Abstract
In women, medroxyprogesterone acetate (MPA) is the most commonly used progestin component of HT. In vitro, MPA negatively impacts markers of neuronal health and exacerbates experimentally-induced neurotoxicity. There is in vitro evidence that these factors are driven by GABAergic and neurotrophic systems. Whether these effects translate to a negative impact on brain function has not been tested in vivo, clinically or preclinically. Here we evaluate the mnemonic and neurobiological effects of MPA in the surgically menopausal rat. Aged ovariectomized (OVX) rats were given subcutaneous vehicle, natural progesterone, low-dose MPA or high-dose MPA. Multiple cognitive domains were analyzed via the water radial-arm maze (WRAM), and Morris maze (MM). Cognitive brain regions were assayed for changes in the GABAergic system by evaluating GAD protein, the synthesizing enzyme for GABA, and neurotrophins. On the WRAM, both progestin types impaired learning. Further, high-dose MPA impaired delayed memory retention on the WRAM, and exacerbated overnight forgetting on the MM. While neurotrophins were not affected by progesterone or MPA treatment, both progestin types altered GAD levels. MPA significantly and progesterone marginally decreased GAD levels in the hippocampus, and both MPA and progesterone significantly increased GAD levels in the entorhinal cortex. These findings suggest that MPA, the most commonly used progestin in HT, is detrimental to learning and two types of memory, and modulates the GABAergic system in cognitive brain regions, in aged menopausal rats. These findings, combined with in vitro evidence that MPA is detrimental to neuronal health, indicates that MPA has negative effects for brain health and function.
Keywords: Hormone Therapy, Cognition, Progestins, Menopause, Aging, Learning and Memory
Introduction
By the year 2050 there will be an estimated 90 million people in the United States who are over the age of 65, and over half of these individuals will be postmenopausal women (U.S. Census Bureau, 2007). Menopause, occurring typically in the fifth decade of life, is characterized by loss of ovary-derived circulating hormones, including estrogen and progesterone (Timaras, Quay, and vernadakis, 1995). Menopause-induced hormone loss has been linked to many symptoms that affect quality of life in women including hot flashes, urogenital atrophy, and memory decline (Freedman, 2002; Nappi, Sinforiani, Mauri, Bono, Polatti, and Nappi, 1999; Sherwin, 1988). Hormone therapy (HT) is given to women to attenuate menopause-induced symptoms. Premarin, a complex combination of horse-derived estrogens, is the most widely used estrogenic component of HT (Hersh, Stefanick, and Stafford, 2004). Women with a uterus that are taking estrogens must include a progestin in their regimen because of increased risk of endometrial hyperplasia associated with unopposed estrogen treatment (Smith, Prentice, Thompson, and Herrmann, 1975; Ziel and Finkle, 1975). Prempro (Premarin + medroxyprogesterone acetate; MPA) is the most widely prescribed progestin-containing HT in the United States, with an estimated 20 million prescriptions written per year within the last decade (Hersh et al., 2004). Further, over 10 million women in the United States have been prescribed MPA as the injectable contraceptive Depo Provera (Mosher, Martinez, Chandra, Abma, and Willson, 2004). Thus, MPA is widely clinically utilized.
Several clinical studies in menopausal and postmenopausal women have demonstrated positive effects of estrogen-containing HT on memory and cognition (Campbell and Whitehead, 1977; Duka, Tasker, and McGowan, 2000; Kantor, Michael, and Shore, 1973; Ohkura, Isse, Akazawa, Hamamoto, Yaoi, and Hagino, 1995; Phillips and Sherwin, 1992; Sherwin, 1988; Wolf, Kudielka, Hellhammer, Torber, McEwen, and Kirschbaum, 1999). However, recently the cognitive effectiveness of HT has been of much debate, due to the unexpected findings of the large, placebo-controlled, randomized Women’s Health Initiative Memory Study (WHIMS) conducted by the National Institute of Health. Menopausal women taking Premarin alone did not differ significantly from those taking placebo for dementia diagnoses (Shumaker, Legault, Kuller, Rapp, Thal, Lane, Fillit, Stefanick, Hendrix, Lewis, Masaki, and Coker, 2004). In contrast, twice as many women receiving Prempro were diagnosed with dementia as compared to the placebo group, a significant effect (Shumaker, Legault, Rapp, Thal, Wallace, Ockene, Hendrix, Jones, Assaf, Jackson, Kotchen, Wassertheil-Smoller, and Wactawski-Wende, 2003).
MPA may be a key factor in Prempro that caused cognitive impairments, although many variables could be involved in this negative outcome (Sherwin, 2005). There has been no study directly testing the hypothesis that MPA is detrimental to cognition in women or an animal model, however, there is indirect evidence that MPA is detrimental to the brain and its function. Indeed, MPA exacerbated neuronal death by glutamate-induced excitotoxicity (Nilsen, Morales, and Brinton, 2006), reduced estrogen-mediated neural protection against excitotoxicity (Nilsen and Brinton, 2002b), and completely blocked the glutamate-stimulated calcium increase produced by 17 β-estradiol, a positive mechanism by which estrogens may modulate cognitive functioning (Nilsen and Brinton, 2002a). In both clinical and preclinical studies, progesterone has been associated with detrimental cognitive effects. In healthy women, a large oral progesterone dose is detrimental to memory (Freeman, Weinstock, Rickels, Sondheimer, and Coutifaris, 1992). High circulating progesterone levels are observed in most rats following estropause (reproductive senescence) (Lu, Hopper, Vargo, and Yen, 1979). It is noteworthy that ovariectomy (Ovx) in aged rats improves cognition (Bimonte-Nelson, Singleton, Hunter, Price, Moore, and Granholm, 2003), which is likely related to progesterone removal, since progesterone administration reverses the beneficial effects of Ovx (Bimonte-Nelson, Singleton, Williams, and Granholm, 2004b). Additionally, administration of the ring-A reduced progesterone metabolite, allopregnanolone, can impair cognition in healthy women (Kask, Backstrom, Nilsson, and Sundstrom-Poromaa, 2008) and young rats (Frye and Sturgis, 1995; Johansson, Birzniece, Lindblad, Olsson, and Backstrom, 2002).
Because the ring-A reduced metabolites of progesterone have a very high affinity for the GABAA receptor (Paul and Purdy, 1992), we hypothesize that progesterone-induced memory impairments are related to GABAergic system alterations. Providing support for this hypothesis, the hippocampus and related brain regions affected by aging and mediating memory processing are largely controlled by the GABAergic system (Izquierdo, Medina, Bianchin, Walz, Zanatta, Da Silva, Bueno e Silva, Ruschel, and Paczko, 1993; Mora, Segovia, and del Arco, 2007), and experimental manipulation of progesterone alters the GABAergic system. For example, progesterone administration decreases glutamic acid decarboxylase (GAD), the synthesizing enzyme and rate limiting step of GABA production, activity in the rodent dorsal hippocampus (Wallis and Luttge, 1980). Additionally, after 17 β-estradiol priming, progesterone but not MPA decreased mRNA expression of the α4 subunit of the GABAA receptor in the CA1 of Ovx rats (Pazol, Northcutt, Patisaul, Wallen, and Wilson, 2009). Indeed, MPA results in different ring-A reduced metabolites (dihydroMPA and tetrahydroMPA) that do not influence binding at the benzodiazepine site of the GABAA receptor as the progesterone ring-A reduced metabolites do, as seen with allopregnanelone (McAuley, Kroboth, Stiff, and Reynolds, 1993).
Neurotrophins, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), is another system that is influenced by progesterone, is involved in neuronal function and in age-related memory changes (Backman, Rose, Hoffer, Henry, Bartus, Friden, and Granholm, 1996). Progesterone counteracts estrogen-induced increases in entorhinal and frontal cortex neurotrophin levels in aged Ovx rats (Bimonte-Nelson, Nelson, and Granholm, 2004a). This same effect is seen in hippocampal slice cultures for BDNF (Aguirre & Baudry, 2009); however, in cerebral cortex slice cultures, progesterone treatment alone increases BDNF levels (Kaur, Jodhka, Underwood, Bowles, de Fiebre, de Fiebre, and Singh, 2007). Thus, alteration of neurotrophin levels is a neuronal mechanism by which progesterone-induced memory changes may be mediated.
While studies suggest that progesterone has a profound impact on GABAergic and neurotrophic systems, as well as cognition, these findings cannot be extrapolated to effects of the synthetic progesterone, MPA. Given that MPA is the progestin component in Prempro, the most widely used combination HT, the negative in vitro findings regarding this hormone are particularly salient. Methodical investigations are warranted to determine whether these detrimental in vitro effects translate to brain functions such as learning and memory. The goal of the present study was to determine whether MPA exerts detrimental effects on the brain and its function. To do this, we tested the cognitive, GABAergic, and neurotrophic effects of MPA in the aged surgically menopausal rat, comparing effects to natural progesterone and vehicle control Ovx and Sham animals.
Methods and Materials
Subjects
Subjects were 37 eighteen month-old Fischer-344 female rats born and raised at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). Rats were acclimated for several weeks before surgery, had access to food and water ad-lib, and were maintained on a 12-hour light/dark cycle at the Arizona State University animal facility. All procedures were approved by the local IACUC committee and adhered to NIH standards.
Ovariectomy and Hormone Treatment
Rats were randomly assigned to one of five treatment groups: Sham (ovary-intact), OVX, OVX+PROG, OVX+Low MPA, and OVX+High MPA. Approximately two months before behavioral testing, all rats received OVX or sham surgery. All rats were anesthetized via isofluorene inhalation. Rats receiving OVX underwent bilateral dorsolateral incisions in the skin and peritoneum, and the ovaries and tips of uterine horns were ligatured and removed. Muscle and skin were then sutured. Rats receiving sham surgery underwent identical skin incision and suture. At the time of surgery, Alzet osmotic pumps (2ML4; Durect Co., Cupertine, CA) containing either proplyene glycol (vehicle, Sigma-Aldrich, St. Louis, MO, USA), progesterone (PROG; 21 mg dissolved in 2 mL propylene glycol, Sigma-Aldrich, St. Louis, MO, USA), or MPA (low dose: 14 mg; high dose: 21 mg, dissolved in 2 mL propylene glycol (Sigma-Aldrich, St. Louis, MO, USA)) were implanted in the neck scruff. Hormone administration continued throughout behavior testing and sacrifice. Doses were based on prior research (Zhang, Fishman, and Huang, 1999), multiplied by a factor of 10 to account for the increased weight from the mouse to the rat. After surgery, rats received Rimadyl (5 mg/mL/kg) for pain and saline (2 mL) to prevent dehydration. Animals underwent pump reinsertion surgery every 31–32 days; behavioral testing began 66 days after the first pump insertion. Thus, hormone administration continued throughout behavior testing and sacrifice.
Vaginal Smears and Uterine Weights
Vaginal smears were taken 16 days after OVX and pump insertion. Smears were classified as proestrus, estrous, metestrus or diestrus, per prior protocols (Acosta, Mayer, Talboom, Zay, Scheldrup, Castillo, Demers, Enders, and Bimonte-Nelson, 2009; Goldman, Murr, and Cooper, 2007). As expected, all OVX animals, regardless of treatment, showed leukocytic smears, while sham animals showed one of the four phases of the estrous cycle. To examine drug effects on uterine tissues, at sacrifice the uteri of all subjects were removed, trimmed of visible fat and immediately weighed (wet weight).
Water Radial-Arm Maze
Subjects were tested for 13 days on the eight-arm win-shift WRAM to evaluate spatial working and reference memory, including performance as working memory load increased, as described previously (Bimonte and Denenberg, 1999; 2000; Bimonte, Granholm, Seo, and Isacson, 2002; Bimonte, Hyde, Hoplight, and Denenberg, 2000). The maze contained escape platforms hidden under the water surface in the ends of 4 of the 8 arms. Each subject had different platform locations that remained fixed throughout the experiment. A subject was released from the start arm and had 3 minutes (m) to locate a platform. Once a platform was found, the animal remained on it for 15 seconds (s), and was then returned to its heated cage for a 30s inter-trial interval (ITI) until its next trial. During the interval, the just-chosen platform was removed from the maze. The animal was then placed again into the start alley and allowed to locate another platform. For each animal a daily session consisted of four trials, with the number of platformed arms reduced by one on each subsequent trial. Thus, the working memory system was increasingly taxed as trials progressed, allowing us to access working memory load. Each subject was given one session a day for 12 consecutive days.
Quantification and blocking were based on prior studies (Bimonte and Denenberg, 1999; 2000; Bimonte et al., 2002; Bimonte et al., 2000; Hyde, Hoplight, and Denenberg, 1998; Hyde, Sherman, and Denenberg, 2000). An arm entry was counted when the tip of a rat’s snout reached a mark delineated on the outside of the arm (11 cm into the arm). Errors were quantified using the orthogonal measures of working and reference memory errors (Jarrard, Okaichi, Steward, and Goldschmidt, 1984), as done previously in WRAM studies (Bimonte et al., 2002; Bimonte et al., 2000; Hyde et al., 2000). Working Memory Correct (WMC) errors were the number of first and repeat entries into any arm from which a platform had been removed during that session. Trial 1 is not analyzed for WMC errors because no platform has yet been removed. Reference memory (RM) errors were the number of first entries into any arm that never contained a platform. Working Memory Incorrect (WMI) errors were repeat entries into a RM arm.
Morris Maze
The MM was tested for 6 trials/day for 5 days using a tub (188 cm diameter) filled with black water made opaque using non-toxic paint. A hidden platform (10 cm wide) remained in a fixed location, thereby testing spatial reference memory (Bimonte-Nelson, Francis, Umphlet, and Granholm, 2006; Morris, Garrud, Rawlins, and O'Keefe, 1982). The rat was placed in the maze from the North, South, East, or West location, and had 60s to locate the hidden platform (10 cm wide), which remained in a fixed location (Northeast quadrant) throughout testing. Once the rat found the platform the trial was terminated. After 15s platform time, the rat was placed into its heated cage until its next trial. The approximate ITI was 15 m. To evaluate whether rats localized the platform to the spatial location, after all test trials on day 5, a 60s probe trial was given whereby the platform was removed. For each trial, a camera suspended above the maze tracked each rat’s path and a tracking system (Ethovision 3.1, Noldus Instruments) analyzed each rat’s tracing.
MM performance was assessed by swim path distance (cm) and latency (s) to the platform. For probe trial data, percent of total distance in the previously platformed (target) quadrant was compared to the quadrant diagonally opposite the platform. Rats that learned the platform location were expected to spend the greatest percent distance in the target quadrant (Stavnezer, Hyde, Bimonte, Armstrong, and Denenberg, 2002).
Visible Platform Maze
Since the MM and WRAM rely on spatial navigation, it was necessary to confirm that all subjects had intact vision and could perform the procedural task components without difficulty. A visible platform (VP) water-escape task was used in this regard. A rectangular tub (39 × 23 in) was filled with clear water and a black platform (10 cm wide) with a white flag (2 × 3 in) was elevated above the water surface. Opaque curtains covered extramaze cues. The drop off location remained the same across trials, and the platform location for each trial varied in space semi-randomly. Animals had to locate the flagged platform protruding from the water, and were given 8 trials/day for 2 days. Performance was assessed by latency (s) to the platform.
Brain Dissections
Two days after conclusion of behavior testing, animals were anesthetized, decapitated and brains rapidly dissected and frozen. Dissected tissues were stored in preweighed microcentrifuge tubes at −70°C until analysis. Dissections were according to plate designations in Paxinos and Watson (1998) and were as follows: frontal cortex (plates 5–14), cingulate cortex (plates 5–14), basal forebrain (plates 14–16), perirhinal cortex (plates 39–42), entorhinal cortex (plates 39–42), and CA1/CA2 region of the dorsal hippocampus (plates 33–35). For each brain the frontal cortex was taken first from the dorsal aspect of the intact brain. Next, the cingulate cortex was taken with the longitudinal fissure as the medial border, and the medial border of the frontal cortex cut as the lateral border. Next, the brain was cut in the coronal plane to obtain access to the basal forebrain. For the basal forebrain, both medial septum and ventral diagonal band were included with the posterior landmark being the crossing point of the anterior commissure. The brain was then cut in the coronal plane to obtain access to the last three brain regions. For the CA1/CA2 region of the hippocampus, the dentate gyrus and the alveus were excluded. For the entorhinal cortex, the tissue was dissected from the same slice as the hippocampal sample, taking a 2- to 3-mm sample ventral to the hippocampus. The perirhinal cortex was also collected from this same slice, taking a 2- to 3-mm sample around the perirhinal fissure.
Western Blot Analyses of GAD 65+67
GAD 65+67 protein expression levels were analyzed in frontal, cingulate, perirhinal, and entorhinal cortices, and hippocampus, from the right hemisphere, and the basal forebrain. Samples were sonicated in RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% Na Deoxycholate, 50 mM Tris) equivalent to 10 times the weight of the sample and centrifuged at 10,000 RPM for 10 m at 4°C. Protein concentrations were determined using BCA protein assays (ThermoFisher Scientific, Pittsburgh, PA, USA) and 10 µg of protein from each sample was run on a NuPAGE Bis-Tris gel, using the SureLock mini-cell (Invitrogen, Carlsbad, CA, USA). Gels were counterbalanced by group and each region was run on two gels. Approximately half of each group was loaded onto a single gel, corresponding to 2–4 subjects from each treatment group. Proteins were transferred onto a PVDF membrane (Millipore, Bedford, MA, USA) and immunoblotting was performed with working dilutions of a rabbit anti-GAD 65+67 (ab11070) and rabbit anti-beta Actin (ab25894) primary antibodies (Abcam Inc., Cambridge, MA, USA). Antibody dilutions were 1:10,000 for GAD 65+67 and 1:2,000 for beta Actin primary antibodies. Membranes were then exposed to a peroxidase-conjugated goat anti-rabbit secondary antibody, 1:10,000 dilution (111-035-003; Jackson Immuno Research, West Grove, PA, USA) and visualized using Pierce ECL Western Blotting Substrate (ThermoScientific, Rockford, IL, USA) on a Biospectrum Biochemi 500 Imaging System (UVP, Upland, CA, USA). Bands were identified as the protein of interest, based on molecular weight, using Precision Plus Protein WesternC Standards (Bio Rad, Hercules, CA, USA). The density of GAD 65+67 and beta Actin (control protein) were then quantified using ImageJ software (Rasband, 1997–2004). The dependent measure was a proportion of each subject’s GAD 65+67 density to their beta Actin density, brought to percent control of OVX subjects run on the same gel, per prior protocols (Pandey, Zhang, Mittal, and Nayyar, 1999).
Growth Factor ELISAs
NGF and BDNF levels were assessed in frontal, cingulate, and entorhinal cortex, and hippocampus, from the left hemisphere. Using Promega kits (Madison, WI), NGF and BDNF can be quantified in the range of 7.8–500 pg/mL, and cross-reactivity with other trophic proteins is < 2–3%. This procedure has been utilized routinely in our laboratory with excellent replicability (Bimonte-Nelson et al., 2004a). In brief, flat-bottom plates were coated with the corresponding capture antibody, which binds the neurotrophin of interest. The captured neurotrophin was bound by a second specific antibody, which was detected using a species-specific antibody conjugated to horseradish peroxidase as a tertiary reactant. All unbound conjugates were removed by subsequent wash steps according to the Promega protocol. After incubation with chromagenic substrate, color change was measured in an ELISA plate reader at 450nm. The dependent measure was pg of BDNF or NGF/mg tissue.
Blood Serum Analyses for MPA
Liquid-liquid extraction of aliquots (1 mL) from serum samples was performed according to (Kim and Kim, 2001) after adding 10 ng/mL of d9-progesterone (C/D/N Isotopes, Pointe-Claire, Quebec, Canada) as an internal reference compound. MPA concentrations were determined by liquid chromatography–atmospheric-pressure ionization tandem mass spectrometry. A reversed-phase column and isocratic elution were used for chromatographic separation of MPA and internal reference. Measurements were performed on a Surveyor – LTQ-FT system from Thermo Fisher (San Jose, CA). In 13×100 mm glass tubes, aliquots (1 mL) of serum were diluted with identical volumes of potassium phosphate buffer (100 mM, pH 7.0) followed by addition of an internal reference compound (d9-progesterone, 10 µL from an 1-µg/mL solution in ethanol). Pentane (4 mL) was then added and the mixture was vortexed for 1 min. After centrifugation at 1000g for 10 min, the organic layer was removed and the extraction was repeated. The pooled organic extracts were dried under nitrogen stream at room temperature and, then, reconstituted in 100 µL acetonitrile before analysis. MPA concentrations were determined by liquid chromatography–atmospheric-pressure ionization tandem mass spectrometry (LC–APCI-MS/MS). Analytes were separated on a 50 mm × 2.1 mm i.d. Supelco (Bellefonte, PA) Discovery HS C18 reversed-phase column. Isocratic elution was used with a mobile phase of water (22.5% v/v), acetonitrile (77% v/v) and acetic acid (0.5% v/v) delivered at 0.25 mL/min flow rate by a Surveyor MS pump (Thermo Fisher, San Jose, CA). Injections (6 µL each) were made by an autosampler (Leap Technologies, Carrboro, NC). The entire column effluent was introduced to the APCI source of an LTQ-FT instrument (Thermo Fisher) whose linear ion trap was used to perform MS/MS data acquisitions. Selected-reaction monitoring (SRM) was employed to record MPA and d9-progesterone signals. MPA and d9-progesterone were measured through SRM chromatograms obtained by monitoring the fragments m/z 327 and m/z 306, respectively, which represented fragments of the protonated compounds (m/z 387 for MPA and m/z 324 for d9-progesterone). MPA concentrations were determined by performing calibrations from the analysis of serum samples spiked with 1, 2, 5 and 10 ng/mL of analyte, respectively, and using the added d9-progesterone as an internal reference to compensate for slight changes in extraction efficiency and LC–APCI-MS/MS conditions among samples. The dependent measure was ng/mL of MPA in serum.
Statistical Analyses
For behavior assessments, data were analyzed separately for each maze, first using an omnibus repeated measures ANOVA with Treatment as the between variable and Days and/or Trials as the within variable/s, as appropriate for the specific maze test. This was done to allow interpretation of Days and/or Trials repeated measures effects in the context of potentially complex Treatment interactions, and for these omnibus analyses all Treatment groups were included. Since our interest was to evaluate effects of OVX, progesterone, and dose-specific MPA administration, two-group planned comparisons were run on the specific measures of the last WRAM testing day, the delay WRAM testing day, and Morris maze overnight forgetting (described below), noting that Type I error correction is not necessary with orthogonal planned comparisons (Keppel and Wickens, 2004).
For WRAM analyses, for all three orthogonal measures of WMC, RM and WMI, learning across all testing days was evaluated in order to detect Day × Treatment interactions. Performance on the final day of regular testing, Day 12, was evaluated at the highest working memory load (Trial 4), as this has revealed Ovx-induced benefits and progesterone-induced impairments in aged animals in our laboratory (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b). On Day 13, a two hour delay was imposed between trials 2 and 3 to assess memory retention for numerous items of information (i.e., the found platform locations on trials 1 and 2). Treatment effects and Trial × Treatment interactions were analyzed for the post-delay trials (Trials 3 and 4) in order to detect impairing effects of the delay within that day. Errors committed on the post-day trials were also compared to errors on the last day of regular testing, Day 12 (baseline) in order to detect impairing effects of a two hour delay versus the 30s ITI.
For MM, in addition to the learning evaluation across all days, in order to test overnight forgetting of the platform location on the MM, we compared distance scores from the last trial of each day (Trial 6) to the first trial of the following day (Trial 1), collapsed across all testing days, as done previously (Acosta et al., 2009; Bimonte-Nelson et al., 2006; Markham, Pych, and Juraska, 2002). Using a priori two-group planned comparisons we examined Treatment × Trial interactions for effects of Ovx, and progesterone and MPA administration across the overnight interval (Trial 6 to Trial 1). We also examined within group trial comparisons of the overnight interval (Trial 6 to Trial 1) in order to determine which treatment groups increased their distance swum across the overnight interval, thus showing overnight forgetting of the platform location.
Uterine weight, neurotrophins, and GAD protein levels were assessed via a priori planned comparisons using t-tests. Alpha was set at 0.05, two-tailed, for all analyses except for the Sham vs. OVX working memory load effect, as we have previously established that OVX decreases errors on this measure (Bimonte-Nelson et al., 2003).
Results
Water Radial-Arm Maze
There were no Treatment main effects or interactions across the 12 testing days for WMC, WMI, or RM errors.
Working memory load effects
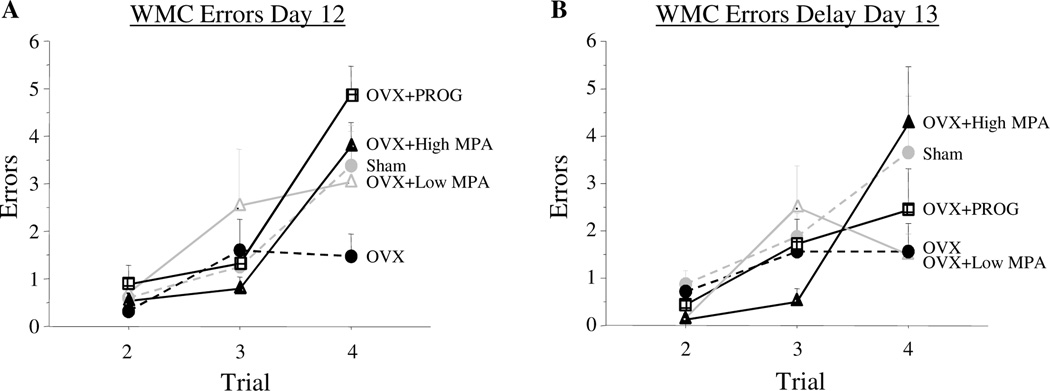
On the final testing day with a 30s ITI (Day 12), for the omnibus ANOVA there was a Treatment × Trial interaction for WMC errors [F(8, 64) = 2.078, p = .05]; both progesterone and high MPA impaired ability to handle numerous items of information as working memory load increased [Treatment × Trial interaction: OVX vs. OVX+PROG: F(2, 24) = 11.01; p < .0005; OVX vs. OVX+High MPA: F(2, 26) = 8.671; p < .005]. At the highest memory load on Trial 4 there was a Treatment main effect for the omnibus ANOVA [F(4, 32) = 2.77; p < .05]. As we have shown previously in aged rats, OVX improved performance [Sham vs. OVX: t(14) = 1.77; p < .05], and OVX+PROG impaired performance [OVX vs. OVX+PROG: t(12) = 4.483; p < .001] (Figure 1a). High MPA impaired memory on the trial with the highest working memory load as well [OVX vs. OVX+High MPA: t(13) = 3.357; p < .01] (Figure 1a). There was not a significant difference between OVX+PROG and OVX+High MPA treated animals. Also, there were no group differences for RM or WMI errors.
Figure 1.
Mean error scores for WMC (±SE) on the water radial-arm maze for Day 12 and delay Day 13. Figure 1a shows the working memory load effect where Sham, OVX+PROG, and OVX+High MPA animals exhibited more errors than OVX animals. Figure 2b shows the trial by treatment interaction between OVX and OVX+High MPA where OVX+High MPA animals increased in errors across trials during the delay day of testing while OVX animals did not.
Delayed memory retention on the WRAM
On Day 13 a two hour delay was given between Trials 2 and 3. There was a Treatment × Trial interaction on Day 13 for WMC errors for the omnibus ANOVA with Trials 2–4 included [F(8, 64) = 2.540; p < .05]. High MPA impaired performance on Day 13, as compared to OVX [Treatment × Trial interaction including trials 2–4: F(2, 26) = 6.448; p < .01] (Figure 1b). Further, there was a Treatment × Trial interaction for WMC errors across Trials 3 and 4 for Day 13 [F(4, 32) = 2.752; p < .05; OVX vs. OVX+High MPA F(1, 13) = 7.626; p < .05], where OVX+High MPA animals showed an increase in WMC errors across these post-delay trials [t(9) = 3.109; p < .05], while errors committed by OVX animals remained stable across these postdelay trials (ns) (Figure 1b). There was no effect of low MPA, progesterone or Ovx on Day 13, nor were there treatment group differences for WMI or RM errors.
It is important to note that OVX+High MPA animals did not commit any more errors after the delay of two hours as they did on the previous baseline day (Day 12) with an ITI of 30 s. Indeed, scores on Day 12 did not differ from scores on Day 13 for OVX+High MPA animals. OVX+PROG animals improved performance from baseline Day 12 to the delay Day 13 [t(6) = 3.158; p < .05], and were no longer different from OVX animals (ns), showing no delay induced impairment but instead a continuation of their learning curve. There were also no longer differences between Sham and OVX groups (ns), again showing no delay-induced impairment.
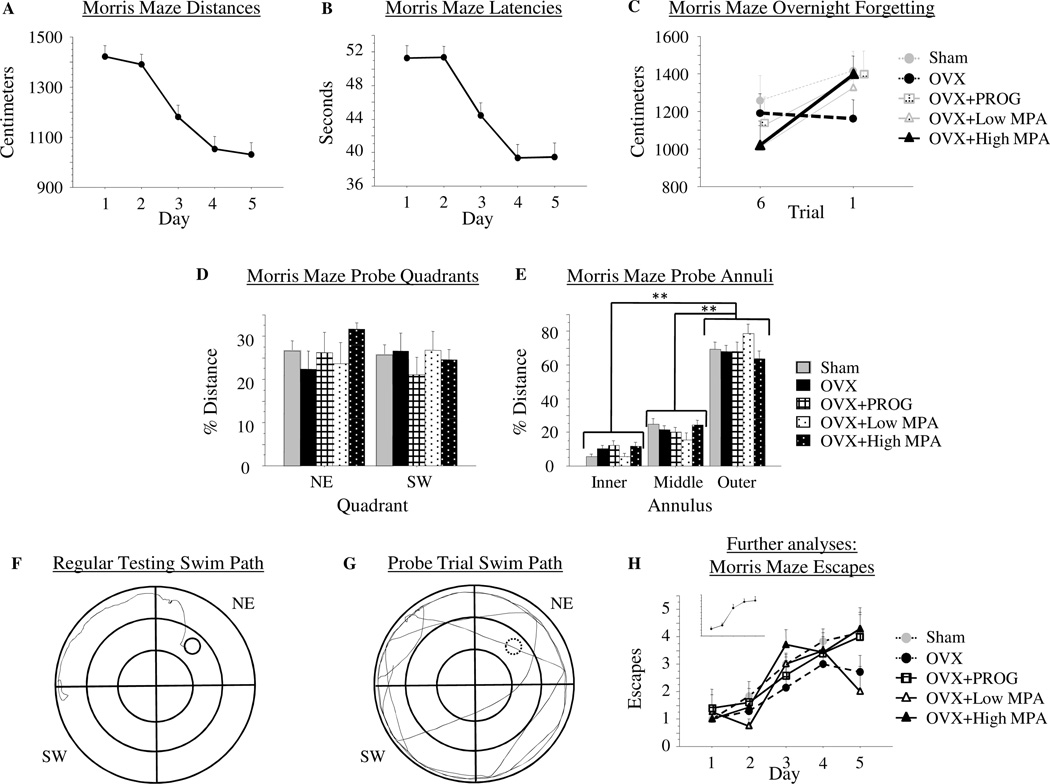
Morris Maze
There were no Treatment effects for MM performance as measured by Distance or Latency to reach the platform (data not shown). There was a Day main effect for each measure for the omnibus ANOVA [Distance: F(4, 24) = 12.153; p < .0001 (Figure 2a); Latency: F(4, 24) = 10.526; p < .0001 (Figure 2b)], with Distance and Latency scores decreasing across days, and no Treatment × Day interaction for either variable. OVX+High MPA animals showed overnight forgetting, while OVX animals did not, as evidenced by a Treatment × Trial interaction for the OVX vs. OVX+High MPA comparison [F(1, 12) = 8.526; p < .05]. Distance scores remained stable after the overnight interval in the OVX group [t(6) = .307; p = .77], and increased after the overnight interval in the OVX+High MPA group [t(6) = 4.059; p < .01] (Figure 2c). There were no interactions between OVX and OVX+PROG [F(1, 10) = 3.166; p = .11], OVX+Low MPA [F(1, 9) = 2.417; p = .15], or Sham [F(1, 11) = 1.01; p = .34] animals, nor was there a significant increase across the overnight interval for any of these groups [Sham: t(5) = .96; p = .38; OVX+PROG: t(4) = 2.02; p = .11; OVX+Low MPA: t(3) = 1.32; p = .28], indicating that only the OVX+High MPA group showed an impaired ability to remember the location of the platform overnight.
Figure 2.
2a) Mean distance scores in centimeters (+SE) on Morris maze days 1–5. 2b) Mean latency scores in seconds (+SE) on Morris maze days 1–5. There were no group differences on distance swum or latency to locate the platform on the Morris Maze. 2c) Mean distance scores in centimeters (+SE) on Morris maze representing overnight forgetting. The figure depicts the collapsed total mean distance score from trial 6 of the previous day to trial 1 of the next day. The OVX+High MPA group significantly increased distance swum across the overnight interval while the OVX group did not; thus, OVX animals remembered the platform location from one day to the next, while OVX+High MPA animals did not. 2d) Mean % distance scores (+SE) spent in the quadrants of the Morris maze during the probe trial. There was no preference, as represented by % distance swum, for the previously platformed quadrant (NW) over the opposite quadrant (SE) by any group. 2e) Mean % distance scores (+SE) spent in the annuli of the Morris maze during the probe trial. All groups exhibited a preference for the outer annulus over the inner and middle annuli confirming animals were not using a motoric strategy of circling the platformed annulus (middle annulus) to solve the task. 2f) A swim path of an animal during regular testing. 2g) A swim path of an animal during the probe trial. 2h) Mean escape scores (+SE) on Morris maze days 1–5. To confirm that animals did learn the platform location, the number of trials within a day that each animal successfully found the platform (and thus escaped) within the 60 second time limit was quantified. Every group showed a significant increase in number of escapes across days. ** p < .0001
Traditional probe analyses comparing distance swum in the target quadrant (NE) to the opposite quadrant (SW) showed no overall Quadrant preference (Quadrant main effect, ns) indicating animals did not localize to the previously platformed spatial location. There was a null Treatment × Quadrant interaction suggesting groups did not differ in this pattern (Figure 2d). Because the probe trial indicated that animals did not localize to the target quadrant, we further assessed probe trial data by analyzing the annuli. Indeed, we and others have shown that animals can use a motoric pattern of circling the middle annulus to locate the platform (Bimonte-Nelson et al., 2006; Stavnezer et al., 2002). This was not the case in the current study, however. Analysis of the probe trial showed an Annulus main effect [F(2, 24) = 112.785; p < .0001], and no Treatment main effect nor Treatment × Annulus interaction (ns), suggesting that treatments did not differ in annuli preference. Animals showed a preference for the outer annulus when compared to both the inner [t(24) = 12.766; p < .0001] and middle [t(24) = 8.898; p < .0001] annuli (figure 2e). This was further confirmed by visual, qualitative inspection of the swim paths during regular testing (figure 2f) and the probe trial (figure 2g). It appeared that animals circled in the outer annulus until they made a direct trajectory to the platform.
The decrease in Distance scores across days described above, suggests that animals swam a lesser distance within the allotted maximum search time, or until the platform was located. To confirm that animals were not floating or showing a decrease in movement within the maximum search time, and that this was not why distance scores decreased across days, we quantified the number of trials within a day that each animal successfully found the platform (and thus escaped) within the 60s time limit. The number of trials escaped increased across days [Day main effect: F(4, 24) = 22.92; p < .0001] (Figure 2h inset), with a null Treatment × Day interaction. Every group showed an increase in the number of Escapes across days, suggesting learning by every group [Sham: F(4, 5) = 7.899; p < .0001; OVX: F(4, 6) = 2.984; p < .05; OVX+PROG: F(4, 4) = 3; p = .05; OVX+Low MPA: F (4, 3) = 4.123; p < .05; OVX+High MPA: F(4, 6) = 13.552; p < .0001] . There was no effect of Treatment on Escapes across all testing days, and no group significantly differed from any other group. Please see Figure 2H for Escapes.
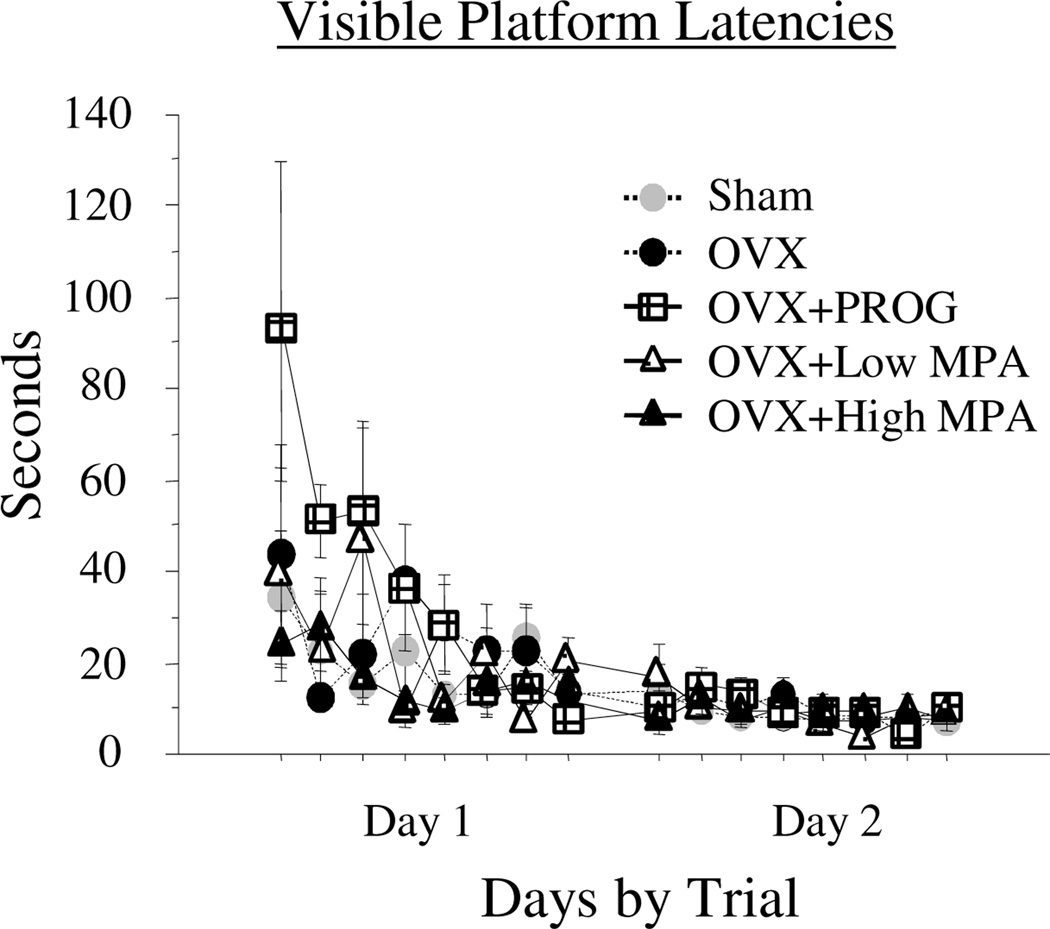
Visible Platform
There were no Treatment main effects or interactions. There was a Day × Trial interaction [F(7, 168) = 3.483; p < .0016], with Time decreasing across all trials of testing (Figure 3). By the second day of testing, all subjects found the visible platform within 6 seconds. Together, these data confirm visual and motor competence to perform the maze tasks.
Figure 3.
Mean latency scores in seconds (+SE) on the visible platform maze days 1–2. There was no Treatment effect for this task, nor were there interactions with Treatment. There was a significant Day × Trial interaction with latencies to locate the platform decreasing across days and trials. These data confirm visual and motor competence by all subjects for platform search.
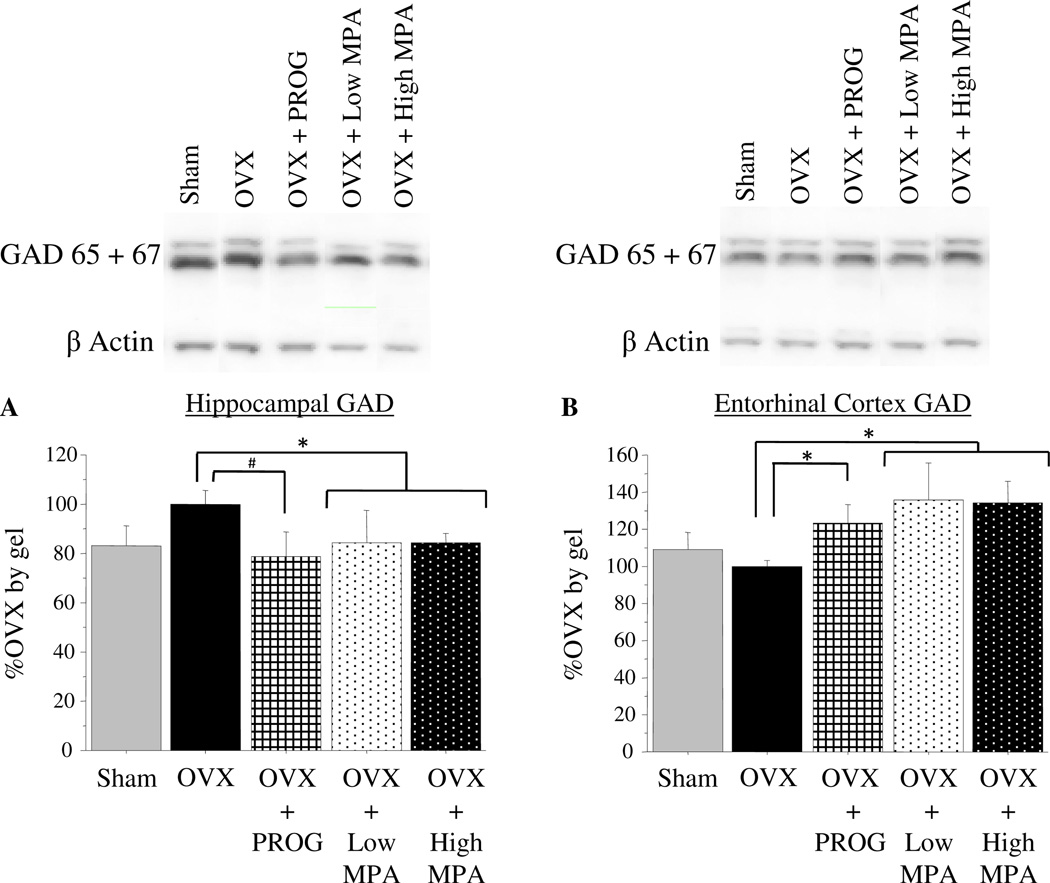
GAD 65 & 67
Differences in GAD levels across groups were present in the hippocampus and entorhinal cortex. Because GAD protein levels did not differ between the OVX+Low MPA and OVX+High MPA groups, they were combined for statistical analyses. In the hippocampus, MPA treatment (doses combined) significantly [t(15) = 2.251; p < .05], and PROG treatment marginally [t(10) = 1.96; p < .10], decreased GAD levels as compared to OVX (Figure 4a). However, in the entorhinal cortex, both progesterone and MPA (doses combined) treatment increased GAD levels as compared to OVX [OVX vs. OVX+MPA: t(15) = 2.542; p < .05; OVX vs. OVX + PROG: t(9) = 2.428; p < .05] (Figure 4b). There was no Treatment effect or any group differences for the loading control, beta Actin (ns).
Figure 4.
4a) Mean %OVX by gel (+SE) of GAD protein expression and representative bands, as determined by Western blot luminescence, in the hippocampus. Combined, the OVX+Low MPA and OVX+High MPA groups had significantly and OVX+PROG group had marginally decreased levels of GAD in the Hippocampus compared to the OVX group. 4b) Mean %OVX by gel (+SE) of GAD luminescence and representative bands, in the entorhinal cortex. The combined OVX+MPA group and the OVX+PROG group had significantly increased levels of GAD in the Entorhinal Cortex compared to the OVX group. * p < .05; # p < .10.
Neurotrophins
There were no group differences in BDNF or NGF levels in any brain region analyzed (Table I).
Table I. Neurotrophin Levels (pg/mg).
Mean neurotrophin levels in pg/mg by treatment group in the Cingulate Cortex, Hippocampus, Entorhinal Cortex, and Frontal Cortex. There were no group differences in neurotrophin levels in any brain region.
| Cingulate Cortex | Hippocampus | Entorhinal Cortex | Frontal Cortex | |||||
|---|---|---|---|---|---|---|---|---|
| BDNF | NGF | BDNF | NGF | BDNF | NGF | BDNF | NGF | |
| Sham | 1.36 ±.38 | 4.71 ±.97 | 6.30 ±1.21 | 3.56 ±.47 | 1.44 ±.44 | 1.49 ±.34 | .77 ±.10 | 1.39 ±.24 |
| OVX | 1.10 ±.12 | 4.47 ±.64 | 6.03 ±1.32 | 3.05 ±.47 | 1.34 ±.16 | 1.49 ±.20 | .79 ±.15 | 1.18 ±.25 |
| OVX+PROG | 1.58 ±.17 | 4.78 ±.97 | 5.90 ±1.45 | 3.21 ±.50 | 1.31 ±.30 | 1.38 ±.20 | .90 ±.15 | 1.07 ±.11 |
| OVX+Low MPA | 1.58 ±.56 | 5.55 ±1.24 | 3.95 ±.30 | 2.07 ±.16 | 1.34 ±.18 | 1.68 ±.33 | .79 ±.19 | 1.16 ±.24 |
| OVX+High MPA | 1.19 ±.30 | 4.05 ±.80 | 5.03 ±.53 | 3.28 ±.62 | 1.69 ±.24 | 1.56 ±.15 | 1.13 ±.27 | 1.56 ±.47 |
Blood Serum Levels of MPA
Low MPA (mean = 4.9 ng/mL) and High MPA (mean = 6.67 ng/mL) -treated groups were the only groups to show MPA concentrations in serum, as expected, since this is not a naturally occurring hormone. Therefore, this confirms presence of the drug (Table II).
Table II. MPA Serum Levels (ng/ml) and Uterine Weights (g).
Mean blood serum concentrations of MPA in ng/mL (+SE) and Mean uterine weights in g (+SE). MPA treated animals were the only groups to show MPA concentrations in blood serum. Uterine weights were significantly greater in Sham animals than all other treatment groups. Uterine weights were also greater in the OVX+Low MPA and OVX+High MPA groups compared to the OVX group.
| Sham | OVX | OVX+ PROG |
OVX+ Low MPA |
OVX+ High MPA |
|
|---|---|---|---|---|---|
| MPA Serum Levels | Not Detected | Not Detected | Not Detected | 4.90 ± .56 | 6.67 ± 1.08 |
| Uterine Weights | .47 ± .03 | .16 ± .01 | .20 ± .03 | .25 ± .02 | .22 ± .01 |
Uterine Weights
Uteri of Sham animals weighed more than those of all other treatment groups [Sham vs. OVX: t(11) = 9.239; p < .0001; Sham vs. OVX+PROG: t(9) = 6.057; p < .0005; Sham vs. OVX+Low MPA: t(8) = 5.028; p < .001; Sham vs. OVX+High MPA: t(11) = 7.208; p < .0001]. MPA uterine weights were greater than those of OVX [OVX vs. OVX+Low MPA: t(9) = 4.079; p < .005; OVX vs. OVX+High MPA: t(12) = 3.523; p < .005] (Table II).
Discussion
The current study is the first to test the synthetic progestin MPA, the most commonly utilized progestin component of HT given to women in the United States (Hersh et al., 2004), for learning and memory in the female rodent. We found that MPA impairs cognition and alters the GABAergic system in the aged surgically menopausal rat. Specifically, MPA impaired the ability to handle an increasing working memory load on the WRAM across 30 second and 2 hour delays, and exacerbated overnight forgetting on the MM spatial reference memory task. MPA effects were dose specific, with only the highest dose showing detrimental effects. Natural progesterone also impaired performance on the WRAM at the highest working memory load with a 30 second delay, which is in agreement with our prior findings of progesterone administration to aged Ovx rats (Bimonte-Nelson et al., 2004b). We also replicated our previous findings, the herein being our third report, that Ovx is beneficial in aged rats, by improving performance on the WRAM at the highest working memory load at the lattermost portion of testing (Bimonte-Nelson et al., 2003; Bimonte-Nelson et al., 2004b), likely mediated in part by the removal of high endogenous progesterone levels (Bimonte-Nelson et al., 2004b). It is noteworthy that the current working memory load findings in aged female rats correspond with prior findings that decrements at the highest working memory load on the WRAM are exacerbated with aging (Bimonte, Nelson, and Granholm, 2003). Additionally, working memory deficits associated with an elevated demand on the system via increasing the number of items to be remembered (Bimonte and Denenberg, 1999) or extended temporal delays (Engler-Chiurazzi, Tsang, Nonnenmacher, Liang, Corneveaux, Prokai, Huentelman, and Bimonte-Nelson, 2009; Markowska and Savonenko, 2002) can be attenuated by 17 β-estradiol or conjugated equine estrogen administration, further demonstrating that different types of working memory demand increases are sensitive to steroid hormones.
Our data also concur with findings that the progesterone metabolite, allopregnanolone, impairs reference memory in young male rats (Johansson et al., 2002) and can impair reference memory and working memory in female rats (Frye and Sturgis, 1995). Progesterone’s detrimental effects on cognition have also been seen in women. Data suggest that in pregnant women the “maternal amnesia” phenomenon is due to high circulating progesterone levels (Brett and Baxendale, 2001; Freeman et al., 1992). Moreover, as demonstrated by the WHIMS collective findings, there is evidence that the combination of estrogen and MPA has a greater negative impact on cognition (Shumaker et al., 2003), than estrogen alone (Shumaker et al., 2004).
The current findings demonstrate that both natural progesterone and the synthetic MPA either significantly or marginally alter GAD levels in the hippocampus and entorhinal cortex. GAD is the synthesizing enzyme and rate limiting step of GABA synthesis. There is a strong positive correlation between GAD mRNA levels and GABA neuronal activity (Erlander and Tobin, 1991), making GAD a good neuronal marker for GABAergic function (Bauer, Brozoski, Holder, and Caspary, 2000; Milbrandt, Holder, Wilson, Salvi, and Caspary, 2000; Raol, Zhang, Budreck, and Brooks-Kayal, 2005). Our findings that progesterone marginally, and MPA significantly, decreases GAD in the hippocampus are consistent with the literature for progesterone. Others have shown that progesterone decreased GAD activity in the dorsal hippocampus (Wallis and Luttge, 1980), and reversed 17 β-estradiol-induced increases in GAD mRNA in hippocampal CA1 and hilus (Weiland, 1992). This same decrease of GAD in the hippocampus is observed after treatment with the GABAA agonist diazepam (Raol et al., 2005), suggesting that progesterone- and MPA-induced GAD decreases may result from increased GABAA receptor activation. Recent work testing both progesterone and MPA found that after 17 β-estradiol priming, progesterone but not MPA decreased mRNA expression of the α4 subunit of the GABAA receptor in the CA1 of young Ovx rats (Pazol et al., 2009). Our effects somewhat conflict with these findings in that both progesterone and MPA impacted the GABAergic system; however, our animals were not estradiol primed and were much older.
To our knowledge, the current report is the first to measure the impact of progesterone or MPA on the GABAergic system in the entorhinal cortex. In contrast to the progesterone- and MPA- mediated decreases seen in the hippocampus, both progesterone and MPA treatment increased GAD levels in the entorhinal cortex. Although no study has measured progesterone or MPA-related changes in the GABAergic system in the entorhinal cortex, it has been shown that alteration of the GABAergic system in this region affects memory function. Post-training infusion of the GABAA agonist muscimol into the entorhinal cortex blocked memory formation (Ferreira, Da Silva, Medina, and Izquierdo, 1992), and lesions of the GABAergic system in entorhinal cortex layer III disrupted hippocampal CA1 place fields, causing impaired spatial representation (Brun, Leutgeb, Wu, Schwarcz, Witter, Moser, and Moser, 2008). The entorhinal cortex and the hippocampus are intimately associated (Knowles, 1992), and both play crucial roles in memory processing, which increasing evidence suggests is facilitated in part through the GABAergic system (Izquierdo et al., 1993).
Given the strong links between progesterone and memory, and the GABAergic system and memory, it is tempting to speculate that the progesterone- and MPA-induced alterations of the GABAergic system begin to explain the cognitively-impairing effects of progestins. However, our data do not allow clear interpretations of these links. Indeed, in the current study there was a dissociation of progestin-induced memory changes and progestin-induced GAD changes. Specifically, while GAD levels were altered with the low-dose and high-dose MPA treatments, low-dose MPA had no impact on behavior, while high-dose MPA did. Thus, it is not clear whether the alterations in GAD levels underlie the observed differences in cognitive performance. Progesterone and MPA both bind to the progesterone receptor, and progesterone is known to interact with several other steroid receptors (Pluchino, Luisi, Lenzi, Centofanti, Begliuomini, Freschi, Ninni, and Genazzani, 2006; Pridjian, Schmit, and Schreiber, 1987), presumably all of which may lead to altered synaptic function through changes in gene transcription. Several of progesterone’s ring-A reduced metabolites are potent agonists of the GABAA receptor (Paul and Purdy, 1992). While MPA’s ring-A reduced metabolites do not seem to influence the GABAA receptor (McAuley et al., 1993), MPA has been shown to alter progesterone’s metabolic conversions (Penning, Sharp, and Krieger, 1985), wherein these alterations might lead to enhanced synaptic and extrasynaptic GABAA receptor-mediated inhibition (Belelli and Herd, 2003). However, at supraphysiological levels, MPA moderately inhibits benzodiazepine binding at the GABAA receptor (McAuly et al., 1993). Additionally, MPA pretreatment exacerbates neuronal death induced by glutamate excitotoxicity in cultured hippocampal neurons (Nilsen et al., 2006). Therefore, while the specific mechanism(s) by which progestins alter GAD levels in the hippocampus and entorhinal cortex is unknown, several neuronal actions of these compounds are documented which could independently or interactively contribute to altered GAD levels. Further studies manipulating the GABAergic system after progestin administration will be integral to this mechanistic understanding.
Although previous literature shows that progesterone increases neurotrophins in cerebral cortex slice cultures (Kaur et al., 2007) and counteracts estrogen-induced increases in neurotrophins in the entorhinal and frontal cortices (Bimonte-Nelson et al., 2004a) and hippocampal slice cultures (Aguirre & Baudry, 2009), we found no effects of progesterone or MPA treatment on neurotrophin levels in several cognitive brain regions. Perhaps in vivo, progestins do not have independent, but only regulatory actions, of estrogen’s effects on neurotrophins. More research is necessary to investigate this hypothesis.
We were able to confirm presence of MPA in serum with mass spectrometry. Not only was this verification of hormone administration, but it also allowed us to compare resulting hormone levels in our animals to known serum concentrations of hormones in women taking HT that includes MPA. According to the prescribing information of Prempro (Prempro Prescribing Information, 2009), women administered the most common dose of MPA given as HT (5 mg), achieved peak plasma concentrations of 4.8 ng/mL. This corresponds almost exactly to our low-dose MPA serum blood levels (4.9 ng/ml) and is still strikingly similar to our high-dose MPA (6.67 ng/ml). These data confirm the clinical relevance of the MPA doses used in this study.
Several lines of evidence converge to suggest that progestins such as MPA have a negative consequence on neuronal health yielding detrimental effects on brain functions such as learning and memory. This is exemplified across multiple realms of the literature, from clinical to basic science. Clinical evidence shows detrimental cognitive effects of combined HT (Shumaker et al., 2003). Basic science evidence shows that both MPA (current report) and progesterone (Bimonte-Nelson et al., 2004b) impair cognition, and that progesterone abolishes 17 β-estradiol-induced memory enhancements (Bimonte-Nelson et al., 2006; Harburger et al., 2007; but see Gibbs, 2000) and attenuates 17 β-estradiol’s neurotrophic effects in vivo (Bimonte-Nelson et al., 2004a) and in cell culture (Aguirre & Baudry, 2009). Moreover, when comparing MPA and progesterone, it is noteworthy that MPA does not demonstrate neuroprotective properties, while progesterone does (Nilsen and Brinton, 2002b), and that MPA causes a greater attenuation of 17 β-estradiol’s neurotrophic actions (Nilsen and Brinton, 2002a). This in vitro work is in accordance with our current findings that MPA has broader detrimental effects for learning and memory, as compared to progesterone. In fact, progesterone’s detrimental effect on learning of the WRAM seemed to be more of a transient nature, as effects were only seen on the last testing day with a 30 second ITI, and not after a two-hour delay to challenge extended memory retention. Furthermore, while no study has directly evaluated the impact of MPA on cognitive health in younger women using this pharmaceutical as a contraceptive, it should be noted that these effects could translate to cognitive impairments in these younger women as well, and account for some of the negative effects reported with use of this drug. Indeed, a documented case study reports amnesic effects corresponding with use of Depo Provera, a contraceptive wherein MPA is the sole hormone component (Gabriel and Fahim, 2005).
In conclusion, the current study is the first to show that not only natural progesterone, but also the synthetic progestin MPA, the most common progestin included in HT, impairs memory and alters the GABAergic system in cognitive brain regions in the aged surgically menopausal rat. To our knowledge, there has been no study directly evaluating MPA-mediated cognitive effects in younger, or older, women. Increasing evidence suggests that this is clearly warranted. The current study has significant implications for the components of HT that might impact cognitive functioning, and suggest that MPA is detrimental within this domain.
Acknowledgements
We are grateful to Cynthia Zay for expert technical assistance and to Dr. Petr Fryčak for performing the LC–APCI-MS/MS assay. This research was funded by grants awarded to HAB-N from the National Institute on Aging (AG028084), state of Arizona, ADHS and the Arizona Alzheimer’s Disease Core Center. LK recognizes support by the NIH grant AG027956 and an endowment (BK-0031) from the Welch Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have nothing to disclose.
References
- U.S. Census Bureau Word population information. [Retrieved on April 21. 2007];2007 from http://www.census.gov/ipc/www/world.html.
- Prempro Prescribing Information. [Retrieved on November 23. 2009];2009 from http://www.wyeth.com/content/showlabeling.asp?id=133.
- Acosta JI, Mayer L, Talboom JS, Zay C, Scheldrup M, Castillo J, Demers LM, Enders CK, Bimonte-Nelson HA. Premarin improves memory, prevents scopolamine-induced amnesia and increases number of basal forebrain choline acetyltransferase positive cells in middle-aged surgically menopausal rats. Horm Behav. 2009;55:454–464. doi: 10.1016/j.yhbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre CC, Baudry M. Progesterone reverses 17beta-estradiol-mediated neuroprotection and BDNF induction in cultured hippocampal slices. Eur J Neurosci. 2009;29:447–454. doi: 10.1111/j.1460-9568.2008.06591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman C, Rose GM, Hoffer BJ, Henry MA, Bartus RT, Friden P, Granholm AC. Systemic administration of a nerve growth factor conjugate reverses age-related cognitive dysfunction and prevents cholinergic neuron atrophy. J Neurosci. 1996;16:5437–5442. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CA, Brozoski TJ, Holder TM, Caspary DM. Effects of chronic salicylate on GABAergic activity in rat inferior colliculus. Hear Res. 2000;147:175–182. doi: 10.1016/s0378-5955(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABA(A) receptor-mediated inhibitory neurotransmission in the rat hippocampus: evidence for endogenous neurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004a;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Hunter CL, Price KL, Moore AB, Granholm AC. Ovarian hormones and cognition in the aged female rat: I. Long-term, but not short-term, ovariectomy enhances spatial performance. Behav Neurosci. 2003;117:1395–1406. doi: 10.1037/0735-7044.117.6.1395. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behav Neurosci. 2004b;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav. 2000;68:495–499. doi: 10.1016/s0031-9384(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Granholm AC, Seo H, Isacson O. Spatial memory testing decreases hippocampal amyloid precursor protein in young, but not aged, female rats. Neurosci Lett. 2002;328:50–54. doi: 10.1016/s0304-3940(02)00442-1. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Nelson ME, Granholm AC. Age-related deficits as working memory load increases: relationships with growth factors. Neurobiol Aging. 2003;24:37–48. doi: 10.1016/s0197-4580(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Brett M, Baxendale S. Motherhood and memory: a review. Psychoneuroendocrinology. 2001;26:339–362. doi: 10.1016/s0306-4530(01)00003-8. [DOI] [PubMed] [Google Scholar]
- Brun VH, Leutgeb S, Wu HQ, Schwarcz R, Witter MP, Moser EI, Moser MB. Impaired spatial representation in CA1 after lesion of direct input from entorhinal cortex. Neuron. 2008;57:290–302. doi: 10.1016/j.neuron.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Campbell S, Whitehead M. Oestrogens for menopausal flushing. Br Med J. 1977;1:104–105. doi: 10.1136/bmj.1.6053.104-f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Tasker R, McGowan JF. The effects of 3-week estrogen hormone replacement on cognition in elderly healthy females. Psychopharmacology (Berl) 2000;149:129–139. doi: 10.1007/s002139900324. [DOI] [PubMed] [Google Scholar]
- Engler-Chiurazzi E, Tsang C, Nonnenmacher S, Liang WS, Corneveaux JJ, Prokai L, Huentelman MJ, Bimonte-Nelson HA. Tonic Premarin dose-dependently enhances memory, affects neurotrophin protein levels and alters gene expression in middle-aged rats. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlander MG, Tobin AJ. The structural and functional heterogeneity of glutamic acid decarboxylase: a review. Neurochem Res. 1991;16:215–226. doi: 10.1007/BF00966084. [DOI] [PubMed] [Google Scholar]
- Ferreira MB, Da Silva RC, Medina JH, Izquierdo I. Late posttraining memory processing by entorhinal cortex: involvement of NMDA and GABAergic receptors. Pharmacol Biochem Behav. 1992;41:767–771. doi: 10.1016/0091-3057(92)90225-5. [DOI] [PubMed] [Google Scholar]
- Freedman MA. Quality of life and menopause: the role of estrogen. J Womens Health (Larchmt) 2002;11:703–718. doi: 10.1089/15409990260363661. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo-controlled study of effects of oral progesterone on performance and mood. Br J Clin Pharmacol. 1992;33:293–298. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Sturgis JD. Neurosteroids affect spatial/reference, working, and long-term memory of female rats. Neurobiol Learn Mem. 1995;64:83–96. doi: 10.1006/nlme.1995.1046. [DOI] [PubMed] [Google Scholar]
- Gabriel A, Fahim G. Do Depot Medroxyprogesterone Acetate Contraceptive Injections Cause Mood Changes and Memory Impairment? Primary Psychiatry. 2005;12:59–60. [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Denenberg VH. Non-spatial water radial-arm maze learning in mice. Brain Res. 2000;863:151–159. doi: 10.1016/s0006-8993(00)02113-2. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH, Bianchin M, Walz R, Zanatta MS, Da Silva RC, Bueno e Silva M, Ruschel AC, Paczko N. Memory processing by the limbic system: role of specific neurotransmitter systems. Behav Brain Res. 1993;58:91–98. doi: 10.1016/0166-4328(93)90093-6. [DOI] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002;934:125–131. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Kask K, Backstrom T, Nilsson LG, Sundstrom-Poromaa I. Allopregnanolone impairs episodic memory in healthy women. Psychopharmacology (Berl) 2008;199:161–168. doi: 10.1007/s00213-008-1150-7. [DOI] [PubMed] [Google Scholar]
- Kantor HI, Michael CM, Shore H. Estrogen for older women. Am J Obstet Gynecol. 1973;116:115–118. doi: 10.1016/0002-9378(73)90894-6. [DOI] [PubMed] [Google Scholar]
- Kaur P, Jodhka PK, Underwood WA, Bowles CA, de Fiebre NC, de Fiebre CM, Singh M. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85:2441–2449. doi: 10.1002/jnr.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis : a researcher's handbook. 4th ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- Kim SM, Kim DH. Quantitative determination of medroxyprogesterone acetate in plasma by liquid chromatography/electrospray ion trap mass spectrometry. Rapid Commun Mass Spectrom. 2001;15:2041–2045. doi: 10.1002/rcm.478. [DOI] [PubMed] [Google Scholar]
- Knowles WD. Normal anatomy and neurophysiology of the hippocampal formation. J Clin Neurophysiol. 1992;9:252–263. [PubMed] [Google Scholar]
- Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod. 1979;21:193–203. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the morris water maze. Horm Behav. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley JW, Kroboth PD, Stiff DD, Reynolds IJ. Modulation of [3H]flunitrazepam binding by natural and synthetic progestational agents. Pharmacol Biochem Behav. 1993;45:77–83. doi: 10.1016/0091-3057(93)90089-c. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Holder TM, Wilson MC, Salvi RJ, Caspary DM. GAD levels and muscimol binding in rat inferior colliculus following acoustic trauma. Hear Res. 2000;147:251–260. doi: 10.1016/s0378-5955(00)00135-0. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004:1–36. [PubMed] [Google Scholar]
- Nappi RE, Sinforiani E, Mauri M, Bono G, Polatti F, Nappi G. Memory functioning at menopause: impact of age in ovariectomized women. Gynecol Obstet Invest. 1999;47:29–36. doi: 10.1159/000010058. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002a;13:825–830. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002b;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Morales A, Brinton RD. Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol. 2006;22:355–361. doi: 10.1080/09513590600863337. [DOI] [PubMed] [Google Scholar]
- Ohkura T, Isse K, Akazawa K, Hamamoto M, Yaoi Y, Hagino N. Long-term estrogen replacement therapy in female patients with dementia of the Alzheimer type: 7 case reports. Dementia. 1995;6:99–107. doi: 10.1159/000106929. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang D, Mittal N, Nayyar D. Potential role of the gene transcription factor cyclic AMP-responsive element binding protein in ethanol withdrawal-related anxiety. J Pharmacol Exp Ther. 1999;288:866–878. [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 4 ed. 4 th edition ed. New York: Academic Press; 1998. [Google Scholar]
- Pazol K, Northcutt KV, Patisaul HB, Wallen K, Wilson ME. Progesterone and medroxyprogesterone acetate differentially regulate alpha4 subunit expression of GABA(A) receptors in the CA1 hippocampus of female rats. Physiol Behav. 2009;97:58–61. doi: 10.1016/j.physbeh.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning TM, Sharp RB, Krieger NR. Purification and properties of 3 alpha-hydroxysteroid dehydrogenase from rat brain cytosol. Inhibition by nonsteroidal anti-inflammatory drugs and progestins. J Biol Chem. 1985;260:15266–15272. [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Luisi M, Lenzi E, Centofanti M, Begliuomini S, Freschi L, Ninni F, Genazzani AR. Progesterone and progestins: effects on brain, allopregnanolone and beta-endorphin. J Steroid Biochem Mol Biol. 2006;102:205–213. doi: 10.1016/j.jsbmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Pridjian G, Schmit V, Schreiber J. Medroxyprogesterone acetate: receptor binding and correlated effects on steroidogenesis in rat granulosa cells. J Steroid Biochem. 1987;26:313–319. doi: 10.1016/0022-4731(87)90095-1. [DOI] [PubMed] [Google Scholar]
- Raol YH, Zhang G, Budreck EC, Brooks-Kayal AR. Long-term effects of diazepam and phenobarbital treatment during development on GABA receptors, transporters and glutamic acid decarboxylase. Neuroscience. 2005;132:399–407. doi: 10.1016/j.neuroscience.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Rasband W. ImageJ. Bethesda, Maryland, USA: National Institute of Health; 1997–2004. http://rsb.info.nih.gov/ij/: [Google Scholar]
- Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 2005;47:371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous estrogen and endometrial carcinoma. N Engl J Med. 1975;293:1164–1167. doi: 10.1056/NEJM197512042932302. [DOI] [PubMed] [Google Scholar]
- Stavnezer AJ, Hyde LA, Bimonte HA, Armstrong CM, Denenberg VH. Differential learning strategies in spatial and nonspatial versions of the Morris water maze in the C57BL/6J inbred mouse strain. Behav Brain Res. 2002;133:261–270. doi: 10.1016/s0166-4328(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Timaras P, Quay W, vernadakis A, editors. Hormones and aging. Boca Raton, NY, London, Tokyo: CRC Press; 1995. [Google Scholar]
- Wallis CJ, Luttge WG. INfluence of estrogen and progesterone on glutamic acid decarboxylase activity in discrete regions of rat brain. J Neurochem. 1980;34:609–613. doi: 10.1111/j.1471-4159.1980.tb11187.x. [DOI] [PubMed] [Google Scholar]
- Weiland NG. Glutamic acid decarboxylase messenger ribonucleic acid is regulated by estradiol and progesterone in the hippocampus. Endocrinology. 1992;131:2697–2702. doi: 10.1210/endo.131.6.1446611. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kudielka BM, Hellhammer DH, Torber S, McEwen BS, Kirschbaum C. Two weeks of transdermal estradiol treatment in postmenopausal elderly women and its effect on memory and mood: verbal memory changes are associated with the treatment induced estradiol levels. Psychoneuroendocrinology. 1999;24:727–741. doi: 10.1016/s0306-4530(99)00025-6. [DOI] [PubMed] [Google Scholar]
- Zhang L, Fishman MC, Huang PL. Estrogen Mediates the Protective Effects of Pregnancy and Chorionic Gonadotropin in a Mouse Model of Vascular Injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:2059–2065. doi: 10.1161/01.atv.19.9.2059. [DOI] [PubMed] [Google Scholar]
- Ziel HK, Finkle WD. Increased risk of endometrial carcinoma among users of conjugated estrogens. N Engl J Med. 1975;293:1167–1170. doi: 10.1056/NEJM197512042932303. [DOI] [PubMed] [Google Scholar]