Summary
Background
Secretions within the adult female reproductive tract mediate sperm survival, storage, activation and selection. Drosophila female reproductive gland secretory cells reside within the adult spermathecae and parovaria, but their development remains poorly characterized.
Results
With cell-lineage tracing, we found that precursor cells down-regulate lozenge and divide sterotypically to generate three-cell secretory units during pupal development. The NR5A-class nuclear hormone receptor Hr39 is essential for precursor cell division and secretory unit formation. Moreover, ectopic Hr39 in multiple tissues generates reproductive gland-like primordia. Rarely, in male genital discs these primordia can develop into sperm-filled testicular spermathecae.
Conclusion
Drosophila spermathecae provide a powerful model for studying gland development. Hr39 functions as a master regulator of a program that may have been conserved throughout animal evolution for the production of female reproductive glands and other secretory tissues.
Keywords: Hr39, Lz, LRH-1, spermathecae, reproductive tract, gland, steroid hormone
Introduction
In species where fertilization takes place internally, including mammals and insects, the penetration of an egg only culminates a sperm’s long and obstacle-filled journey through the female reproductive tract. Prior to reaching its target, both paternal [1] and maternal [2–4] reproductive tissues deploy mechanisms that strongly influence an individual sperm’s chances for success. In particular, specialized glands in female reproductive tracts produce mucus-rich secretions that capacitate sperm to fertilize successfully, inhibit infection, and provide nutritional, maintenance, and storage factors. The interactions of sperm and seminal fluid with the female reproductive tract and its secretions in Drosophila offer an opportunity to genetically analyze these complex processes [3, 5–7].
Two paired glands, spermathecae (SPs) and parovaria (POs), are the primary sources of secretions encountered by sperm within the Drosophila female reproductive tract (Fig. 1A). mRNAs encoding serine proteases, serpins, anti-oxidants, immune proteins, and enzymes involved in mucus production are found in SPs [5, 8–10]. While two SPs arise from the engrailed− (en−) and en+ domains of the A8 segment, both POs originate in the en+ domain of the A9 segment in the female genital disc during pupal development (Fig. 1B–C) [11, 12]. Both types of mature gland contain large, polyploid secretory cells (SCs). Each SC connects with the gland lumen via a specialized cuticular canal equipped with a secretion-collecting “end apparatus” (Fig. 1D) [13]. Anatomically related secretory units are found in SPs from other species [14] and in insect epidermal glands that produce pheromones, venoms, and many other products [15, 16]. Despite their ubiquity, insect epidermal gland development has not been well characterized at the molecular genetic level.
Figure 1. Structure and origin of Drosophila female reproductive glands.
(A) Diagram of the female lower reproductive tract showing the paired SPs (yellow) and POs (purple). (B) Diagram of the dorsal view of an early pupal genital disc (8 h APF) showing the location of SP and PO primordia relative to zones of En and Wg expression in the A8 and A9 segments. (C) Time course of reproductive gland (green) protrusion and morphogenesis during pupation. Diagrams show dorsal-lateral view of the genital discs. (D) Diagram showing a SP in cross section revealing the duct and the gland lumen, lined by chitin, underlying epithelial cells (green) and numerous secretory units consisting of a gland cell (purple) connected to the lumen by a chitinous canal (tan). Gland cell secretions are released via abundant microvilli into an “end apparatus (EA),” a specialized collecting zone of the canal.
Studies of genital disc development and patterning [17, 18] have identified multiple genes important for reproductive gland formation. lozenge (lz), encoding a runt-domain transcription factor, is essential for both SP and PO formation [19] and may be directly regulated by the sex determination pathway [20]. Homologous to mammalian AML-1, Lz also supports developing blood precursors and prepatterns ommatidial cells in the developing eye [21]. The dachshund (dac) gene also acts in multiple imaginal discs and is specifically needed for spermathecal duct development [22]. Mutations that disrupt sphingolipid metabolism [23] also cause abnormalities in spermathecal number and structure.
One of the most interesting genes needed to form reproductive glands encodes the nuclear hormone receptor Hr39 [5]. Hr39 and Ftz-f1 are the only two NR5A class nuclear hormone receptors in Drosophila, a class that in mammals includes Steroidogenic factor 1 (SF-1) and Liver receptor homolog 1 (LRH-1). All four of these proteins share 60–90% sequence identity within their DNA binding domains, and bind in vitro to identical sequences. SF-1 is a master regulator of steroidogenesis and sex hormone production [24], while LRH-1 is required in the ovary for female fertility [25], in ES cells for pluripotency [26, 27], and in endodermal tissues for metabolic homeostasis [28, 29]. Weak Hr39 mutations alter the production of some SP gene products [5], while LRH-1 directly controls major secretory proteins of the exocrine pancreas [30]. Thus, NR5A class hormone receptors may play a conserved role controlling secretions from certain tissues, including female reproductive glands.
Here, we characterize the cell lineage of developing reproductive glands and clarify the roles of lz and Hr39. Hr39 is expressed sex-specifically in lz-positive female gland primordia beginning shortly after the ecdysone pulse that initiates prepupal development. When levels of Hr39 are reduced, lz-expressing precursors fail to protrude, divide or remain viable, suggesting that Hr39 expression orchestrates reproductive gland development. Mouse LRH-1, but not SF-1, can partially replace Hr39 function in gland formation. Ectopic expression of Hr39 in male larvae can induce a pigmented SP-like structure containing sperm to develop in the male reproductive tract. We propose that Hr39 acts as a master regulator of reproductive gland development and that the production of sperm-interacting proteins in the female reproductive tract under the control of NR5A proteins has been conserved during evolution. These findings suggest new targets for controlling agriculture pests and human-disease vectors.
Results
Reproductive glands develop from imaginal precursors marked by lz expression
We initially investigated reproductive gland development at the cellular level using gene expression markers. With a lz-Gal4 driven UAS-GFP transgene [31], lz expression was first detected in the genital imaginal disc about 0 hour after puparium formation (APF; Fig. S1A–C). Expression occurs within the four gland precursor domains, as evidenced by their location relative to en expression and by the protrusion of lz+ cells at 8 h APF (Fig. 2A). The same expression pattern was seen using a specific anti-Lz antibody (Fig S1A–C), except that lz-GAL4/UAS-GFP expression, but not antibody staining, variegated. By contrast, no lz expression was seen in similarly aged male genital discs (data not shown), consistent with a recent report [20]. Thus, lz expression can be used to precisely mark early female reproductive gland precursor populations and to investigate how subsequent gland development takes place.
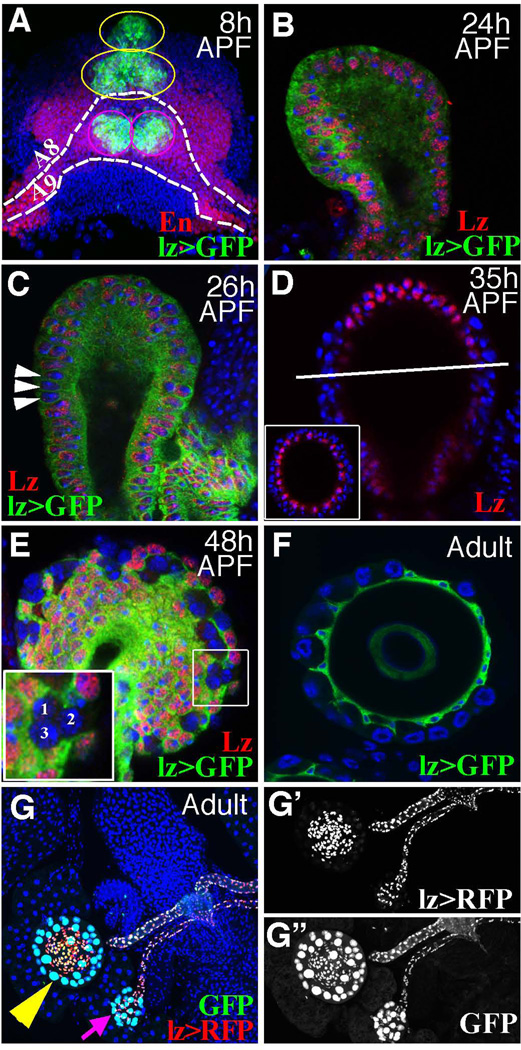
Figure 2. Reproductive glands originate from lz-expressing cells in the female genital disc.
(A) The dorsal view of a female genital disc at 8 h APF showing En (red) and lz expression (green; lz>GFP = lz-Gal4::UAS-GFP) to reveal the four reproductive gland primordia (green cells). Precursors of the two SPs straddle the midline in the anterior and posterior regions of the A8 segment (yellow circles). PO precursors lie laterally off the midline within the posterior domain of the A9 segment (magenta circles). (B–C) A sagittal section of spermathecal head with lz expression (green) and Lz antibody staining (red). Cells lacking Lz (arrowheads) arise basally between 24–26 h APF. (D) Cells at the top of the spermathecal head continue to express Lz (red) at 35 h APF; sagital section, inset: cross section (white bar). (E) By 48 h APF, basal clusters each contain three lz− cells (see inset). (F) lz is expressed in epithelial cells but not SCs of adult SPs. (G–G”) Adult SP (yellow arrowhead) and PO (magenta arrow) epithelial and duct cells express lz (RFP in red), whereas lineage tracing (GFP, Methods) shows that all SP and PO cells, including SCs, but not other cells of the female reproductive tract, derive from lz+ progenitors. Blue = DAPI staining of DNA in this and all subsequent figures. See also Figure S1.
Two subpopulations of cells emerge within both SP and PO gland heads at about 24 h APF when they become multi-layered (Fig. 2B). Many basally located cells lose lz expression beginning at 26 h APF (Fig 2C). By 35 h APF, individual lz− cells ring the central part of the spermathecal head basally, while apical cells continue to express lz (Fig 2D). Cells around the top of the spermathecal head (where secretory cells do not form) and all duct cells remain lz+. This suggests that the new lz− cells correspond to secretory unit precursors (SUPs). By 48 h APF lz− cells in both SPs and POs have become organized into three-cell clusters separated by lz+ epithelial cells (Fig 2E). Luminal and ductal epithelial cells, but not secretory cells, express lz in adult female reproductive glands (Fig 2F), consistent with the idea that lz shuts off in secretory precursors,
Secretory cell progenitors downregulate lz and proliferate more than other gland cells
We used a dual lineage tracing system [32] that marks ongoing lz expression (with RFP) and the lineal derivatives of all lz-expressing cells (with GFP) to confirm these conclusions (Fig. 2G). GFP was detected in all reproductive gland cells, including secretory cells (Fig. 2G”), but not in any other genital disc derivatives, which verified that lz+ cells in the female genital disc give rise to all gland cells, but to no other disc-derived parts of the reproductive tract. Only gland epithelial and ductal cells expressed RFP (Fig. 2G). Thus, all female reproductive gland cells derive from lz+ cells, but lz expression is down-regulated in the secretory cell lineage.
Secretory cells were much more dependent on cell division than other gland cells. We used lz-GAL4 to drive RNAi constructs that target the key cell cycle genes cycA, cdc2 or stg to block cell proliferation (Fig. S1D–F). The stunted SPs that resulted from knocking down either cycA or cdc2 virtually lack secretory cells, but the dimensions of the lumen were only reduced by 25% and the ducts were only shortened by half (Fig. S1G–I). Thus, secretory cell production entirely depends on precursor cell proliferation, while luminal and duct cell number is only augmented two fold or less after puparium formation.
Secretory cells differentiate as part of three-cell secretory units with a defined cell lineage
Detailed insight into post-larval divisions was obtained from further lineage analysis. A few cycling cells were marked randomly at specific times by transiently inducing FLP via a brief heat shock, and the daughters of these marked cells were mapped at various intervals thereafter (Fig 3A). The low labeling frequency of less than one clone per gland ensured that each cluster of labeled cells derived from a single progenitor. We categorized the clones in the gland head into SC or non-SC clones (containing or not containing a secretory cell respectively; Fig. 3B). As expected from the results of blocking cell division, non-SC clones induced at 14 h APF contained fewer than 1.5 cells on average and consisted of either one or two lz+ epithelial cells adjacent to the lumen (Fig. 3B–C). Cells giving rise to such clones were termed “luminal epithelial precursors” (LEPs; Fig. 3L). Clones of epithelial cells were only observed infrequently, and contained just one cell when inductions were carried out at 24 h and scored at 72 h APF (abbreviated 24–72h), indicating that most LEPs cease dividing by 24 h APF.
Figure 3. Lineage analysis of secretory unit formation.
(A) Schematic lineage experiments in which marked clones were induced by heat shock (“labeling”) and scored (“score”) later in pupal development. (B) Mean clone sizes in seven treatment groups. Each group is summarized on the x-axis by “L–S” which indicates clone induction at time L and scoring at time S (in h AFP). The average clone size is plotted (± standard deviation) separately for SC (blue) and no-SC (red) clones. The number of clones analyzed is above in parentheses. (C) An example of a SC (bottom circle) and a non-SC (upper circle) clone in 14–72 h APF treatment group. Clone marker β–Gal is in green and Lz staining in red. Note the Lz+ cell in the bottom circle is β–Gal-negative. AC: apical cell; BC: basal cell; SC: secretory cell. The boxed area in inset shows the clone location in the entire SP. (D–K) Examples of SC clones from the indicated treatment groups following staining with β–Gal (green). (L) A model showing the lineage of female reproductive gland. Cell divisions are shown on a developmental timeline (top) in hours after puparium formation (APF). Times of major events: orange- ecdysone pulses; light orange- high ecdysone level; green- precursor protrusion, proliferation, and specification; red- secretory unit production; blue- secretory unit maturation. See also Figure S2.
The behavior of SC clones was very different. 14–72h SC clones were larger, and almost always consisted of exactly three lz− cells (Fig 3C–D). These three-cell clones were highly structured and spatially ordered along the apical-basal axis. Usually, such clones have a small oval-shaped cell positioned adjacent to the lumen (the apical cell, AC). Next to the AC lies a large, polyploidy SC, while even more basally a third cell of intermediate size is present (the basal cell, BC; Fig 3D). Partner cells to the SC are lacking in adult glands. We verified the transient nature of ACs and BCs by further clonal analysis. In 24–72 h clones, all SC clones contained a typical three-cell unit (Fig. 3B). In 24–92 h clones, the number of labeled cells decreased to two (Fig. 3B), which always consisted of a SC and a BC (Fig. 3E). However, 24-adult clones contained only a single cell, the SC (Fig. 3B and 3F). These experiments precisely map the time of loss of both ACs (72–92 h APF) and BCs (92 h APF-one day of adulthood). Likewise, we determined the order of divisions giving rise to the three-cell units. 31–72h clones contained exactly two cells, one SC and one BC (Fig. 3G). In addition, a new class of single-cell, non-luminal, non-SC clones was also observed, likely corresponding to single ACs (Fig. 3B). In 42–72 h clones, no multi-cellular clones were obtained. These observations indicate a precise lineage defined by an initial division of the SUP, and a subsequent division of one of the daughters (pIIa; Fig. 3L). No evidence for division of the second daughter (pIIb) was found even in14–48h SC clones (Fig. 3B and H).
We next analyzed earlier clones to investigate whether SUP and LEP specification is coordinated. 8–72h clones that mark cells upstream from the SUP fell into three categories (Fig 3I–K). Clones of four- or five-cells contained a typical three-cell SC unit (from one SUP) plus one or two cells whose high β–Gal expression marked them as epithelial cells outside the secretory lineage (Fig 3I–J). Thus, more than 70% of the time (N = 14), the precursor of the SUP undergoes a differential division resulting in a lz+ LEP and a lz− SUP (Fig. 3L). However, the remaining cases consisted of six-cell clones made up of two typical three-cell SC-containing units (Fig 3K). Overall clone induction was too infrequent for these to be adjacent, independent three-cell clones. Thus, about 30% of the time a symmetric division generates two daughter SUPs.
During SP formation in some insects, a cilium from one of the accessory cells is reported to participate in canal formation during secretory unit morphogenesis [33]. To investigate whether Drosophila secretory units transiently utilize cilia, we carried out an electron microscopic study of the forming secretory units. No evidence of cilia was observed (data not shown). Furthermore, secretory cells formed normally within flies in which the ciliary genes unc or Pkd2 were knocked down using actin-GAL4 driven RNAi constructs (Fig. S2A–B). The constructs were effective since males of the same genotype were sterile, like unc and Pkd2 mutants [34–36].
Hr39 functions cell-autonomously in gland and secretory cell formation
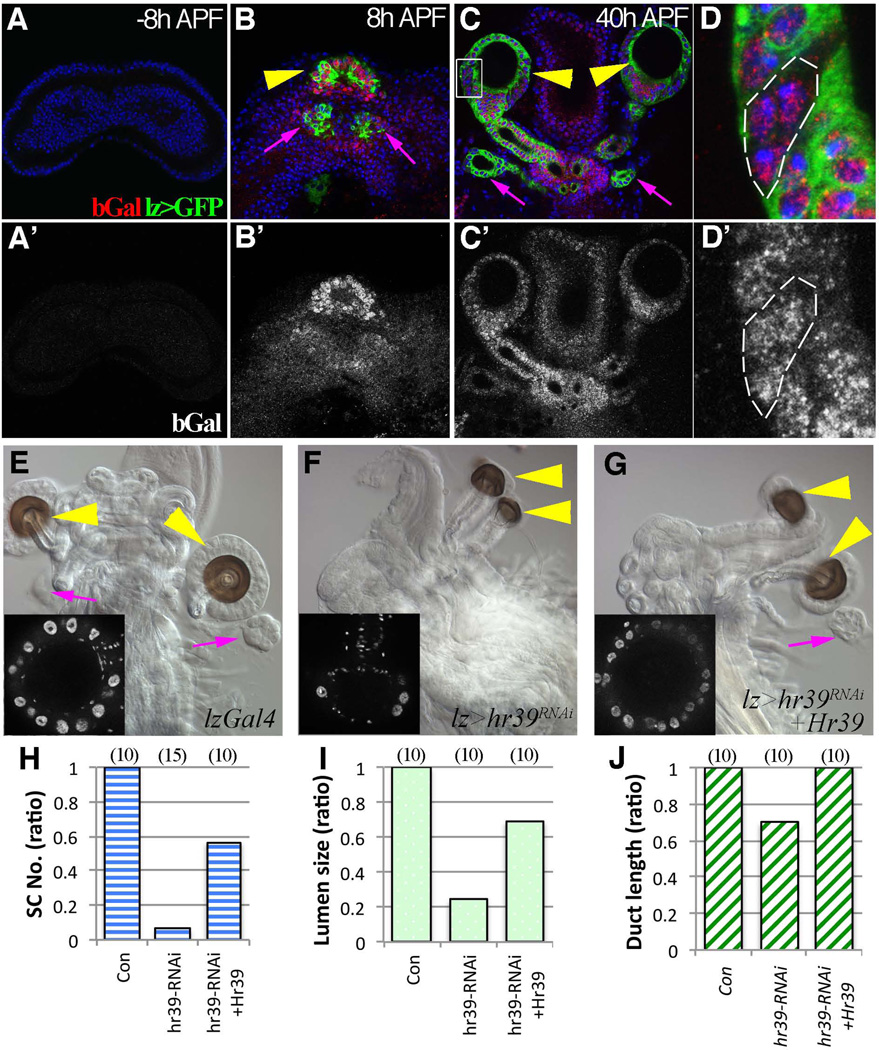
The finding that secretory cells arise from lz− precursors suggested that Hr39 might regulate their development. A good anti-Hr39 antibody is lacking and it has been difficult to follow Hr39 RNA expression within pupal imaginal tissue. To obtain greater sensitivity, we analyzed pupal Hr39-lacZ expression using an anti-β-Gal antibody that had been pre-absorbed against control imaginal discs lacking a lacZ gene to remove background. No Hr39 expression was detected in female genital discs from late larvae (Fig 4A). However, Hr39-lacZ expression is enriched in the spermathecal and parovarian domains defined by lz-GAL4::UAS-GFP expression at 8 h and 20 h APF (Fig. 4B and S3B). Lower level of expression was also detected in all other female genital disc cells (Fig. 4B’) and within the male genital disc inner layer (Fig S3A). At 40 h APF, Hr39 continued to be expressed in all reproductive gland cells, including the developing secretory units (Fig. 4C–D). Thus, unlike lz, Hr39 expression is not shut off within the secretory lineage.
Figure 4. Hr39 is expressed and required in gland progenitor cells.
(A–D’) Hr39 transcription indicated by β–Gal expression (red in A–D and white in A’–D’) in female genital discs at −8h APF (A), 8 h APF (B), or 40 h APF (C and D) with one copy of the enhancer trap (Hr39-lacZ). Hr39 transcription is highly enriched in the spermathecal (yellow arrowhead) and parovarian (magenta arrow) domains of genital discs at 8 h APF and 40 h APF. D is the higher magnification of boxed area in C showing a three-cell secretory unit with Hr39 expression (without lzGal4:: UASGFP, outlined). (E–G) DIC images showing adult female reproductive tract of lz-Gal4 control (E), Hr39 knock down (F), and Hr39 rescue (G) flies. Yellow arrowheads point to SP and magenta arrows point to PO. Inserts show the DAPI signal of the spermathecal head; the large bright DAPI signals are secretory cell nuclei. (H–J) Quantification of SC number (H), lumen area size (I), and duct length (J) in SPs of different genotypes. lz-Gal4 serves as a control. The number of females analyzed is in parentheses. See also Figure S3.
We depleted Hr39 function via lz-Gal4 driven RNAi to investigate if Hr39 functions directly within gland precursor cells. Parovaria did not form at all, while spermathecae were altered in structure (Fig 4E and 4F). Secretory cells were absent, lumen size was reduced five-fold, and duct length was about one third of normal (Fig 4H–J). Thus, Hr39 functions in early precursors of all gland cell types and is strongly required in secretory cells. These defects were largely reversed by simultaneously expressing Hr39 cDNA along with Hr39 RNAi (Fig. 4G), arguing that they represent specific Hr39 functions.
The ability of mammalian NR5A genes to substitute for Hr39 was also tested. Even in strong Hr39 mutant females, 32% still retain one or both SP ducts, and the frequency of such ducts is readily quantified (Fig. S3C–D). Duct formation in such animals was completely rescued by expressing Hr39 cDNA; glands also formed but were frequently abnormal, most likely due to altered temporal expression (Fig. S3E). When mouse LRH-1 cDNA was driven by lz-GAL4 in this background, SP duct formation was restored in more than 70% of females (Fig. S3F–G). Small gland lumens formed occasionally, and sometimes contained one or two SCs (Fig. S3F). In contrast, expressing mouse SF-1 cDNA using the same driver completely suppressed SP duct formation (Fig S3H).
Hr39 is required for protrusion, proliferation, and survival of lz+ precursors during early pupation
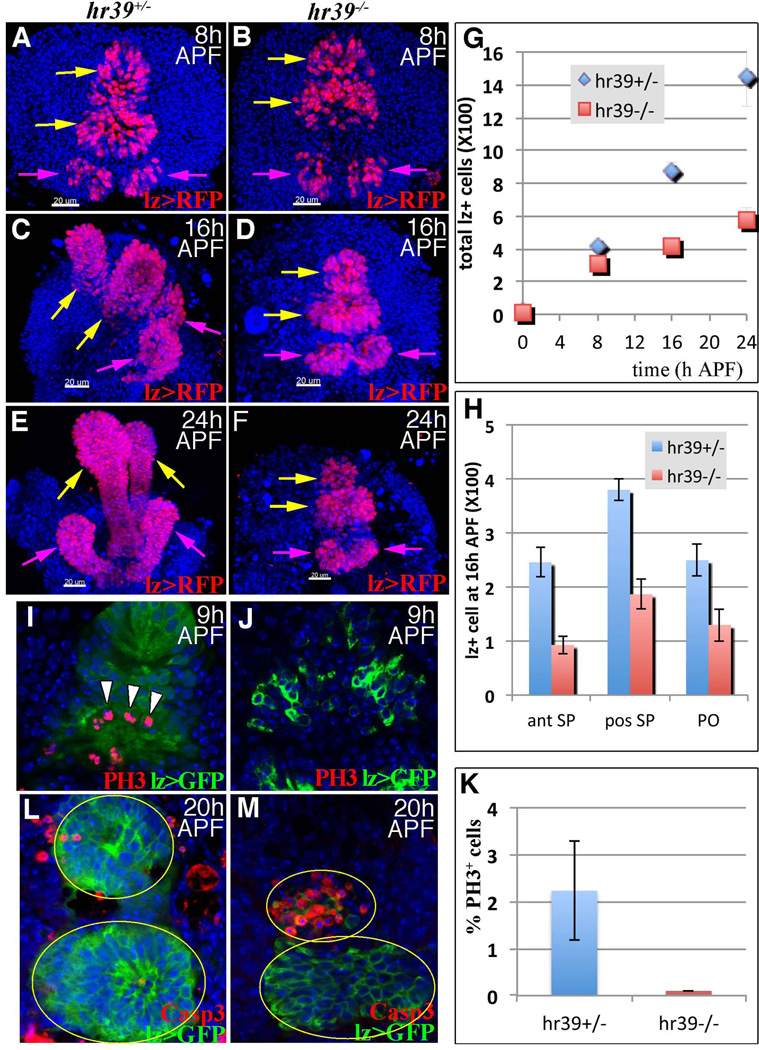
We analyzed the behavior of reproductive gland precursors in Hr39 mutant genital discs to better understand Hr39 function. Patterning genes were expressed normally in Hr39 mutant larval genital discs (Fig. S4) and lz-expressing gland precursors were present in normal numbers (Fig 5A–B), confirming that Hr39 neither patterns the disc nor establishes initial precursor pools. However, by 8 h APF Hr39 mutant gland precursors protruded much less than control (Fig 5B). At 16 and 24 h APF, wild-type but not Hr39 mutant gland precursors had rounded up and protruded extensively (Fig 5C–F). Moreover, the number of lz+ precursors was clearly reduced in Hr39 mutant discs by 16 h (Fig 5G) in both the spermathecal and parovarian domains (Fig 5H).
Figure 5. Hr39 mediates the protrusion, proliferation and survival of gland precursors.
(A–F) The dorsal-lateral view of female genital discs showing cells expressing lz (lz >RFP= lzGal4::UAS-nlsRFP) in pupal genital discs at the indicated times from control (A, C, and E) and Hr39 mutant (B, D, and F) females. SP (yellow arrows) and PO (magenta arrows) primordia are indicated. The scale bar = 20 µm. (G) The average number of total lz+ cell in female genital discs at different times is plotted. Cells from five animals /genotype /time point were counted. Error bar = standard deviation. (H) The number of lz+ cells in anterior (ant) and posterior (pos) SPs and PO domains at 16 h APF is plotted. (I–K) Dividing cells were labeled using phopho-histone H3 (PH3) staining (red) in control (I) or Hr39 mutant (J) genital discs and the percentage of PH3+ cells in lz+ cell group is plotted in (K). (L–M) Apoptotic cells were labeled using active Caspase 3 staining (Casp3, red) in control (L) and Hr39 mutant (M) genital discs at 20 h APF. See also Figure S4.
We measured the frequency of cell division to verify that Hr39 mutant gland precursors fail to proliferate. Staining of the M-phase marker phospho-histone H3 was readily detected at 9 h APF in lz+ precursors in control genital discs, but not in Hr39 mutant discs (Fig 5I–K). No apoptosis of lz+ cells was observed in either control or Hr39 mutant genital disc up to 16 h APF (data not shown). However, by 20 h APF, Hr39 mutant (but not control) cells within both the anterior spermathecal domain and the two parovarian domains began to undergo apoptosis (Fig 5L–M). By 48 h APF, all lz+ cells had disappeared from these domains in Hr39 mutants (data not shown). Therefore, Hr39 is required for the protrusion, proliferation, and survival of reproductive gland precursors prior to the differentiation of secretory cells.
Ectopic Hr39 is sufficient to stimulate protrusion and to generate spermatheca-like structures in the male reproductive tract
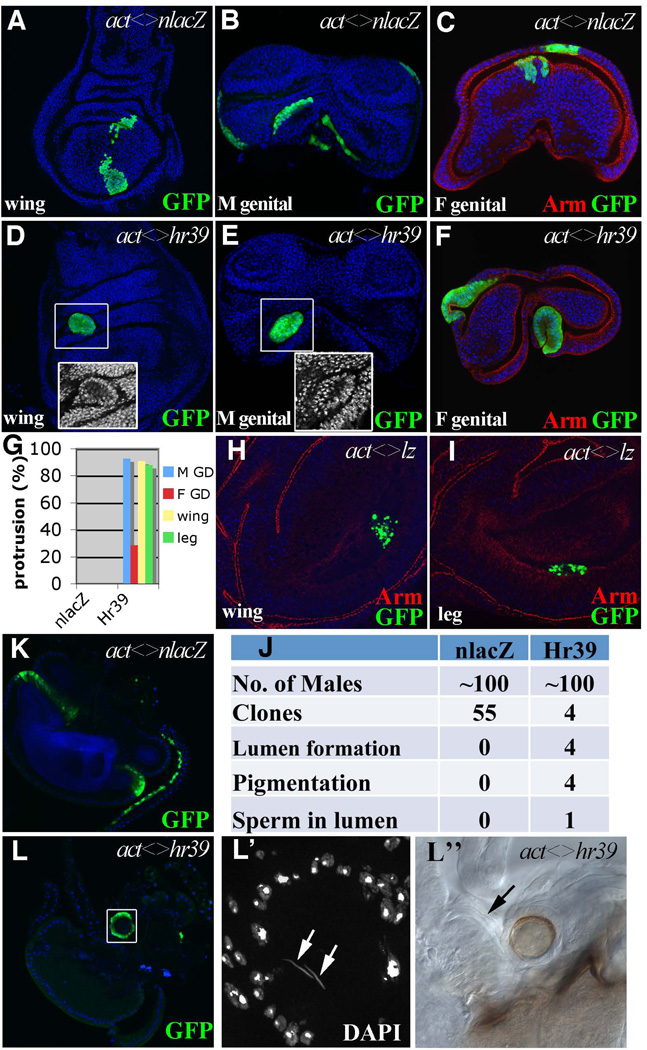
To test whether Hr39 is sufficient to trigger gland formation, we examined clones of marked cells that misexpress Hr39 in late larval imaginal discs. Interestingly, in contrast to control clones (Fig. 6A–C), Hr39-expressing clones in the wing, leg, eye-antennal, and genital discs developed a distinctive shape similar to early stages of gland protrusion (Fig 6D–F and data not shown). Not only did the cells bulge out from the disc, but they frequently developed a central lumen (Fig 6D–F). About 90% of clones in the wing, leg and male genital disc clones protruded. About 28% of similarly sized clones in the female genital disc protruded, but none of the control clones did so (Fig 6G). By contrast, misexpression of lz in clones within these same tissues did not cause similar protrusion or lumen formation (Fig. 6H–I). Unlike Hr39, lz misexpression was toxic and many cells died prior to pupation.
Figure 6. Hr39 mis-expression induces protrusion and ectopic spermatheca formation.
(A–F) Morphology of flip-out clones (marked by GFP) expressing nlacZ (A–C) or Hr39 (D–F) in wing (A and D), male (B and E) and female (C and F) genital discs at the late 3rd instar larval stage. Armadillo (Arm, red) highlights the apical side of the epithelia. Insets show higher magnification of DAPI signal in the boxed area. (G) Quantification of protruding clones of control and Hr39-misexpressing imaginal discs. 30–60 clones were analyzed in each disc. (H–I) Cells misexpressing Lz show apoptosis with fragmented GFP signals in wing (H) and leg (I) discs of 3rd instar larvae. (J) A summary of phenotypes of the Hr39-misexpressing clones in the adult male reproductive tract. (K) Control clones in adult male reproductive tract. Clones are in the ejaculatory bulb and duct. (L and L”) A Hr39-expressing clone in the male ejaculatory bulb shows a circular shape with a lumen in the center. L’ shows a higher magnification of the DAPI signal in the boxed area to highlight sperm nuclei (arrows) in the lumen. The lumen has brown pigmentation (DIC image in L”) with a tiny duct (arrow) connected to the ejaculatory bulb.
Hr39-misexpressing cells usually underwent apoptosis during pupal development. Clones in the wing, leg, and eye-antennal discs were never detected in corresponding adult structures. Surprisingly, however, a few clones induced in the male genital disc did survive and grow further. In three separate experiments, in which a total of 100 males were examined following clone induction, four examples of surviving adult clones were observed (Fig. 6J). Two were located near the junction between the ejaculatory duct and penis, one was loosely attached to the ejaculatory duct, and the fourth clone was near the ejaculatory bulb. The surviving cells were organized in a round structure (Fig. 6L), entirely different in appearance from control clones (Fig 6K). All four contained a central lumen covered by a brown-pigmented cell layer (Fig 6L”). Most strikingly, the clone at the ejaculatory bulb was connected to the bulb by a duct (Fig. 6L”, arrow), and sperm were present inside the lumen, as revealed by DAPI staining (Fig 6L’, arrowheads). The ejaculatory bulb in this male also contained sperm, which is not normally observed. The shape, pigmentation, and sperm content of the Hr39-induced structures were characteristic of spermathecae. Thus, clonal ectopic Hr39 expression in the male genital disc at low frequency generates a spermatheca-like structure in the male reproductive tract that when properly connected can attract and store sperm.
Discussion
The Drosophila spermatheca as a model insect gland
Our studies define the cellular events of reproductive gland formation and reveal that lz and Hr39, despite their nearly identical loss-of-function phenotypes, have distinctive expression patterns during gland development. All gland precursors express both genes following puparium formation, but within 24 hours divide to produce lz+ epithelial precursors apically and lz− SUPs basally. SUPs then differentiate according to a stereotyped program involving production of two transient accessory cells and a single polyploid secretory cell.
Our studies show that reproductive secretory cells arise in a superficially similar manner to sensory bristles and multiple classes of mechanosensory and chemosensory sensilla [37]. Both utilize short fixed cell lineages that employ transient accessory cells to generate permanent extracellular structures (secretory canal, sensory bristle, etc), but the three-cell secretory lineage analyzed here differs from the four asymmetric divisions producing five different cells typical of PNS differentiation [38]. Many other insect epidermal glands probably develop in a generally similar manner but the precise cell lineages and mechanisms documented here for Drosophila reproductive glands (3 cells, absence of ciliary involvement) differ from previous models [15].
Drosophila secretory units provide a powerful system for analyzing insect gland development. Studies in other insects suggested that an accessory cell utilizes a ciliary process to prevent the SCs from being sealed off by cuticle-secreting epithelial cells [14, 16, 33]. We found no morphological or genetic evidence that cilia are involved in forming Drosophila secretory units (Fig. S2A–B). However, the AC may fulfill this same role using normal microtubules, in much the same way that the anterior polar cells in egg chambers template the micropyle channel during oogenesis [39]. Membranes from the BC likely surround this AC process, secrete the cuticular canal, and join it to the luminal cuticle. Concomitantly, the BC likely secretes the end apparatus around a large apical segment of the SC, which it surrounds.
Hr39 regulates gland and secretory cell development at multiple levels
The NR5A hormone receptor Hr39 plays multiple roles in reproductive gland development. Initially, Hr39 orchestrates gland protrusion and in the absence of Hr39 protrusion fails to occur. Among Drosophila imaginal discs, gland protrusion in genital discs is a unique process that leads to the differentiation of a gland capsule connected to the nascent reproductive tract by a tubular duct. When Hr39 is misexpressed, patches of cells within multiple imaginal discs that do not normally express Hr39 undergo changes reminiscent of early protrusion.
Hr39, a known member of the ecdysone response pathway [41], is likely to time reproductive gland cell divisions during pupal development. The initial Hr39 expression we observed in the genital disc was detected shortly after the prepupal ecdysone pulse. Several additional peaks of ecdysone titer during pupal development [40] correspond closely with the timing we measured of the secretory cell divisions. These observations suggest that external hormonal signals rather than internal autonomous mechanisms sometimes drive precise cell lineages. In addition, to its requirement within cellular precursors, Hr39 mutations alter SP secretory gene mRNA levels [5], suggesting that Hr39 also regulates secretory gene expression within SCs.
Finally, Hr39 acts as a high level “master regulator” by integrating individual pathways to elicit the production of an entire gland. Most cells expressing ectopic Hr39 could not progress past the initial stage of eversion, but in male genital discs Hr39-positive clones sometimes generated integrated structures that strongly resembled small spermathecae. They contained round heads with lumens, a pigmented layer, and rarely were connected to the male reproductive tract by ducts through which sperm were taken up (Fig. 6L). Thus, Hr39 (but not lz) can reprogram male genital cells to generate ectopic spermathecae that likely synthesize and secrete products attractive to sperm.
Female reproductive glands differentiate from developmentally sensitive precursor pools
Drosophila reproductive gland development is unusually susceptible to perturbation. Rare adults in some wild strains contain an extra spermatheca, and females bearing weak alleles of either lz or Hr39 lose POs entirely and produce fewer SPs, which vary dramatically in size and cellular content [5, 19]. These effects probably result from the disparate sizes of the precursor pools for individual organs (Fig. 5). PO pools are very small, while the exceptionally large posterior SP primordium may easily split in two under conditions where precursor proliferation is perturbed. The effects of dac mutations on duct structure [22] are probably also due to altered precursor pools. Sphingolipids may affect gland development [24] by serving as endogenous Hr39 ligands, consistent with reports that SF-1 can bind sphingolipids [41, 42].
Development and function of female reproductive glands may be conserved
In mammals, sperm interact with female secretory products at multiple locations. Glands within the uterine endometrium are hypothesized to govern selective passage through the cervix, uterus, and subsequently, the utero-tubal junction [4]. Following entry into the oviduct, sperm induce and interact with the products of specialized tubal secretory cells that likely mediate capacitation [43]. In some species these products also allow sperm to be stored in the oviduct while retaining their ability to fertilize an egg [4]. Mammalian female reproductive glands continue to nurture preimplantation embryos and are likely essential for successful pregnancy [44].
Drosophila is emerging as a valuable model with which to study multiple aspects of reproductive physiology, some of which may have been conserved during evolution [3, 5–7]. The mouse lz homolog Aml1 (Runx1) is expressed in the Müllerian ducts and genital tubercle [45], but its role in fertility is unknown. The murine Hr39 homolog LRH-1 is required for female fertility [25], but whether it plays a role in reproductive gland secretion has yet to be tested. However LRH-1 is required for the development of several exocrine tissues [46], and in the pancreas is directly involved in the transcription of major secretory products [30]. Thus, LRH-1 and Hr39 may both govern the formation and secretory function of exocrine tissue.
Our studies provide further support for the idea that an NR5a-dependent program of secretory cell development has been conserved in evolution. Murine LRH-1 can partially replace Hr39 function in Drosophila reproductive gland formation. Similar rescue with two other NR5A members (mammalian SF-1 or Drosophila Ftz-F1) failed and instead, suppressed all gland formation (Fig S3F–H and data not shown). This is consistent with previous findings that Hr39 and Ftz-F1 have opposing roles in alcohol dehydrogenase and EcR expression [47, 48]. Antagonistic roles in gene regulation by the two NR5A family members may be evolutionarily conserved. Further study of the roles of Hr39 and LRH-1 should help define a fundamental program of secretory cell development that may be widely used.
Experimental Procedures
Drosophila genetics
Drosophila were reared on standard cornmeal-molasses food at 25 °C unless otherwise indicated. White prepupae (designated as 0 h APF) were collected over a 30-minute (min) period and aged to the desired stage. All stocks not otherwise indicated were from the Bloomington Drosophila Stock Center. UAS-mCD8:GFP, lz-Gal4 and lz-Gal4;UAS-GFP stocks [31] were used to map lz expression in genital discs by staining with anti-GFP antibodies. lz-Gal4 bearing flies were crossed to UAS-RedStinger, UAS-FLP, ubi<Stop<Stinger to monitor real-time expression and lineage expression of lz [32]. Clonal labeling was initiated by heat shocking (37 °C 60 min) pupae of genotype hsFLP; X-15-29/X-15-33 [49]. RNAi lines targeting cyclin A (V32421), cdc2 (V41838), stg (V17760), and Hr39 (V37694) were obtained from the Vienna Drosophila RNAi Center and used at 29 °C. Hr3907154, a strong loss-of-function allele caused by a lacZ enhancer trap insertion [5], was used to map Hr39 expression and is designated Hr39-lacZ. Unless otherwise denoted, Hr39 mutant animals were Hr3907154 homozygotes.
For clonal misexpression, hsFLP; act<CD2<Gal4, UAS-GFP [50] flies were crossed to either UAS-nlacZ or UAS-Hr39 [5]. To induce such clones, embryos aged from 16 h to 24 h after egg laying were heat-shocked for 45 min in a 37 °C water bath. For rescue experiments, UAS-mCD8:GFP, lz-Gal4; Hr3907154/CyO flies were crossed to 1) Hr3907154/CyO, 2) Hr3907154/CyO;UAS-Hr39,, 3) Hr3907154/CyO;UAS-mLRH-1 (a generous gift from Leslie Pick), or 4) Hr3907154/CyO;UAS-mSF-1 [51].
Immunostaining and microscopy
Larval and pupal imaginal discs and adult reproductive tracts were dissected in cold Grace’s medium, then fixed in 4% formaldehyde + 0.2% Triton X-100 for 20 min at room temperature. Tissues were vigorously shaken after fixation to loosen attached fat tissues, blocked in antibody buffer (PBS+ 0.3% Triton X-100 + 0.5% BSA+ 2% normal goat serum), and stained with primary antibodies overnight at 4 °C followed by secondary antibody staining. Chicken anti-β–Gal antibody and all secondary antibodies were preabsorbed to minimize background. Primary antibodies used: mouse anti-En (1:20), anti-Lz (1:15), anti-Arm (1:40), anti-Wg (1:30), anti-Dac (1:20) from Developmental Studies Hybridoma Bank; mouse anti-GFP (1:2000, Invitrogen), anti-phospho-H3 Ser10 (1:1000, Cell Signaling Technology); rabbit anti-GFP (1:4000, Invitrogen), anti-RFP (1:1000, MBL international), anti-cleaved Caspase 3 (1:100, Cell Signaling Technology); and chicken anti-β–Gal (1:1000, Abcam). The secondary antibodies were Alexa 488 or 568 goat anti-mouse, anti-rabbit, and anti-chicken antibodies (1:1000, Invitrogen). DAPI staining was at 5µg/ml for 10 minutes. All fluorescent images were acquired on a Leica TCS SP5 confocal microscope and DIC images on a Zeiss Axioimager ZI microscope. Images were assembled using Imaris 3D software, Image J, and Photoshop.
Quantitation of spermathecal cell number
Adult SCs within SPs were identified based on their elevated ploidy and distinctive nuclear morphology, and manually counted. Adult luminal or ductal epithelial cell numbers were estimated from the gland luminal area or duct length, respectively, based on a constant ratio of cells per unit area or length that was determined empirically. The total duct length and lumen area was calculated by using Image J software to acquire the maximal projection of the Z-series of DIC images. The number of lz+ cells in genital discs at particular pupal stages was determined using Imaris 3D software. lz+ cells within the anterior SP, posterior SP, or POs were distinguished using position filters and manual examination.
Supplementary Material
Highlights.
-
▪
Defines a stereotyped secretory lineage within female reproductive glands
-
▪
Delineates major roles nuclear receptor Hr39 plays during gland development
-
▪
Supports a conserved role for NR5A nuclear receptors in gland formation
ACKNOWLEDGEMENTS
We thank Bruce Baker and Audrey Christiansen for helpful discussion on genital disc development and lzGal4 expression; Leslie Pick for sending flies with UAS-mSF-1 and UAS-mLRH-1 constructs; Utpal Banerjee for sending lz alleles and sharing information about Lz antibody staining; Mike Sepanski for the EM study of the developmental secretory unit; Don Fox for technical discussion on pupal dissection; Becky Frederick, Ming-Chia Lee, Robert Levis, Vicki Losick, and Matt Sieber for comments on the manuscript. A.C.S. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests statement
The authors declare that they have no competing financial interests.
References
- 1.Avila FW, Sirot LK, LaFlamme BA, Rubinstein CD, Wolfner MF. Insect seminal fluid proteins: identification and function. Annu Rev Entomol. 2011;56:21–40. doi: 10.1146/annurev-ento-120709-144823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang MC. The meaning of sperm capacitation. A historical perspective. J Androl. 1984;5:45–50. doi: 10.1002/j.1939-4640.1984.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 3.Bloch Qazi MC, Heifetz Y, Wolfner MF. The developments between gametogenesis and fertilization: ovulation and female sperm storage in Drosophila melanogaster. Dev Biol. 2003;256:195–211. doi: 10.1016/s0012-1606(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 4.Holt WV, Fazeli A. The oviduct as a complex mediator of mammalian sperm function and selection. Mol Reprod Dev. 2010;77:934–943. doi: 10.1002/mrd.21234. [DOI] [PubMed] [Google Scholar]
- 5.Allen AK, Spradling AC. The Sf1-related nuclear hormone receptor Hr39 regulates Drosophila female reproductive tract development and function. Development. 2008;135:311–321. doi: 10.1242/dev.015156. [DOI] [PubMed] [Google Scholar]
- 6.Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 7.Heifetz Y, Rivlin PK. Beyond the mouse model: using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology. 2010;73:723–739. doi: 10.1016/j.theriogenology.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS. A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development. 2004;131:2007–2021. doi: 10.1242/dev.01077. [DOI] [PubMed] [Google Scholar]
- 9.Lawniczak MK, Begun DJ. Molecular population genetics of female-expressed mating-induced serine proteases in Drosophila melanogaster. Mol Biol Evol. 2007;24:1944–1951. doi: 10.1093/molbev/msm122. [DOI] [PubMed] [Google Scholar]
- 10.Prokupek AM, Kachman SD, Ladunga I, Harshman LG. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster. Insect Mol Biol. 2009;18:465–475. doi: 10.1111/j.1365-2583.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 11.Keisman EL, Christiansen AE, Baker BS. The sex determination gene doublesex regulates the A/P organizer to direct sex-specific patterns of growth in the Drosophila genital imaginal disc. Dev Cell. 2001;1:215–225. doi: 10.1016/s1534-5807(01)00027-2. [DOI] [PubMed] [Google Scholar]
- 12.Epper F. The Evagination of the Genital Imaginal Discs of. Drosophila melanogaster I. Morphogenesis of the Female Genital Disc. Roux's Arch Dev Biol. 1983;192:275–279. doi: 10.1007/BF00848660. [DOI] [PubMed] [Google Scholar]
- 13.Filosi M, Perotti ME. Fine structure of the spermatheca of Drosophila melanogaster Meig. J Submicr Cytol. 1975;7:259–270. [Google Scholar]
- 14.Lococo D, Huebner E. The development of the female accessory gland in the insect Rhodnius prolixus. Tissue Cell. 1980;12:795–813. doi: 10.1016/0040-8166(80)90030-0. [DOI] [PubMed] [Google Scholar]
- 15.Noirot C, Quennedey A. FINE STRUCTURE OF INSECT EPIDERMAL GLANDS. Ann Rev Entomol. 1974;19:61–80. [Google Scholar]
- 16.Büning J. The Insect Ovary. London: Chapman and Hall; 1994. p. 400. [Google Scholar]
- 17.Estrada B, Casares F, Sanchez-Herrero E. Development of the genitalia in Drosophila melanogaster. Differentiation. 2003;71:299–310. doi: 10.1046/j.1432-0436.2003.03017.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen EH, Christiansen AE, Baker BS. Allocation and specification of the genital disc precursor cells in Drosophila. Dev Biol. 2005;281:270–285. doi: 10.1016/j.ydbio.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Anderson RC. A Study of the Factors Affecting Fertility of Lozenge Females of Drosophila Melanogaster. Genetics. 1945;30:280–296. doi: 10.1093/genetics/30.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee SS, Uppendahl LD, Chowdhury MA, Ip PL, Siegal ML. The female-specific Doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development. 2011;138:1099–1109. doi: 10.1242/dev.055731. [DOI] [PubMed] [Google Scholar]
- 21.Canon J, Banerjee U. Runt and Lozenge function in Drosophila development. Semin Cell Dev Biol. 2000;11:327–336. doi: 10.1006/scdb.2000.0185. [DOI] [PubMed] [Google Scholar]
- 22.Keisman EL, Baker BS. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development. 2001;128:1643–1656. doi: 10.1242/dev.128.9.1643. [DOI] [PubMed] [Google Scholar]
- 23.Phan VH, Herr DR, Panton D, Fyrst H, Saba JD, Harris GL. Disruption of sphingolipid metabolism elicits apoptosis-associated reproductive defects in Drosophila. Dev Biol. 2007;309:329–341. doi: 10.1016/j.ydbio.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schimmer BP, White PC. Minireview: steroidogenic factor 1: its roles in differentiation, development, and disease. Mol Endocrinol. 2010;24:1322–1337. doi: 10.1210/me.2009-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggavathi R, Volle DH, Mataki C, Antal MC, Messaddeq N, Auwerx J, Murphy BD, Schoonjans K. Liver receptor homolog 1 is essential for ovulation. Genes Dev. 2008;22:1871–1876. doi: 10.1101/gad.472008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo G, Smith A. A genome-wide screen in EpiSCs identifies Nr5a nuclear receptors as potent inducers of ground state pluripotency. Development. 2010;137:3185–3192. doi: 10.1242/dev.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, Orlov YL, Huss M, Yang L, Lufkin T, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010;6:167–174. doi: 10.1016/j.stem.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Lee JM, Lee YK, Mamrosh JL, Busby SA, Griffin PR, Pathak MC, Ortlund EA, Moore DD. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–510. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Front Biosci. 2008;13:5950–5958. doi: 10.2741/3128. [DOI] [PubMed] [Google Scholar]
- 30.Holmstrom SR, Deering T, Swift GH, Poelwijk FJ, Mangelsdorf DJ, Kliewer SA, Macdonald RJ. LRH-1 and PTF1-L coregulate an exocrine pancreas-specific transcriptional network for digestive function. Genes Dev. 2011;25:1674–1679. doi: 10.1101/gad.16860911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crew JR, Batterham P, Pollock JA. Developing compound eye in lozenge mutants of Drosophila: lozenge expression in the R7 equivalence group. Dev Genes Evol. 1997;206:481–493. doi: 10.1007/s004270050079. [DOI] [PubMed] [Google Scholar]
- 32.Evans CJ, Olson JM, Ngo KT, Kim E, Lee NE, Kuoy E, Patananan AN, Sitz D, Tran P, Do MT, et al. G-TRACE: rapid Gal4-based cell lineage analysis in Drosophila. Nat Methods. 2009;6:603–605. doi: 10.1038/nmeth.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sreng L, Quennedey A. Role of a temporary ciliary structure in the morphogenesis of insect glands. An electron microscope study of the tergal glands of male Blattella germanica L. (Dictyoptera, Blattellidae) J Ultrastruct Res. 1976;56:78–95. doi: 10.1016/s0022-5320(76)80142-6. [DOI] [PubMed] [Google Scholar]
- 34.Baker JD, Adhikarakunnathu S, Kernan MJ. Mechanosensory-defective, male-sterile unc mutants identify a novel basal body protein required for ciliogenesis in Drosophila. Development. 2004;131:3411–3422. doi: 10.1242/dev.01229. [DOI] [PubMed] [Google Scholar]
- 35.Gao Z, Ruden DM, Lu X. PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol. 2003;13:2175–2178. doi: 10.1016/j.cub.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 36.Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol. 2004;269:1–17. doi: 10.1016/j.ydbio.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Orgogozo V, Schweisguth F, Bellaiche Y. Lineage, cell polarity and inscuteable function in the peripheral nervous system of the Drosophila embryo. Development. 2001;128:631–643. doi: 10.1242/dev.128.5.631. [DOI] [PubMed] [Google Scholar]
- 39.Montell DJ, Rorth P, Spradling AC. slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell. 1992;71:51–62. doi: 10.1016/0092-8674(92)90265-e. [DOI] [PubMed] [Google Scholar]
- 40.Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev Biol. 1982;93:73–82. doi: 10.1016/0012-1606(82)90240-8. [DOI] [PubMed] [Google Scholar]
- 41.Urs AN, Dammer E, Kelly S, Wang E, Merrill AH, Jr, Sewer MB. Steroidogenic factor-1 is a sphingolipid binding protein. Mol Cell Endocrinol. 2007;265–266:174–178. doi: 10.1016/j.mce.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Urs AN, Dammer E, Sewer MB. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- 43.Georgiou AS, Snijders AP, Sostaric E, Aflatoonian R, Vazquez JL, Vazquez JM, Roca J, Martinez EA, Wright PC, Fazeli A. Modulation of the oviductal environment by gametes. J Proteome Res. 2007;6:4656–4666. doi: 10.1021/pr070349m. [DOI] [PubMed] [Google Scholar]
- 44.Dunlap KA, Filant J, Hayashi K, Rucker EB, 3rd, Song G, Deng JM, Behringer RR, DeMayo FJ, Lydon J, Jeong JW, et al. Postnatal deletion of Wnt7a inhibits uterine gland morphogenesis and compromises adult fertility in mice. Biol Reprod. 2011;85:386–396. doi: 10.1095/biolreprod.111.091769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simeone A, Daga A, Calabi F. Expression of runt in the mouse embryo. Dev Dyn. 1995;203:61–70. doi: 10.1002/aja.1002030107. [DOI] [PubMed] [Google Scholar]
- 46.Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14:250–260. doi: 10.1016/j.tcb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Boulanger A, Clouet-Redt C, Farge M, Flandre A, Guignard T, Fernando C, Juge F, Dura JM. ftz-f1 and Hr39 opposing roles on EcR expression during Drosophila mushroom body neuron remodeling. Nat Neurosci. 2011;14:37–44. doi: 10.1038/nn.2700. [DOI] [PubMed] [Google Scholar]
- 48.Ayer S, Walker N, Mosammaparast M, Nelson JP, Shilo BZ, Benyajati C. Activation and repression of Drosophila alcohol dehydrogenase distal transcription by two steroid hormone receptor superfamily members binding to a common response element. Nucleic Acids Res. 1993;21:1619–1627. doi: 10.1093/nar/21.7.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- 50.Sun J, Deng WM. Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development. 2005;132:4299–4308. doi: 10.1242/dev.02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yussa M, Lohr U, Su K, Pick L. The nuclear receptor Ftz-F1 and homeodomain protein Ftz interact through evolutionarily conserved protein domains. Mech Dev. 2001;107:39–53. doi: 10.1016/s0925-4773(01)00448-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.