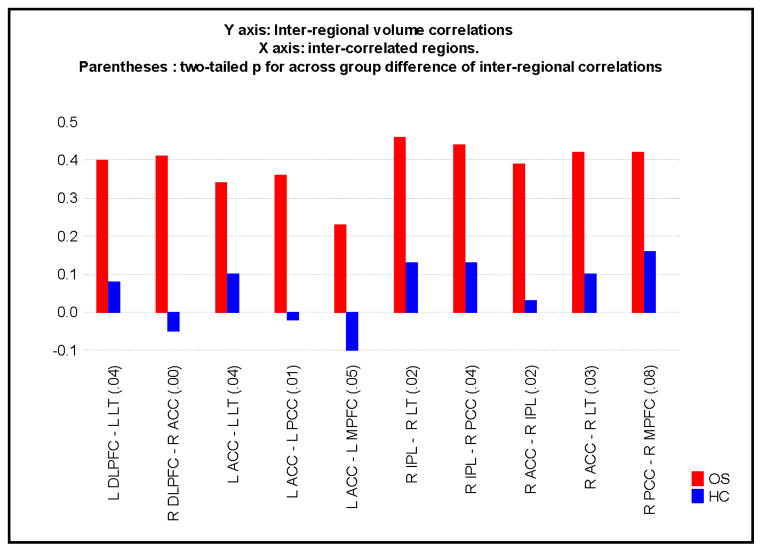
Inter-regional correlations for two DLPFC-DR and 7 DR-DR region pairs were significant at p < 0.05 (two tailed) in OS subjects. The inter-regional correlation between the L ACC and L MPFC in OS was significant at p=0.071 (two tailed). Inter-correlations for none of the region-pairs reached significance in HC subjects. OS had stronger inter-regional correlations between the DLPFC and DRs and between DRs as revealed by difference of r tests (see figure 1). The difference of r tests for the DLPFC-DR inter-correlations, revealed the inter correlation between the left DLPFC and the left lateral temporal and the inter correlation between the right DLPFC and the right anterior cingulate cortex to be significantly greater in OS compared to HC at p < 0.05 (two tailed). The difference of r tests revealed 7 inter-regional correlations between DRs [L ACC- L LT, L ACC- L PCC, L ACC- L MPFC, R IPL- R LT, R IPL- R PCC, R ACC- R LT, R IPL- R ACC] to be were significantly different across groups at p < 0.05 (two tailed) (see figure 1). The inter-correlation for the R PCC-R MPFC differed across groups at p=0.08 (two-tailed). The across group difference of correlation for the inter correlation between the right DLPFC and the right anterior cingulate cortex correlation survived the Bonferroni correction (see figure 1).
FIGURE 1.
Inter-regional correlations for each group for each pair of regions are depicted. The X-axis denotes each pair of inter correlated regions. Inter-regional correlations are plotted on the Y-axis with the height of the bars equaling the magnitude of the inter-regional correlation for that pair of regions. Red and blue bars denote inter-regional correlations for OS and HC respectively. While the correlations in OS were significant, none of the inter-correlations for HC reached significance. All inter-regional correlations except that for the L ACC- L MPFC region-pair were significant at p < 0.05 (two tailed) in OS subjects. Inter-correlations for none of the region-pairs in HC subjects reached significance. Difference of r tests comparing the inter-regional correlations between OS and HC were performed and the two tailed p values for these across group difference for inter-regional correlations are provided in parentheses on the X-axis. MPFC = medial prefrontal cortex, ACC = anterior cingulate cortex, PCC = posterior cingulate cortex, IPL= inferior parietal lobule and DLPFC = dorso lateral prefrontal cortex. LT= Lateral temporal cortex
4. Discussion
pAs predicted, OS had gray matter volume reductions in DRs and the right dorso lateral prefrontal cortex. Altered functional connectivity, for DRs such as the lateral temporal, medial prefrontal cortex, cingulated and parietal cortices and also for the DLPFC in relatives has been proposed as an inherited trait-marker of schizophrenia (Whalley et al., 2007; Whalley et al., 2005; Whalley et al., 2008; Whalley et al., 2004; Whalley et al., 2009; Whalley et al., 2006; Whitfield-Gabrieli et al., 2009; Whyte et al., 2006). Our findings are consistent with the possibility that structural deficits of the DRs and DLPFC may underlie functional abnormalities in relatives (Buckholtz et al., 2007; Lv et al., 2008; Park et al., 2006). To our knowledge, previous studies have not examined DRs, in particular the precuneus and the posterior cingulate cortex in non-psychotic high-risk relatives. Prefrontal and temporal cortical volumetric deficits may occur in relatives showing sub-threshold psychotic symptoms (Lawrie et al., 2002; Lawrie et al., 1999). A study has assessed gray-matter volume inter-regional correlations in schizophrenia patients (Mitelman et al., 2005). Although functional connectivity in the DRs and DLPFC and their relation to cognition has been assessed using functional imaging (fMRI) in patients(Buckner et al., 2008) and in their adolescent relatives (Whitfield-Gabrieli et al., 2009), gray-matter volume inter-regional correlations for these regions have not been hitherto reported.
Gray-matter volume inter-correlations have been previously interpreted as possible indices of functional connectivity (DaSilva et al., 2008; Mitelman et al., 2005; New et al., 2007). Gray-matter volume inter-correlations were stronger between DR and between DR and DLPFC in offspring compared to HC and hence mirrored functional-connectivity patterns shown previously in offspring (Whitfield-Gabrieli et al., 2009). As functional-connectivity is a correlation of the BOLD (blood oxygenation level dependent) responses of two regions (Fox et al., 2005; Fransson, 2005; Fransson and Marrelec, 2008), metabolic activities and oxygen consumptions (Kida and Hyder, 2006) of functionally connected regions may be correlated. Regional metabolism may impact regional gray-matter volume through use dependent plasticity (DaSilva et al., 2008; Mitelman et al., 2005; New et al., 2007). Functional connectivity between two regions may hence cause correlated changes in volumes of those regions due to use dependent plasticity (DaSilva et al., 2008; Mitelman et al., 2005; New et al., 2007). Gray-matter volume inter-regional correlations may hence form approximately proportionately to inter-regional functional connectivity. Alternative interpretations of volume inter-correlations however exist. Compensatory hyperactivity and trophic volumetric increase may occur in a region due to inefficiency in a volumetrically reduced area with a similar function. This may cause negative inter-regional correlations, in the absence of any inverse functional connectivity between regions (Mitelman et al., 2005). This is however unlikely to account for abnormally increased positive inter-regional correlations in offspring compared to healthy control subjects. Further, a common vulnerability to certain insults may cause concurrent volumetric reductions in two regions in offspring, causing positive volume inter-correlations in the absence of any positive functional connectivity between regions (Mitelman et al., 2005). This mechanism would explain abnormally increased positive inter-regional correlations between regions, both of which show a volumetric deficit in offspring. Coincident volumetric deficits in both inter-correlated regions cannot however underlie increased inter-correlations across region pairs where one or both regions are volumetrically intact in offspring. Of the ten region pairs noted to show abnormally heightened inter-regional correlation, only the R LT-R IPL pair comprises of two regions showing volumetric deficits in offspring. While concurrent volumetric deficits due to a shared vulnerability may explain increased the inter-regional correlation across the R LT and R IPL, it cannot account for the abnormally increased inter-correlations for the other nine region pairs in offspring subjects. Altered functional connectivity may hence be a plausible explanation for abnormally increased structural inter-regional correlations in offspring subjects. Recent evidence of altered functional connectivity across the default network regions and the DLPFC in young relatives of schizophrenia patients (Whitfield-Gabrieli et al., 2009) also implies the viability of this interpretation. The establishment of gray-matter volume inter-regional correlations may require the presence of neuroanatomical connectivity (for example, through white matter tracts (Burns et al., 2003; Kanaan et al., 2009; Kyriakopoulos et al., 2009; Zanetti et al., 2008)) in addition to functional connectivity between those regions. Volume inter-correlations may hence fail to develop between functionally connected, but neuroanatomically discontinuous regions (Mitelman et al., 2005). Volume inter-correlations may hence underestimate the extent of functional connectivity due to neuroanatomical constraints such as the presence of white-matter tracts inter-connecting the correlated regions. This underestimation may be compounded by the reduced white-matter volumes and structural connectivity in schizophrenia (Burns et al., 2003; Kanaan et al., 2009; Kyriakopoulos et al., 2009; Zanetti et al., 2008). While this may render volume inter-correlations relatively insensitive indices of functional connectivity when compared to functional imaging techniques, it does not confound our interpretation of volume inter-regional correlation as reflecting functional connectivity.
Limitations
Our findings of altered volume inter-correlations will have to be viewed as preliminary due to the possibility of type-1 error. Comparisons of OS with schizophrenia patients and longitudinal studies are needed to establish if the alterations we noted are trait or state related phenomena, especially given that functional connectivity alterations have been shown to be trait related (Whalley et al., 2007; Whalley et al., 2005; Whalley et al., 2004; Whalley et al., 2009; Whalley et al., 2006).
The use of structural inter-regional correlations as surrogate markers for altered functional connectivity, a putative trait-marker of schizophrenia (Whalley et al., 2004; Whalley et al., 2009), is a new approach which needs to be investigated further.
Acknowledgments
We thank Shreedhar Kulkarni, Diana Mermon, and Vaibhav A Diwadkar for their help with various aspects of this study.
Role of Funding Source
National Institute of Mental Health (MH 64023 and 01180 to M.S.K); National Alliance for Research on Schizophrenia and Depression (Independent Investigator award to M.S.K); National Alliance for Research on Schizophrenia and Depression and General Clinical Research Center (GCRC) (M01 RR00056 to M.S.K)
Footnotes
Disclosure/Conflict of Interest Statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–96. doi: 10.1093/cercor/bhp055. bhp055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: A systematic review. Neurosci Biobehav Rev. 2009;33:279–96. doi: 10.1016/j.neubiorev.2008.09.002. S0149-7634(08)00150-4 [pii] [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A, Honea RA, Straub RE, Pezawas L, Egan MF, Vakkalanka R, Kolachana B, Verchinski BA, Sust S, Mattay VS, Weinberger DR, Callicott JH. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27:1584–93. doi: 10.1523/JNEUROSCI.5112-06.2007. 27/7/1584 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. S1364-6613(06)00327-5 [pii] [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. 1124/1/1 [pii] [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci. 2009;29:1860–73. doi: 10.1523/JNEUROSCI.5062-08.2009. 29/6/1860 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–43. [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008a;29:828–38. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Eichele T, Pearlson G. Functional brain networks in schizophrenia: a review. Front Hum Neurosci. 2009;3:17. doi: 10.3389/neuro.09.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and “default” hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum Brain Mapp. 2008b;29:1265–75. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Kiehl KA, Astur R, Pekar JJ, Pearlson GD. A method for multitask fMRI data fusion applied to schizophrenia. Hum Brain Mapp. 2006;27:598–610. doi: 10.1002/hbm.20204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Becerra L, Pendse G, Chizh B, Tully S, Borsook D. Colocalized structural and functional changes in the cortex of patients with trigeminal neuropathic pain. PLoS ONE. 2008;3:e3396. doi: 10.1371/journal.pone.0003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. S1053-8119(06)00043-7 [pii] [DOI] [PubMed] [Google Scholar]
- Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–28. doi: 10.1016/j.brainres.2009.02.070. S0006-8993(09)00461-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Williams J, Gibbon M. Structured clinical interview for DSM-IV (SCID) American Psychiatric Association; Washington, DC: 1995. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. S089662730200569X [pii] [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–8. doi: 10.1073/pnas.0504136102. 0504136102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. S1053-8119(08)00728-3 [pii] [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortex and memory: emergence of a new paradigm. J Cogn Neurosci. 2009;21:2047–72. doi: 10.1162/jocn.2009.21280. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–7. doi: 10.1176/appi.ajp.164.3.450. 164/3/450 [pii] [DOI] [PubMed] [Google Scholar]
- Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS. Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry. 2001;158:618–24. doi: 10.1176/appi.ajp.158.4.618. [DOI] [PubMed] [Google Scholar]
- Goghari VM, Rehm K, Carter CS, MacDonald AW. Sulcal thickness as a vulnerability indicator for schizophrenia. Br J Psychiatry. 2007;191:229–33. doi: 10.1192/bjp.bp.106.034595. 191/3/229 [pii] [DOI] [PubMed] [Google Scholar]
- Gur RC, Turetsky BI, Loughead J, Waxman J, Snyder W, Ragland JD, Elliott MA, Bilker WB, Arnold SE, Gur RE. Hemodynamic responses in neural circuitries for detection of visual target and novelty: An event-related fMRI study. Hum Brain Mapp. 2007;28:263–74. doi: 10.1002/hbm.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Calhoun VD. Functional classification of schizophrenia using feed forward neural networks. Conf Proc IEEE Eng Med Biol Soc Suppl. 2006:6631–4. doi: 10.1109/IEMBS.2006.260906. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001. S1053-8119(07)01028-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, Picchioni M, Toulopoulou T, McGuire P. White matter microstructure in schizophrenia: effects of disorder, duration and medication. Br J Psychiatry. 2009;194:236–42. doi: 10.1192/bjp.bp.108.054320. 194/3/236 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Spencer SM, Harenski KA, Luna B, Sweeney JA. A preliminary functional magnetic resonance imaging study in offspring of schizophrenic parents. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1143–9. doi: 10.1016/s0278-5846(02)00249-x. S0278-5846(02)00249-X [pii] [DOI] [PubMed] [Google Scholar]
- Kida I, Hyder F. Physiology of functional magnetic resonance imaging: energetics and function. Methods Mol Med. 2006;124:175–95. doi: 10.1385/1-59745-010-3:175. [DOI] [PubMed] [Google Scholar]
- Koski L, Paus T. Functional connectivity of the anterior cingulate cortex within the human frontal lobe: a brain-mapping meta-analysis. Exp Brain Res. 2000;133:55–65. doi: 10.1007/s002210000400. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Perez-Iglesias R, Woolley JB, Kanaan RA, Vyas NS, Barker GJ, Frangou S, McGuire PK. Effect of age at onset of schizophrenia on white matter abnormalities. Br J Psychiatry. 2009;195:346–53. doi: 10.1192/bjp.bp.108.055376. 195/4/346 [pii] [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Miller P, Best JJ, Owens DG, Johnstone EC. Temporal lobe volume changes in people at high risk of schizophrenia with psychotic symptoms. Br J Psychiatry. 2002;181:138–43. doi: 10.1017/s0007125000161860. [DOI] [PubMed] [Google Scholar]
- Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353:30–3. doi: 10.1016/S0140-6736(98)06244-8. S0140-6736(98)06244-8 [pii] [DOI] [PubMed] [Google Scholar]
- Lv YT, Yang H, Wang DY, Li SY, Han Y, Zhu CZ, He Y, Tang HH, Gong QY, Zang YF. Correlations in spontaneous activity and gray matter density between left and right sensoritmotor areas of pianists. Neuroreport. 2008;19:631–4. doi: 10.1097/WNR.0b013e3282fa6da0. 00001756-200804160-00006 [pii] [DOI] [PubMed] [Google Scholar]
- Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2009 doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–88. doi: 10.1016/j.neuroimage.2007.05.019. S1053-8119(07)00409-0 [pii] [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. 315/5810/393 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–98. doi: 10.1016/s0361-9230(00)00437-8. S0361-9230(00)00437-8 [pii] [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead WJ, Harrison LK, Whalley HC, Johnstone EC, Lawrie SM. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- Meda SA, Bhattarai M, Morris NA, Astur RS, Calhoun VD, Mathalon DH, Kiehl KA, Pearlson GD. An fMRI study of working memory in first-degree unaffected relatives of schizophrenia patients. Schizophr Res. 2008;104:85–95. doi: 10.1016/j.schres.2008.06.013. S0920-9964(08)00291-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27:753–70. doi: 10.1016/j.neuroimage.2005.05.024. S1053-8119(05)00323-X [pii] [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Mitelman SA, Newmark R, Trisdorfer R, Haznedar MM, Koenigsberg HW, Flory J, Siever LJ. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–40. doi: 10.1038/sj.npp.1301283. 1301283 [pii] [DOI] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A. Cognitive and emotional modulation of brain default operation. J Cogn Neurosci. 2009;21:1065–80. doi: 10.1162/jocn.2009.21086. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee JD, Chun JW, Seok JH, Yun M, Oh MK, Kim JJ. Cortical surface-based analysis of 18F-FDG PET: measured metabolic abnormalities in schizophrenia are affected by cortical structural abnormalities. Neuroimage. 2006;31:1434–44. doi: 10.1016/j.neuroimage.2006.02.001. S1053-8119(06)00100-5 [pii] [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Petty RG, Ross CA, Tien AY. Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology. 1996;14:1–17. doi: 10.1016/S0893-133X(96)80054-6. S0893-133X(96)80054-6 [pii] [DOI] [PubMed] [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, Guerrero A, Ortiz-Gil J, Sans-Sansa B, Capdevila A, Cebamanos JM, McKenna PJ. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–93. doi: 10.1017/S0033291708003565. S0033291708003565 [pii] [DOI] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, Manoach DS, Belger A, Diaz M, Wible CG, Ford JM, Mathalon DH, Gollub R, Lauriello J, O’Leary D, van Erp TG, Toga AW, Preda A, Lim KO. Working memory and DLPFC inefficiency in schizophrenia: the FBIRN study. Schizophr Bull. 2009;35:19–31. doi: 10.1093/schbul/sbn162. sbn162 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Pearlson GD. Schizophrenia, the heteromodal association neocortex and development: potential for a neurogenetic approach. Trends Neurosci. 1996;19:171–6. doi: 10.1016/s0166-2236(96)10022-9. S0166223696100229 [pii] [DOI] [PubMed] [Google Scholar]
- Sabsevitz DS, Medler DA, Seidenberg M, Binder JR. Modulation of the semantic system by word imageability. Neuroimage. 2005;27:188–200. doi: 10.1016/j.neuroimage.2005.04.012. S1053-8119(05)00255-7 [pii] [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Baeza I, Perez-Gomez M, Vendrell P, Junque C, Bargallo N, Bernardo M. Sustained attention impairment correlates to gray matter decreases in first episode neuroleptic-naive schizophrenic patients. Neuroimage. 2003;19:365–75. doi: 10.1016/s1053-8119(03)00094-6. S1053811903000946 [pii] [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. S1053811904001880 [pii] [DOI] [PubMed] [Google Scholar]
- Spreng RN, Grady CL. Patterns of Brain Activity Supporting Autobiographical Memory, Prospection, and Theory-of-Mind and Their Relationship to the Default Mode Network. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21282. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Calhoun VD. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum Brain Mapp. 2009;30:2356–66. doi: 10.1002/hbm.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008;50:569–81. doi: 10.1007/s00234-008-0383-9. [DOI] [PubMed] [Google Scholar]
- Verduzco-Flores S, Bodner M, Ermentrout B, Fuster JM, Zhou Y. Working memory cells’ behavior may be explained by cross-regional networks with synaptic facilitation. PLoS ONE. 2009;4:e6399. doi: 10.1371/journal.pone.0006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Wang C, Ulbert I, Schomer DL, Marinkovic K, Halgren E. Responses of human anterior cingulate cortex microdomains to error detection, conflict monitoring, stimulus-response mapping, familiarity, and orienting. J Neurosci. 2005;25:604–13. doi: 10.1523/JNEUROSCI.4151-04.2005. 25/3/604 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu X, Guise KG, Knight RT, Ghajar J, Fan J. Effective Connectivity of the Fronto-parietal Network during Attentional Control. J Cogn Neurosci. 2009 doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Harris JC, Lawrie SM. The neurobiological underpinnings of risk and conversion in relatives of patients with schizophrenia. Int Rev Psychiatry. 2007;19:383–97. doi: 10.1080/09540260701496869. 781048804 [pii] [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Marshall I, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128:2097–108. doi: 10.1093/brain/awh556. awh556 [pii] [DOI] [PubMed] [Google Scholar]
- Whalley HC, Mowatt L, Stanfield AC, Hall J, Johnstone EC, Lawrie SM, McIntosh AM. Hypofrontality in subjects at high genetic risk of schizophrenia with depressive symptoms. J Affect Disord. 2008;109:99–106. doi: 10.1016/j.jad.2007.11.009. S0165-0327(07)00396-5 [pii] [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127:478–90. doi: 10.1093/brain/awh070. awh070 [pii] [DOI] [PubMed] [Google Scholar]
- Whalley HC, Gountouna VE, Hall J, McIntosh AM, Simonotto E, Job DE, Owens DG, Johnstone EC, Lawrie SM. fMRI changes over time and reproducibility in unmedicated subjects at high genetic risk of schizophrenia. Psychol Med. 2009;39:1189–99. doi: 10.1017/S0033291708004923. S0033291708004923 [pii] [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Moorhead W, McIntosh A, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. Functional imaging as a predictor of schizophrenia. Biol Psychiatry. 2006;60:454–62. doi: 10.1016/j.biopsych.2005.11.013. S0006-3223(05)01432-0 [pii] [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. 0809141106 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte MC, Whalley HC, Simonotto E, Flett S, Shillcock R, Marshall I, Goddard NH, Johnstone EC, Lawrie SM. Event-related fMRI of word classification and successful word recognition in subjects at genetically enhanced risk of schizophrenia. Psychol Med. 2006;36:1427–39. doi: 10.1017/S0033291706008178. S0033291706008178 [pii] [DOI] [PubMed] [Google Scholar]
- Woodward ND, Waldie B, Rogers B, Tibbo P, Seres P, Purdon SE. Abnormal prefrontal cortical activity and connectivity during response selection in first episode psychosis, chronic schizophrenia, and unaffected siblings of individuals with schizophrenia. Schizophr Res. 2009;109:182–90. doi: 10.1016/j.schres.2008.11.028. S0920-9964(08)00561-6 [pii] [DOI] [PubMed] [Google Scholar]
- Wu SS, Chang TT, Majid A, Caspers S, Eickhoff SB, Menon V. Functional heterogeneity of inferior parietal cortex during mathematical cognition assessed with cytoarchitectonic probability maps. Cereb Cortex. 2009;19:2930–45. doi: 10.1093/cercor/bhp063. bhp063 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti MV, Schaufelberger MS, de Castro CC, Menezes PR, Scazufca M, McGuire PK, Murray RM, Busatto GF. White-matter hyperintensities in first-episode psychosis. Br J Psychiatry. 2008;193:25–30. doi: 10.1192/bjp.bp.107.038901. 193/1/25 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. S0920-9964(07)00239-3 [pii] [DOI] [PubMed] [Google Scholar]