Abstract
Background and Objectives
Traumatic brain injury (TBI) affects millions worldwide and is without effective treatment. One area that is attracting growing interest is the use of transcranial low-level laser therapy (LLLT) to treat TBI. The fact that near-infrared light can penetrate into the brain would allow non-invasive treatment to be carried out with a low likelihood of treatment-related adverse events. LLLT may treat TBI by increasing respiration in the mitochondria, causing activation of transcription factors, reducing inflammatory mediators and oxidative stress, and inhibiting apoptosis.
Study Design/Materials and Methods
We tested LLLT in a mouse model of closed-head TBI produced by a controlled weight drop onto the skull. Mice received a single treatment with continuous-wave 665, 730, 810, or 980 nm lasers (36 J/cm2 delivered at 150 mW/cm2) 4-hour post-TBI and were followed up by neurological performance testing for 4 weeks.
Results
Mice with moderate-to-severe TBI treated with 665 and 810 nm laser (but not with 730 or 980 nm) had a significant improvement in Neurological Severity Score that increased over the course of the follow-up compared to sham-treated controls. Morphometry of brain sections showed a reduction in small deficits in 665 and 810 nm laser treated mouse brains at 28 days.
Conclusions
The effectiveness of 810 nm agrees with previous publications, and together with the effectiveness of 660 nm and non-effectiveness of 730 and 980 nm can be explained by the absorption spectrum of cytochrome oxidase, the candidate mitochondrial chromophore in transcranial LLLT.
Keywords: photobiomodulation, low-level laser therapy, traumatic brain injury, mouse model, Neurological Severity Score
INTRODUCTION
Head injury in humans is the most common neurological disorder under the age of 50. Victims of traumatic brain injury (TBI) suffer short- and long-term physical, cognitive, behavioral, and emotional impairments that depend on the severity of the injury [1,2]. Moderate and severe TBI, accidental or inflicted, is a major health and socio-economic problem throughout the world. In the United States alone, approximately 2 million injuries occur each year resulting in 56,000 deaths and 18,000 survivors suffering from permanent neurological impairment [3–5]. The consequent direct and indirect annual costs in the United States are estimated at $56 billion [6]. The World Health Organization (WHO) has projected that by 2020, road traffic accidents, a major cause of TBI, will rank third as a cause of the global burden of disease and disablement, behind only ischemic heart disease and unipolar major depression [7]. Moreover, military conflicts in recent years have given rise to a large numbers of cases of TBI caused by over-pressure blast injuries from improvised explosive devices [8,9]. Efforts to improve treatment and outcome of TBI must therefore remain the priority for clinicians and researchers [10].
The pathophysiology of TBI is very complex and still poorly understood [11]. Immediately following the primary impact, activation of several different pathways begins, resulting in secondary brain injury. These include inflammation, oxidative stress, ionic imbalance, increased vascular permeability, mitochondrial dysfunction, and excitotoxic damage [12,13]. These processes result in brain edema, increased intracranial pressure (ICP), and impaired cerebral perfusion [14]. This combination of cellular and physiologic disturbances causes increased neuronal cell death, enlargement of infarct size, and neurological, motor, and cognitive impairment. Since there are no approved specific pharmacological agents that block the progression of the secondary injury, the current management of TBI is mainly supportive and aims at treating brain edema, reducing ICP, and combating complications, such as hypoxia and shock [15]. Despite promising preclinical data, most of the clinical trials that have been performed in recent years have failed to demonstrate any significant improvement in outcome [16].
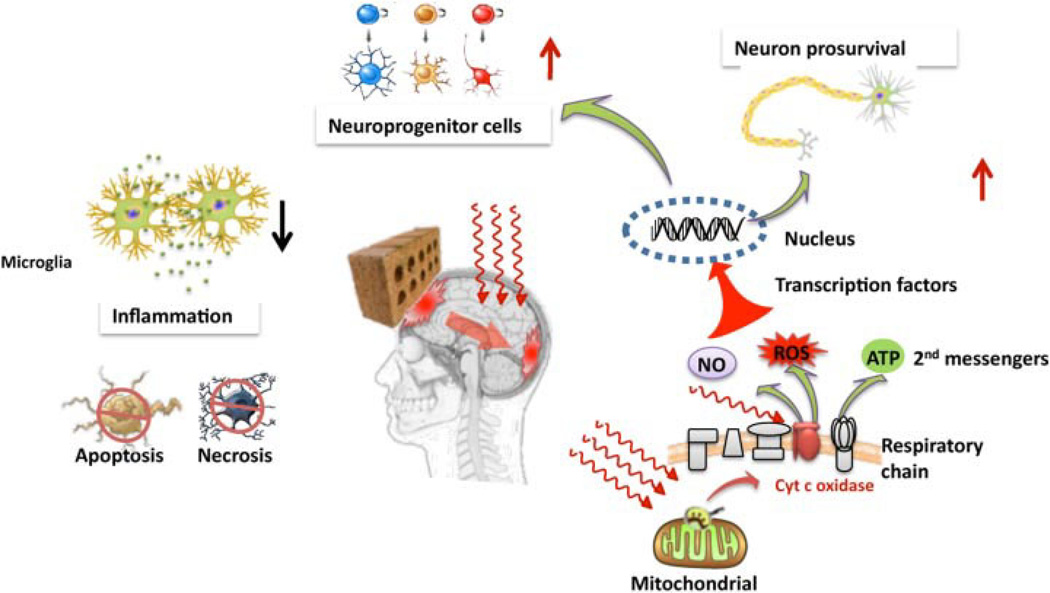
Low-level laser (or light) therapy (LLLT) has been clinically applied for many indications in medicine that require the following processes: protection from cell and tissue death, stimulation of healing and repair of injuries, and reduction of pain, swelling, and inflammation [17,18]. One area that is attracting growing interest [19] is the use of LLLT to treat stroke [20], TBI [21], neurodegenerative diseases [22], and spinal cord injuries [23]. The notable lack of any effective drug-based therapies for most of these diseases has motivated researchers to consider the use of light as an effective approach to mitigating what is considered to be a group of serious diseases. The fact that near-infrared (NIR) light can penetrate into the brain and spinal cord could allow non-invasive treatment to be carried out with a low likelihood of treatment-related adverse events. Although in the past it was generally accepted that the central nervous system could not repair itself, recent discoveries in the area of neuronal stem cells and adult neurogenesis have brought this dogma into question [24]. LLLT may have beneficial effects in the acute treatment of TBI by improving mitochondrial function, stimulating signaling pathways, and transcription factors, inhibiting neuronal apoptosis and upregulating adult neurogenesis (Fig. 1). In this study, the effects LLLT mediated by different wavelengths of laser-light were tested in a mouse model of closed-head TBI and by measuring the neurobehavioral outcome of the traumatized mice.
Fig. 1.
Graphical illustration of the possible mechanisms of transcranial LLLT for TBI. Red or near-infrared light is absorbed in the mitochondria (possible by cyctochrome c oxidase in the respiratory chain) leading to release of ATP, nitric oxide, and modulation of reactive oxygen species. These second messenger molecules can lead to activation of transcription factors that migrate to the nucleus where they alter expression levels of numerous genes. These new gene products may lead to increased survival of neurons in the damaged brain, to an increase in adult neurogenesis in the hippocampus, for example, to reduced levels of inflammation and to an overall reduced level; of apoptotic and necrotic cell death in the brain.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital (protocol #2009N000006) and met the guidelines of the National Institutes of Health. Adult male BALB/c mice (weight 20–25 g; Charles River Laboratories, Wilmington, MA) were used in this study. The animals were housed at one mouse per cage and were maintained on a 12-hour light–12-hour dark cycle with access to food and water ad libitum.
Traumatic Brain Injury Model
Closed-head injury (CHI) was induced under isoflurane anesthesia using a weight drop device. The weight drop apparatus included a vertical 15-cm-long plastic tube (diameter 32 mm) with a 69 g weight (diameter 25 mm) dropped through the upper end of the tube. Briefly, following anesthesia, a midline longitudinal incision in the scalp was performed and the skull was exposed. The mouse was placed on a foam-pad and a Teflon-tipped cone (3 mm diameter) was placed 1 mm lateral to the midline in the mid-coronal plane. The head was manually held in place and a 69 g weight was dropped on the cone from a height that was adjusted to yield a trauma that could be categorized as moderate to severe [Neurological Severity Score (NSS) 6–8] to the left hemisphere. After brain trauma, the skin was sutured. Mice with depressed skull fracture or visible hemorrhage were excluded from the study. The severity of the trauma was confirmed 1 hour later by assessing the NSS score. After recovery from anesthesia, the mice were returned to their cages with post-operative care and ad libitum access to food and water.
Neurobehavioral Evaluation
The neurological status of the traumatized mice was evaluated at different time intervals after CHI according to a NSS. The neurological tests are based on the ability of the mice to perform 10 different tasks (Table 1) that evaluate the balancing, motor ability, and alertness of the mouse. One point is given for failure to perform a task; thus, a normal, uninjured mouse scores 0. The severity of injury is defined by the initial NSS, evaluated 1-hour post-CHI, and is a reliable predictor of the late outcome. Thus, the higher the score, the more severe is the injury; mild injury in mice with an NSS of <5; moderate injury with NSS of 5–6; severe injury in mice with an NSS of 7–8; and fatal or near-fatal injury is defined in mice having an NSS of 9–10. Only mice with an NSS of 6–8 at 1 hour were included in the study.
TABLE 1.
Neurological Severity Score (NSS) for TBI Mice
| Task | NSS |
|---|---|
| Presence of mono- or hemiparesis | 1 |
| Inability to walk on a 3-cm-wide beam | 1 |
| Inability to walk on a 2-cm-wide beam | 1 |
| Inability to walk on a 1-cm-wide beam | 1 |
| Inability to balance on a 1-cm-wide beam | 1 |
| Inability to balance on a round stick (0.5-cm wide) | 1 |
| Failure to exit a 30-cm-diameter circle (for 2 minutes) | 1 |
| Inability to walk straight | 1 |
| Loss of startle behavior | 1 |
| Loss of seeking behavior | 1 |
| Maximum total | 10 |
Mice are awarded 1 point for each failure to complete a task.
Laser Treatment
All mice were subjected to CHI and assessment of NSS was performed 1-hour post-TBI. The NSS scores of the mice ranged from 6 to 8, indicating a moderately severe trauma. The mice were then divided into five groups of 8–12 mice per group, so that the mean NSS in each group were similar, to ensure similar average severity of injury in all groups. One group of mice served as a sham-treated TBI control, which received brain injury and then underwent the same procedures as the laser-treated group, but did not receive actual laser-irradiation. The lasers that used were: 665 nm, model BWF-665-1 from B&W-Tek (Newark, DE); 730 nm, model 730/6 from Diomed Inc (Andover, MA); 810 nm, model DioDent Micro 810, ConBio (Fremont, CA); 980 nm, model V-Raser, ConBio. Mice were immobilized in a plastic holder so their head was exposed. The lasers were coupled into a fiber-optic that was arranged to deliver a 1-cm diameter spot centered on the top of the mouse head over the sutured incision. The power densities generated were measured with a power meter (Model DMM 199 with 201 Standard head, Coherent, Santa Clara, CA) and were adjusted to be 150 mW/cm2. Four laser-treated groups of mice received a single treatment with 665, 730, 810, or 980 nm laser (36 J/cm2 over 4-min) 4-hour post-injury.
Sacrifice and Histomorphometry
At day 28 mice were sacrificed by deep anesthesia (1.5% isoflurane) and transcardial perfusion with phosphate-buffered saline (PBS) followed by fixation by perfusion and immersion in 4% paraformaldehyde. After the brains were removed and further fixed overnight, they were embedded in OCT compound (Sakura Finetek USA Inc., Torrance, CA), frozen in liquid nitrogen, cut into 15-µm-thick axial sections, and stained with hematoxylin and eosin (HE). Because there were no focal lesions visible in this CHI model of TBI but rather a diffuse axonal injury, the following morphometric technique was employed. Brains were imaged with a light microscope (BH-2, Olympus America Inc, Center Valley, PA), with a magnification objective of 4× and photographed using a digital camera (FD400, Sony USA, San Diego, CA). Outlines of the brains were drawn manually to calculate the total pixel area occupied by the brain assuming no small defects, and the actual area of H&E stained pixels was calculated using Image J software (NIH, Bethesda, MD).
Statistical Analysis
Data are presented as mean ± SD. In a two-dimensional coordinate system, the area under the curve (AUC) data, which represent the time courses of NSS in the various groups of mice, were calculated using numerical integration [25]. Differences in the AUC between the untreated TBI and the laser-treated groups and between different laser-treated groups were compared for statistical significance using one-way ANOVA. Significance was defined as P < 0.05. SPSS statistics V17.0 software (IBM Corp, Armonk, NY) was used for statistical analyses.
RESULTS
Neurobehavioral Evaluation After Laser Treatment to the Head
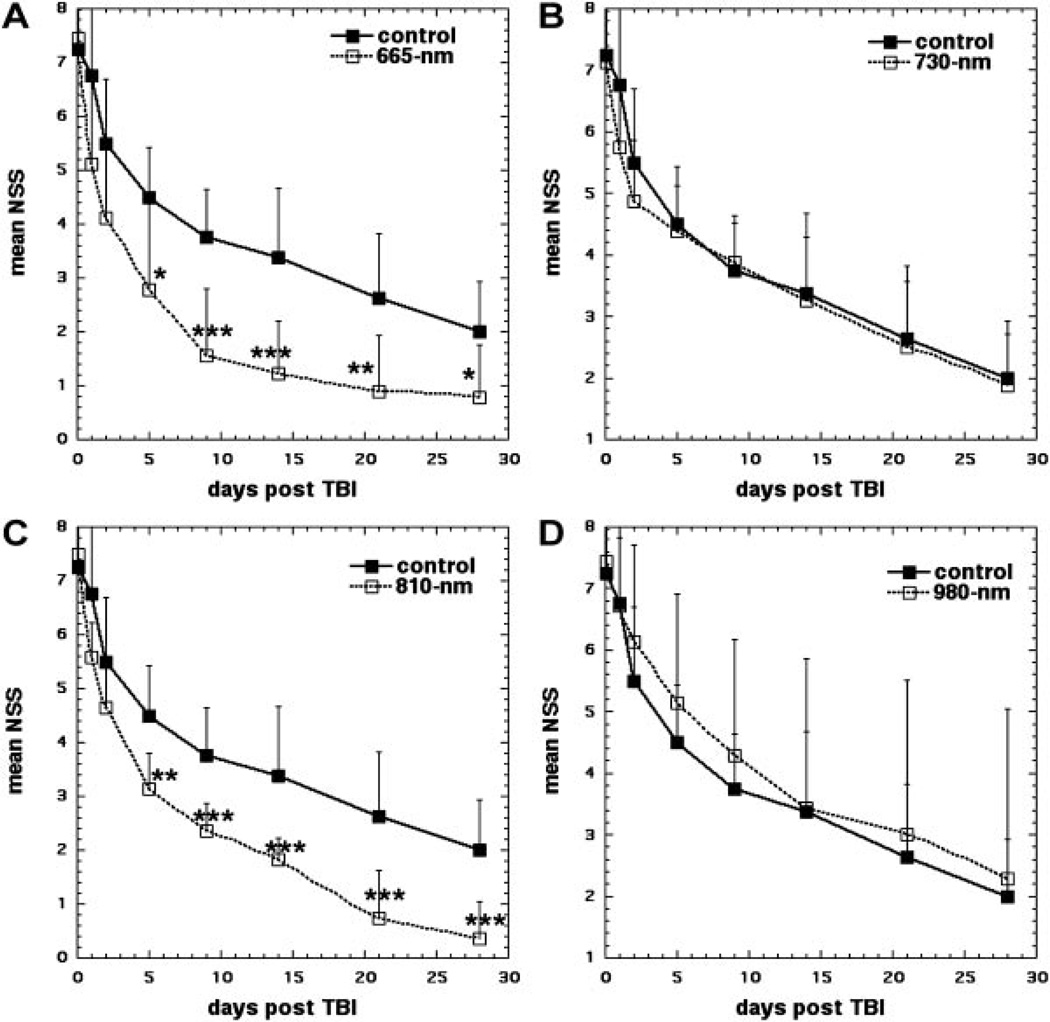
The NSS measurements in control sham-treated TBI mice displayed a gradual improvement from 7.3 at 1-hour post-TBI to 4.5 at 5 days; to 3.4 at 14 days; and to 2 at 28 days. Figure 2A shows that mice treated with 665 nm laser at 4-hour post-injury treatment started to show a significantly greater improvement than sham-treated control mice at day 5 (2.78 vs. 4.5, P < 0.05) and this improvement increased at day 9 (1.55 vs. 3.75, P < 0.001) and remained at this significance level at day 14 (1.22 vs. 3.37, P < 0.001). At day 21 the improvement was a little less significant (0.89 vs. 2.62, P < 0.01) and at day 28 (0.78 vs. 2.0, P < 0.05).
Fig. 2.
Time course of NSS scores of sham and laser-treated mice. A: Sham-treated control versus 665 nm laser. B: Sham-treated control versus 730 nm laser. C: Sham-treated control versus 810 nm laser. D: Sham-treated control versus 980 nm laser. Points are means of 8–12 mice and bars are SD. *P < 0.05; **P < 0.01; ***P < 0.001 (one-way ANOVA).
Figure 2B shows that 730 nm laser had absolutely no beneficial effect on the NSS of the mice, and laser-treated mice improved at the same rate as the sham-treated control mice throughout the entire course of the follow-up.
Figure 2C shows the effect of laser-irradiation at 810 nm on the NSS of mice compared to sham-treated control mice. In a similar fashion to the scores of the 665 nm treated mice, the NSS scores of the 810 nm laser-treated mice showed a significant improvement over sham-treated control mice at day 5 (3.14 vs. 4.5, P < 0.01), and this continued to increase at day 9 (2.8 vs. 3.75, P < 0.001). The improvement remained at this significance level throughout the rest of the follow-up; on day 14 (1.64 vs. 3.37, P < 0.001), on day 21 (0.71 vs. 2.62, P < 0.001), and day 28 (0.35 vs. 2.0, P < 0.001).
Figure 2D shows the effect of laser irradiation at 980 nm on the NSS of mice. In a similar fashion to the response seen with 730 nm, there was no significant difference between 980 nm laser and sham-treated control at any time point.
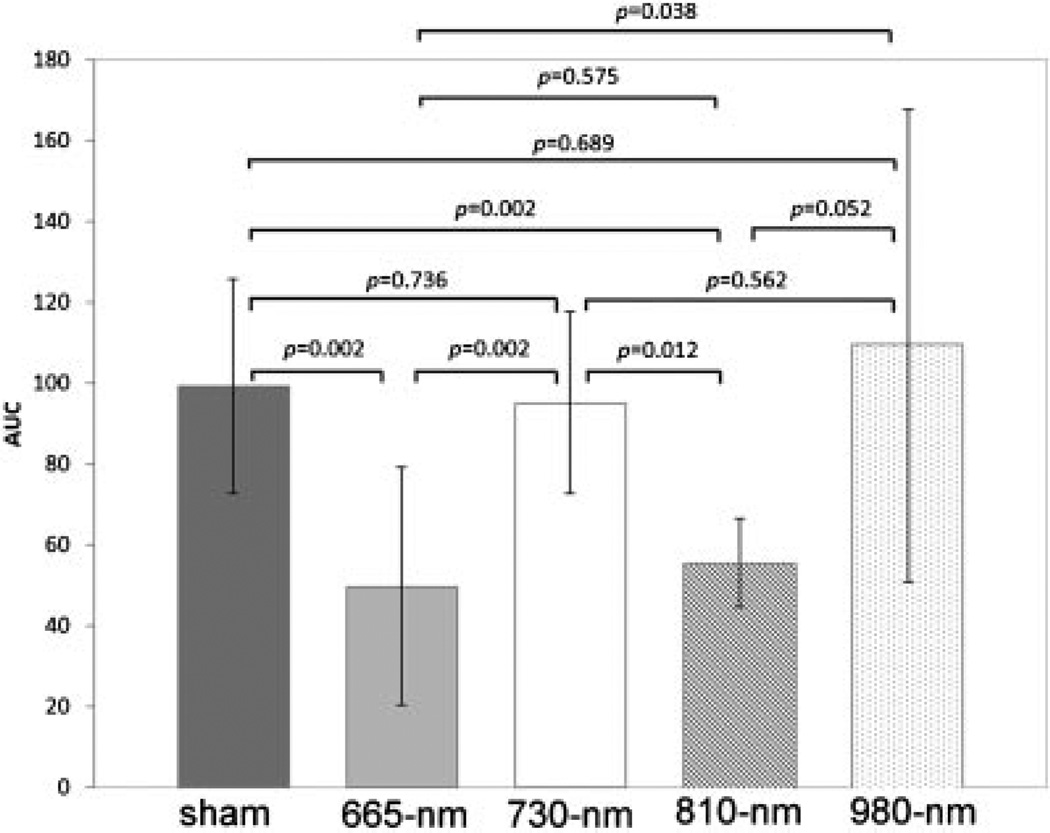
We integrated the NSS values for each mouse over the 28-day time course of the experiment to give an AUC value for each mouse. The means of these AUC values for each group of mice was compared in Figure 3. There was not significant difference between mean AUC values in sham-treated controls and mice treated with 730 nm laser or with 980 nm laser. The mean AUC for 665 nm laser treated mice was approximately half that seen in the sham, 730 nm, and 980 nm groups (P = 0.002–0.038). The mean AUC for 810 nm laser-treated mice was also about half of that seen in sham, 730 nm, and 980 nm groups (P = 0.002–0.032). There was no difference between mice treated with 665 and 810 nm lasers.
Fig. 3.
Means of AUC values for individual mice in the five groups. Values are means of 8–12 mice per group and bars are SD. P-values shown were determined by one-way ANOVA.
Brain Sections and Morphometry
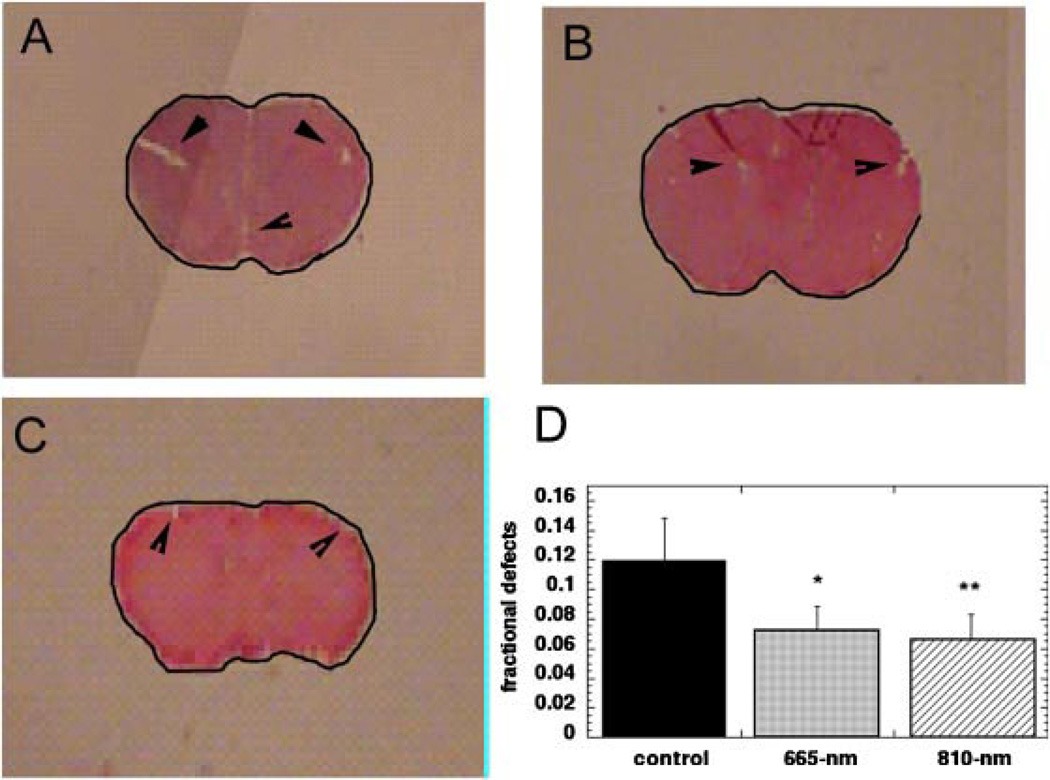
Representative images of the brain sections removed at day 28 are shown in Figure 4A. Because of the absence of focal brain lesions in this closed-head model of TBI it was impossible to distinguish any gross differences between the sham-treated control and the 665 nm or 810 laser-treated groups. There were small defects visible (arrows in Fig. 4A) in the brain parenchyma but it was supposed that these were tissue-processing artifacts. In further studies, these defects should be investigated at higher magnification to establish their exact pathological features. However, when we calculated the pixel area occupied by the entire shape of the brain according to the black outline visible in Figure 4A, and subtracted from this value, the pixel area of the brain stained by H&E using Image J, we arrived at the total area of the small lesions marked by arrows in Figure 4A. This area of deficits was expressed as a fraction of the total area to normalize for the varying sizes of the whole brain images. The values obtained for these parameters in sham, 665 nm, and 810 nm laser-treated mice are shown in Table 2. To our surprise when we compared the means of these fractional lesion areas we found significant differences between groups. Figure 4D shows the mean fractional defect areas for 660 nm laser-treated mice (0.065 ± 0.024, P < 0.05) and for 810 nm laser-treated mice (0.053 ± 0.029, P < 0.01) were significantly lower than that for untreated TBI mice (0.12 ± 0.029).
Fig. 4.
Morphometry of brain sections removed from mice at day 28. Micrographs of typical H&E stained brain sections showing outline to calculate total brain area and small defects marked with arrows. A: Sham-treated control. B: 665 nm laser-treated mouse. C: 810 nm laser-treated mouse. D: Mean fractional defect area in brains of sham, 665 nm, and 810 nm laser-treated mice. Values are means from 8 to 12 mice per group and bars are SD. *P < 0.05; **P < 0.01 (one-way ANOVA).
TABLE 2.
Morphometry Measurements on Areas of Brain Sections Taken at 28 Days From Sham, 665 nm, and 810 nm Laser-Treated Mouse Groups
| Mouse | Whole area (pixels)a |
Stained area (pixels)b |
Area of deficits (pixels)c |
Fractional area that is deficitd |
|---|---|---|---|---|
| Sham 1 | 2,733 | 2,362 | 371 | 0.135 |
| Sham 2 | 2,750 | 2,389 | 361 | 0.131 |
| Sham 3 | 2,763 | 2,342 | 421 | 0.152 |
| Sham 4 | 4,059 | 3,519 | 540 | 0.133 |
| Sham 5 | 4,062 | 3,638 | 424 | 0.104 |
| Sham 6 | 4,056 | 3,565 | 491 | 0.121 |
| Sham 7 | 2,689 | 2,517 | 172 | 0.063 |
| Sham 8 | 2,236 | 1,960 | 276 | 0.123 |
| Sham 9 | 2,741 | 2,439 | 302 | 0.11 |
| Mean ± SD | 0.119 ± 0.029 | |||
| 665 nm 1 | 2,711 | 2,553 | 158 | 0.058 |
| 665 nm 2 | 2,726 | 2,545 | 181 | 0.066 |
| 665 nm 3 | 2,630 | 2,474 | 156 | 0.059 |
| 665 nm 4 | 2,569 | 2,398 | 171 | 0.067 |
| 665 nm 5 | 2,470 | 2,378 | 92 | 0.037 |
| 665 nm 6 | 2,448 | 2,303 | 145 | 0.059 |
| 665 nm 7 | 1,756 | 1,619 | 137 | 0.078 |
| 665 nm 8 | 1,658 | 1,516 | 142 | 0.086 |
| Mean ± SD | 0.064 ± 0.024 | |||
| 810 nm 1 | 1,342 | 1,274 | 68 | 0.051 |
| 810 nm 2 | 1,021 | 936 | 85 | 0.083 |
| 810 nm 3 | 1,086 | 997 | 89 | 0.082 |
| 810 nm 4 | 1,143 | 1,025 | 118 | 0.103 |
| 810 nm 5 | 1,367 | 1,278 | 89 | 0.065 |
| 810 nm 6 | 2,890 | 2,854 | 36 | 0.012 |
| 810 nm 7 | 2,743 | 2,654 | 89 | 0.032 |
| 810 nm 8 | 2,699 | 2,514 | 185 | 0.069 |
| 810 nm 9 | 3,150 | 3,026 | 124 | 0.039 |
| 810 nm 10 | 2,951 | 2,908 | 43 | 0.015 |
| 810 nm 11 | 3,008 | 2,912 | 96 | 0.032 |
| Mean ± SD | 0.053 ± 0.029 |
Calulated by drawing an outline around the brain section and determining pixel area.
Calculated by measuring H&E stained pixel area.
Column 1 – column 2.
Column 3/column 1.
DISCUSSION
This study has shown that both red laser (665 nm) and NIR laser (810 nm) can significantly improve the neurobehavioral performance of mice after CHI TBI. Interestingly however, NIR lasers at 730 or 980 nm did not produce the same beneficial positive effects on NSS improvement. In addition, the error bars were higher for the 980 nm laser-treated mice than for the other wavelengths and this may lessen the reliability of the finding.
The principal tissue chromophore that is proposed to be responsible for photobiomodulation effects is cytochrome c oxidase (CCO). CCO has distinct absorption bands in the red (around 665 nm) and in the NIR (around 810 nm) [26]. There is a minimum in the CCO absorption spectrum at 730 nm, and there have been reports that this wavelength is ineffective in preserving cultured cortical neurons from cyanide toxicity [27], while 670 and 830 nm were highly active in this application. In our hands 980 nm was ineffective. However, it should be mentioned that several reports show that 980 nm is an active wavelength in LLLT applications [28–30]. It is entirely possible that our finding that 980 nm was ineffective only meant that we used the wrong parameters. In other words, if the fluence or irradiance of 980 nm laser was lower than we used (or possibly higher) the effect on TBI might have been positive.
LLLT is increasingly being used for tissue preservation and functional stimulation at the cellular level, after various forms of injury. Stimulated wound healing, reduction of edema and inflammation, pain relief, and prevention of tissue loss are the main applications. During LLLT, absorption of red or NIR photons by CCO in the mitochondrial respiratory chain causes an increase in cellular respiration that continues for much longer than the light is present when delivered at appropriate fluences, irradiances, and exposure durations [31]. Primary cellular effects include increases in mitochondrial activity and ATP levels [32], modulation of reactive oxygen species [33], induction of transcription factors (including the pro-survival NF-κB) [34], and inhibition of apoptosis [35].
Secondary injuries after TBI are attributable to spreading cellular damage that can grow to encompass a much greater area of the brain than was originally damaged and results from excitotoxic cell death after glutamate release and sequelae of inflammation [36]. Secondary injury cascades can affect neurological function unrelated to specific tissue damage. Glutamate depolarizes neurons that suffer a huge influx of sodium and calcium but could survive if they had sufficient ATP to power the Na+/K+-ATPase pumps. ATP is increased by LLLT and could prevent excitotoxicity.
LLLT modulates many biological and disease processes increasing fibroblast migration, proliferation and differentiation, angiogenesis, and healing in ischemic heart and skeletal muscles, tendons, and bones. There are reports of beneficial effects of LLLT on functional recovery of injured peripheral nerves (780 nm) [37,38] and also of injured central neurons in the spinal cord (810 nm) [23,39].
Transcranial LLLT has been used to significantly improve recovery in a number of animal models of stroke [20]. LLLT at 808–810 nm can penetrate the brain and was shown to lead to enhanced production of ATP in the rat cerebral cortex [40]. Findings of increased neurogenesis in the subventricular zone (SVZ) were reported in an ischemic stroke animal model treated with TLT [41]. Based on these findings, it is thought that TLT may have multiple mechanisms of action and could be beneficial in acute ischemic stroke [20]. In a rat model of cerebral ischemia induced by a 1-hour occlusion of middle cerebral artery, LLLT (660 nm laser) delivered immediately after reperfusion through a craniotomy, reduced nitric oxide synthase activity, and upregulated TGF-β1 expression simultaneously [42]. The success of transcranial LLLT in these animal studies of stroke encouraged a clinical trial (NEST-1) in human stroke patients [43] in which only one transcranial NIR (808 nm) laser treatment at ~18 hours after stroke led to significant improvement. A second larger clinical trial (NEST-2) failed to meet its primary endpoint [44], but a subset of patients (moderate strokes) showed significant benefit [45].
Oron et al. [46] carried out a study of transcranial LLLT in a mouse model of closed-head TBI. They used 810 nm laser delivered to the skull (skin removed) 4-hour post-TBI with a total power of 200 mW and a calculated irradiance to the cortical surface of either 10 or 20 mW/cm2 giving a calculated fluence of 1.2–2.4 J/cm2 to the brain. They found an improvement in NSS in the laser-treated mice compared to untreated TBI mice starting at 5 days and increasing as time went on in a similar manner to the improvement in NSS we reported in the present study. However, the extent of improvement was higher in our study than in the Oron study [46]. The model of CHI employed in the Oron study [46] was also somewhat different to ours in that it resulted in pronounced focal brain lesions in the area of brain under the skull impact as described in the protocol by Shapira et al. [47].
Recently, Khuman et al. [48] carried out a study of transcranial LLLT in a mouse model of open skull controlled cortical impact (CCI). They used 800 nm laser applied directly to the contused parenchyma or delivered transcranially beginning 60–80 min after CCI. Injured mice treated with 60 J/cm2 (500 mW/cm2 over 2 min) either transcranially or via an open craniotomy had significantly improved latency to the hidden platform in the Morris Water Maze and probe trial performance compared to non-treated TBI controls. The beneficial effects of LLLT in open craniotomy mice were associated with reduced microgliosis at 48 hours. Little or no effect of LLLT on post-injury cognitive function was observed using other doses, a 4-hour administration time point and 7-day administration of 60 J/cm2. No effect of LLLT (60 J/cm2 open craniotomy) was observed on post-injury motor function (days 1–7), brain edema (24 hours), nitrosative stress (24 hours), or lesion volume (14 days).
We very recently [49] completed a study of transcranial LLLT in mice with CCI-TBI. We used 810 nm laser (36 J/cm2 at 50 mW/cm2) delivered to the head at 4-hour post-TBI and compared CW-laser with pulsed-laser (10 or 100 Hz both at 50% duty cycle). Surprisingly, while all three-laser regimens gave significantly improved NSS scores, the 10 Hz pulse structure was significantly better than the other two laser regimens. Furthermore, significant improvements in mouse performance in the forced swim test and the tail suspension test suggested that psychological problems, such as depression and anxiety associated with TBI might also be helped by LLLT. There was significantly reduced lesion area at 28 days in the 10 Hz laser group.
The fact that three separate groups have now obtained positive results using transcranial LLLT in mouse models of TBI suggests that this is an approach that could rapidly advance to clinical trials. As further preclinical studies are reported the optimum laser parameters and optimum treatment timing will become clearer. The total lack of reports of adverse effects after delivery of LLLT to the head encourages us to believe that transcranial laser could be an extremely promising therapy for brain injury in humans.
Acknowledgments
Contract grant sponsor: NIH; Contract grant number: R01AI050875; Contract grant sponsor: Center for Integration of Medicine and Innovative Technology; Contract grant number: DAMD17-02-2-0006; Contract grant sponsor: CDMRP Program in TBI; Contract grant number: W81XWH-09-1-0514; Contract grant sponsor: Air Force Office of Scientific Research; Contract grant number: FA9950-04-1-0079.
Footnotes
Conflict of interest: None reported.
REFERENCES
- 1.Albensi BC. Models of brain injury and alterations in synaptic plasticity. J Neurosci Res. 2001;65(4):279–283. doi: 10.1002/jnr.1151. [DOI] [PubMed] [Google Scholar]
- 2.Waxweiler RJ, Thurman D, Sniezek J, Sosin D, O’Neil J. Monitoring the impact of traumatic brain injury: A review and update. J Neurotrauma. 1995;12(4):509–516. doi: 10.1089/neu.1995.12.509. [DOI] [PubMed] [Google Scholar]
- 3.Sosin DM, Sniezek JE, Thurman DJ. Incidence of mild and moderate brain injury in the United States, 1991. Brain Inj. 1996;10(1):47–54. doi: 10.1080/026990596124719. [DOI] [PubMed] [Google Scholar]
- 4.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003;10(44 Suppl):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 5.Kraus JF, McArthur DL. Epidemiologic aspects of brain injury. Neurol Clin. 1996;14(2):435–450. doi: 10.1016/s0733-8619(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 6.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Finfer SR, Cohen J. Severe traumatic brain injury. Resuscitation. 2001;48(1):77–90. doi: 10.1016/s0300-9572(00)00321-x. [DOI] [PubMed] [Google Scholar]
- 8.MacGregor AJ, Dougherty AL, Galarneau MR. Injury-specific correlates of combat-related traumatic brain injury in Operation Iraqi Freedom. J Head Trauma Rehabil. 2011;26(4):312–318. doi: 10.1097/HTR.0b013e3181e94404. [DOI] [PubMed] [Google Scholar]
- 9.Risdall JE, Menon DK. Traumatic brain injury. Philos Trans R Soc Lond B Biol Sci. 2011;366(1562):241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marklund N, Hillered L. Animal modelling of traumatic brain injury in preclinical drug development: Where do we go from here? Br J Pharmacol. 2011;164(4):1207–1229. doi: 10.1111/j.1476-5381.2010.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zink BJ, Szmydynger-Chodobska J, Chodobski A. Emerging concepts in the pathophysiology of traumatic brain injury. Psychiatr Clin North Am. 2010;33(4):741–756. doi: 10.1016/j.psc.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: Neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39(1):55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- 13.Bramlett HM, Dietrich WD. Pathophysiology of cerebral ischemia and brain trauma: Similarities and differences. J Cereb Blood Flow Metab. 2004;24(2):133–150. doi: 10.1097/01.WCB.0000111614.19196.04. [DOI] [PubMed] [Google Scholar]
- 14.Nortje J, Menon DK. Traumatic brain injury: Physiology, mechanisms, and outcome. Curr Opin Neurol. 2004;17(6):711–718. doi: 10.1097/00019052-200412000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Protheroe RT, Gwinnutt CL. Early hospital care of severe traumatic brain injury. Anaesthesia. 2011;66(11):1035–1047. doi: 10.1111/j.1365-2044.2011.06874.x. [DOI] [PubMed] [Google Scholar]
- 16.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, Bullock MR, Choi SC, Clifton GL, Contant CF, Coplin WM, Dietrich WD, Ghajar J, Grady SM, Grossman RG, Hall ED, Heetderks W, Hovda DA, Jallo J, Katz RL, Knoller N, Kochanek PM, Maas AI, Majde J, Marion DW, Marmarou A, Marshall LF, McIntosh TK, Miller E, Mohberg N, Muizelaar JP, Pitts LH, Quinn P, Riesenfeld G, Robertson CS, Strauss KI, Teasdale G, Temkin N, Tuma R, Wade C, Walker MD, Weinrich M, Whyte J, Wilberger J, Young AB, Yurkewicz L. Clinical trials in head injury. J Neurotrauma. 2002;19(5):503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamblin MR, Demidova TN. Mechanisms of low level light therapy—An introduction. In: Hamblin MR, Anders JJ, Waynant RW, editors. Proceedings of SPIE. vol 2160. San Jose. Bellingham, WA: The International Society for Optical Engineering; 2006. pp. 61001–61012. [Google Scholar]
- 18.Huang Y-Y, Chen AC-H, Hamblin MR. Advances in low-intensity laser and phototherapy. In: Tuchin VV, editor. Handbook of photonics for medical science. London, UK: Taylor and Francis; 2010. pp. 687–716. [Google Scholar]
- 19.Huang Y-Y, Hamblin MR, De Taboada L. Low-level laser therapy in stroke and central nervous system. In: Tuchin VV, editor. Handbook of photonics for medical science. London, UK: Taylor and Francis; 2010. pp. 717–737. [Google Scholar]
- 20.Lampl Y. Laser treatment for stroke. Expert Rev Neurother. 2007;7(8):961–965. doi: 10.1586/14737175.7.8.961. [DOI] [PubMed] [Google Scholar]
- 21.Naeser MA, Hamblin MR. Potential for transcranial laser or LED therapy to treat stroke, traumatic brain injury, and neurodegenerative disease. Photomed Laser Surg. 2011;29(7):443–446. doi: 10.1089/pho.2011.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T, Streeter J, Kindy MS. Transcranial laser therapy attenuates amyloid-beta peptide neuropathology in amyloid-beta protein precursor transgenic mice. J Alzheimers Dis. 2011;23(3):521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Dmitriev AE, Cardoso MJ, Viers-Costello AG, Borke RC, Streeter J, Anders JJ. 810 nm Wavelength light: An effective therapy for transected or contused rat spinal cord. Lasers Surg Med. 2009;41(1):36–41. doi: 10.1002/lsm.20729. [DOI] [PubMed] [Google Scholar]
- 24.Demir O, Singh S, Klimaschewski L, Kurnaz IA. From birth till death: neurogenesis, cell cycle, and neurodegeneration. Anat Rec (Hoboken) 2009;292(12):1953–1961. doi: 10.1002/ar.20980. [DOI] [PubMed] [Google Scholar]
- 25.Davis PJ, Rabinowitz P. Methods of numerical integration. Mineola, NY: Dover Publications; 2007. p. xii. 612. [Google Scholar]
- 26.Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI. Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B. 2005;81(2):98–106. doi: 10.1016/j.jphotobiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Wong-Riley MT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, Kane M, Whelan HT. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: Role of cytochrome c oxidase. J Biol Chem. 2005;280(6):4761–4771. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 28.Park JJ, Kang KL. Effect of 980-nm GaAlAs diode laser irradiation on healing of extraction sockets in streptozotocin-induced diabetic rats: A pilot study. Lasers Med Sci. 2012;27:223–230. doi: 10.1007/s10103-011-0944-8. [DOI] [PubMed] [Google Scholar]
- 29.Skopin MD, Molitor SC. Effects of near-infrared laser exposure in a cellular model of wound healing. Photodermatol Photoimmunol Photomed. 2009;25(2):75–80. doi: 10.1111/j.1600-0781.2009.00406.x. [DOI] [PubMed] [Google Scholar]
- 30.Al-Watban FA, Zhang XY, Andres BL. Low-level laser therapy enhances wound healing in diabetic rats: A comparison of different lasers. Photomed Laser Surg. 2007;25(2):72–77. doi: 10.1089/pho.2006.1094. [DOI] [PubMed] [Google Scholar]
- 31.Lane N. Cell biology: Power games. Nature. 2006;443(7114):901–903. doi: 10.1038/443901a. [DOI] [PubMed] [Google Scholar]
- 32.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84(5):1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 33.Sharma SK, Kharkwal GB, Sajo M, Huang YY, De Taboada L, McCarthy T, Hamblin MR. Dose response effects of 810-nm laser-light on mouse primary cortical neurons. Lasers Surg Med. 2011;43:851–859. doi: 10.1002/lsm.21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-level laser therapy activates NF-ΚB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Wu S, Xing D. Inhibition of Abeta(25–35)-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012;24:224–232. doi: 10.1016/j.cellsig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Rovegno M, Soto PA, Saez JC, von Bernhardi R. Biological mechanisms involved in the spread of traumatic brain damage. Med Intensiva. 2011 doi: 10.1016/j.medin.2011.06.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Rochkind S, Drory V, Alon M, Nissan M, Ouaknine GE. Laser phototherapy (780 nm), a new modality in treatment of long-term incomplete peripheral nerve injury: A randomized double-blind placebo-controlled study. Photomed Laser Surg. 2007;25(5):436–442. doi: 10.1089/pho.2007.2093. [DOI] [PubMed] [Google Scholar]
- 38.Rochkind S, Leider-Trejo L, Nissan M, Shamir MH, Kharenko O, Alon M. Efficacy of 780-nm laser phototherapy on peripheral nerve regeneration after neurotube reconstruction procedure (double-blind randomized study) Photomed Laser Surg. 2007;25(3):137–143. doi: 10.1089/pho.2007.2076. [DOI] [PubMed] [Google Scholar]
- 39.Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, Anders JJ. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg Med. 2005;36(3):171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- 40.Streeter J, De Taboada L, Oron U. Mechanisms of action of light therapy for stroke and acute myocardial infarction. Mitochondrion. 2004;4(5–6):569–576. doi: 10.1016/j.mito.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 41.Oron A, Oron U, Chen J, Eilam A, Zhang C, Sadeh M, Lampl Y, Streeter J, DeTaboada L, Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37(10):2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- 42.Leung MC, Lo SC, Siu FK, So KF. Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1. Lasers Surg Med. 2002;31(4):283–288. doi: 10.1002/lsm.10096. [DOI] [PubMed] [Google Scholar]
- 43.Lampl Y, Zivin JA, Fisher M, Lew R, Welin L, Dahlof B, Borenstein P, Andersson B, Perez J, Caparo C, Ilic S, Oron U. Infrared laser therapy for ischemic stroke: A new treatment strategy. Results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- 44.Zivin JA, Albers GW, Bornstein N, Chippendale T, Dahlof B, Devlin T, Fisher M, Hacke W, Holt W, Ilic S, Kasner S, Lew R, Nash M, Perez J, Rymer M, Schellinger P, Schneider D, Schwab S, Veltkamp R, Walker M, Streeter J. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40(4):1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]
- 45.Stemer AB, Huisa BN, Zivin JA. The evolution of transcranial laser therapy for acute ischemic stroke, including a pooled analysis of NEST-1 and NEST-2. Curr Cardiol Rep. 2010;12(1):29–33. doi: 10.1007/s11886-009-0071-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oron A, Oron U, Streeter J, de Taboada L, Alexandrovich A, Trembovler V, Shohami E. low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J Neurotrauma. 2007;24(4):651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- 47.Shapira Y, Shohami E, Sidi A, Soffer D, Freeman S, Cotev S. Experimental closed head injury in rats: Mechanical, pathophysiologic, and neurologic properties. Crit Care Med. 1988;16(3):258–265. doi: 10.1097/00003246-198803000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Khuman J, Zhang J, Park J, Carroll JD, Donahoe C, Whalen MJ. Low-level laser light therapy improves cognitive deficits and inhibits microglial activation after controlled cortical impact in mice. J Neurotrauma. 2011 doi: 10.1089/neu.2010.1745. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ando T, Xuan W, Xu T, Dai T, Sharma SK, Kharkwal GB, Huang YY, Wu Q, Whalen MJ, Sato S, Obara M, Hamblin MR. Comparison of therapeutic effects between pulsed and continuous wave 810-nm wavelength laser irradiation for traumatic brain injury inmice. PLoS ONE. 2011;6(10):e26212–e26220. doi: 10.1371/journal.pone.0026212. [DOI] [PMC free article] [PubMed] [Google Scholar]