Abstract
Here we studied the mechanism by which Hsp70 prevents Bax activation during UV-induced apoptosis. UV treatment led to JNK phosphorylation, Bim redistribution and subsequent Bax activation. Bim depletion caused a smaller reduction in apoptosis than that by JNK inhibition, indicating that Bim activation is not entirely responsible for induction of apoptosis and other mechanisms are involved. Hsp70 knockdown resulted in high levels of activated JNK and Bax, while Hsp70 overexpression inhibited these processes. These findings demonstrate that Hsp70 prevented Bax activation via inhibiting the JNK/Bim pathway. Simultaneously, increased binding of Hsp70 to Bax was observed. Collectively, our results for the first time demonstrate that Hsp70 prevents Bax activation both by inhibiting the JNK/Bim pathway and by interacting with Bax in UV-induced apoptosis.
Keywords: Hsp70, Bax, JNK, BimL, UV irradiation
1. Introduction
Apoptosis can be initiated by diverse forms of cell stress such as heat shock and UV irradiation [1]. The Bcl-2 family members play a critical role in regulating apoptosis [2]. Bcl-2 family comprises three subfamilies: (a) antiapoptotic members, such as Bcl-2/Bcl-XL; (b) proapoptotic members, such as Bax, Bak, and Bok; and (c) BH3-only proteins, such as Bid, Bim, Puma, and Bmf [3]. The proapoptotic protein Bax plays an important role in apoptosis [4]. Additionally, the c-Jun N-terminal Kinase (JNK) signaling pathway promotes Bax activation by phosphorylating Bim, suggesting that Bim provides a molecular link between the JNK signaling pathway and the Bax-dependent mitochondrial apoptotic machinery [5]. Following exposure to an apoptotic stimulus, Bax undergoes a conformational change, leading to exposure of its N- and C-termini and to its mitochondrial targeting. Within the mitochondrial membrane, oligomerized Bax facilitates mitochondrial membrane permeabilization, leading to cytochrome c release from mitochondria [4,6]. However, cells have self-repairing system to suppress apoptosis under harmful conditions, which can be accomplished by members of the heat shock protein family [7].
Heat shock proteins (Hsps) are a set of highly conserved proteins and they function as molecular chaperones. A well-characterized subgroup of Hsps is the Hsp70 family [8]. There are several Hsp70 family members, including stress-inducible Hsp70, constitutively expressed Hsp70 (Hsc70), mitochondrial Hsp75, and GRP78 [9]. The expression of Hsp70 can be induced by a variety of stresses, including heat shock, UV irradiation and oxidative stress [8]. Hsp70 has been reported to protect cells from apoptosis induced by various stresses and agents [10]. It can block the apoptotic pathway at different levels [11]. Most importantly, recent studies have suggested that Hsp70 prevents Bax translocation to mitochondria and blocks mitochondrial membrane permeabilization [12-15], although its molecular mechanisms are not clear at present.
The aim of this study is to investigate how Hsp70 inhibits Bax activation in UV-induced apoptosis. To determine the molecular mechanisms involved in this process, this study focuses on: (i) the activation of the JNK/Bim/Bax signaling pathway after UV irradiation; (ii) inhibitory effects of Hsp70 on the JNK/Bim/Bax pathway in UV-induced apoptosis; (iii) the interaction between Hsp70 and Bax.
2. Materials and methods
2.1. Materials and plasmids
We used antibodies against Hsp70, JNK and Bax (Cell Signaling Technology) and p-JNK (BD Biosciences). CFP-Bax was provided by Drs. Streuli and Gilmore (University of Manchester), YFP-Hsp70 was a gift from Dr. Morimoto of Northwestern University, and pDsRed-Mit was supplied by Dr. Gotoh (University of Yokyo). Hsp70 shRNA (short hairpin RNA) and Scr were provided by Dr. Tolkovsky [16]. The oligonucleotides for shRNA Bim were purchased from GenePharma (Shanghai, China) and were used as previously described [17]. GFP-BimL was generated as previously described [18]. Other chemicals were purchased from Sigma-Aldrich (St Louis, MO).
2.2. Cell culture and treatments
The human lung adenocarcinoma cell line (ASTC-a-1) was cultured in DMEM supplemented with 15% fetal calf serum (FCS), penicillin (100 units/ml), and streptomycin (100 mg/ml) at 37 °C with 5% CO2 in a humidified incubator. Transfection was performed with Lipofectamine™ 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Cells were examined at 24-48 hours after transfection. Before the 120 mJ/cm2 UV treatment, medium was removed and collected, and then cells were rinsed with phosphate buffered saline. The medium was restored after treatment. For experiments with the inhibitor, cells were pretreated with 20 μM SP600125 (a specific inhibitor of JNK, Sigma, St. Louis, MO, USA) for 1 h before UV irradiation. SP600125 was kept in the medium throughout the experimental process.
2.3. Cell viability assays
ASTC-a-1 cells were cultured in a 96-well microplate at a density of 5 × 103 cells/well for 24 hours. Cell viability was assessed with Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) at indicated times post UV treatment. OD450, the absorbance value at 450 nm, was read with a 96-well plate reader (DG5032, Huadong, Nanjing, China), to determine the viability and proliferation of the cells.
2.4. Flow cytometry
Annexin V-fluorescein isothiocyanate (FITC; 0.1 μg/ml) was used for the assessment of phosphatidylserine exposure. Propidium iodide (PI; 0.5 μg/ml) was used for cell viability analysis. Cell death was measured in a FACSCanto™ II cytofluorimeter (Becton Dickinson, Mountain View, CA). Compensation was used wherever necessary.
2.5. Subcellular fractionation
Cytosolic and mitochondria-enriched fractions were prepared using Subcellular Proteome Extraction Kit (ProteoExtract™, Calbiochem, Darmstadt, Germany) according to the manufacturer's instructions.
2.6. Bax conformational change analysis
Cells were lysed with ice-cold lysis buffer (150 mM NaCl, 10 mM HEPES (pH 7.4), 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonic acid, and 100 μg/ml PMSF) containing protease inhibitors. For immunoprecipitation, 2.5 μg of anti-Bax 6A7 monoclonal antibody (Abcam, Cambridge) was added into 500 μg of cell lysate. The obtained immune complexes were subjected to western blotting analysis with anti-Bax polyclonal antibody.
2.7. LCSM and fluorescence resonance energy transfer (FRET) acceptor photo-bleaching technique
Fluorescence of cyan fluorescent protein (CFP), green fluorescent protein (GFP), yellow fluorescent protein (YFP), red fluorescent protein (DsRed), and Mitotracker were monitored confocally with laser confocal scanning microscopy (LCSM), using different excitation wavelengths and detection filters as previously described [19].
FRET acceptor photo-bleaching was performed on LCSM to detect the interaction between YFP-Hsp70 and CFP-Bax. For excitation, the 458 nm line of an argon-ion laser was attenuated with an acousto-optical tunable filter and reflected by a dichroic mirror (main beam splitter HFT458), and focused through a Plan-Neofluar 40 × /1.3 NA oil DIC objective (Carl Zeiss) onto the sample. CFP (the donor) and YFP (the FRET acceptor) emissions were collected through 470 to 500 and 535 to 545 nm band pass filters, respectively. YFP was excited at 514 nm, and its emission was detected with 565 to 615 nm band-pass (YFP channel). We bleached the YFP signal (the acceptor) in a certain area within the cell (identified by a rectangle) with 514 nm line of an argon-ion laser at 100% power for 300 iterations.
2.8. CFP-Bax and GFP-BimL translocation assay
To monitor Bax translocation in living cells, cells were transfected with CFP-Bax and were stained by MitoTracker for mitochondrial labeling. The cells exhibiting strong punctuate staining of CFP, which overlapped with the distribution of MitoTracker, were counted as the cells with mitochondrially localized Bax. The analysis of GFP-BimL mitochondrial translocation was similar to that of Bax.
2.9. Co-immunoprecipitation and western blotting assays
Cells were lysed with ice-cold lysis buffer (50 mM Tris.HCl [pH 8.0], 150 mM NaCl, 1 × TritonX-100, 100 μg/ml PMSF and Protease Inhibitor Cocktail Set I) for 45 min on ice. After centrifugation, the supernatant was incubated with the antibody against Bax and subsequently with protein A-Sepharose (50% slurry) at 4 °C overnight. After washed five times, pellet was resuspended with the same volume of SDS sample buffer, and boiled to remove Sepharose beads. Then the cell lysates and immunoprecipitates were analyzed by western blotting [20].
3. Results
3.1. Hsp70 confers resistance against UV-induced apoptosis
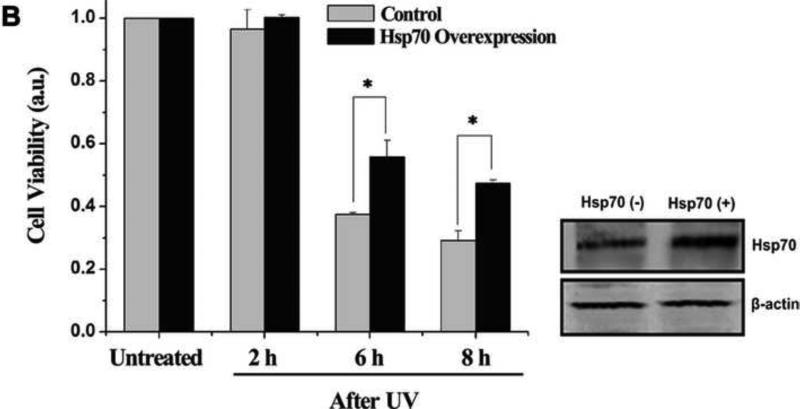
To study Hsp70 expression after UV irradiation, western blotting analysis was performed. The results show that the expression of Hsp70 increased gradually (Fig. 1A). To investigate the cytoprotective function of Hsp70 after UV irradiation, cell viability was analyzed using CCK-8. Overexpressed Hsp70 clearly reduced the level of cell death, compared with the UV-only treatment (Fig. 1B). In addition, western blotting was performed to confirm Hsp70 overexpression (Fig. 1B).
Fig. 1.
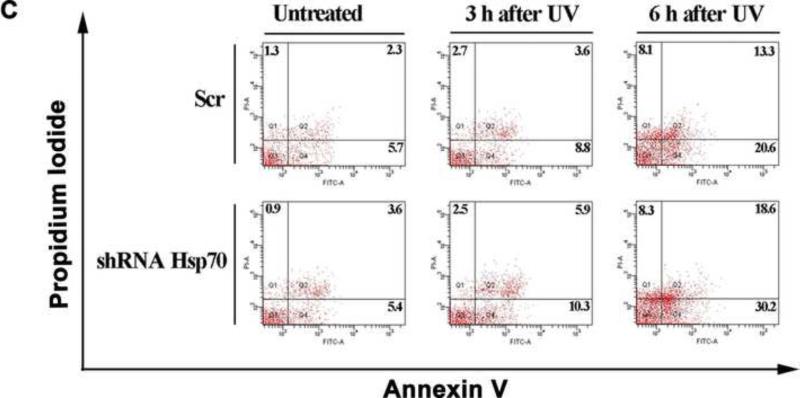
Hsp70 inhibits UV-induced cell apoptosis. (A) Increase in expression of Hsp70 in ATSC-a-1 cells after UV irradiation. β-actin served as a control. (B) Effects of Hsp70 overexpression on cell viability. Viability of ASTC-a-1 cells was assessed by the CCK-8 assays at different time points. Western blotting was performed to confirm Hsp70 overexpression. Data represent mean ± SD of 4 independent experiments, *P < 0.05 vs UV-only treatment. β-actin served as a control. (C) Cell apoptosis in flow cytometry. Numbers represent the percent of cells with positive staining.
We further studied cell apoptosis using flow cytometry after knocking down Hsp70 utilizing RNA interference approach. Scr was used as control. The data show that silencing Hsp70 increased cell apoptosis (Fig. 1C). Statistical results of apoptotic cells under different treatments are given in Fig. S1 (Supplementary Information). Western blotting was also performed to confirm Hsp70 knockdown (Fig. S1). These results clearly suggest that Hsp70 has distinct cytoprotective function in UV-induced apoptosis.
3.2. Hsp70 prevents Bax mitochondrial translocation
Generally, the activation of Bax is inferred by its translocation from cytosol to mitochondria. UV-induced Bax mitochondrial translocation, as well as the activation of Bax, was investigated using western blotting analysis. Conformational changed Bax was detected using 6A7 monoclonal antibody, which could selectively recognize the activated Bax. The results show that Bax translocated to mitochondria after UV irradiation in a time-dependent manner. Simultaneously, the activated Bax on mitochondria increased gradually (Fig. 2A). To determine the impact of Hsp70 on Bax translocation after UV irradiation, single-cell real-time analysis was utilized. Cells were transiently transfected with CFP-Bax alone or co-transfected with CFP-Bax and YFP-Hsp70. MitoTracker was used to label mitochondria. CFP-Bax had a diffuse distribution throughout the cytosol in the untreated cells (Fig. 2B, left panel). After UV irradiation, almost all the CFP-Bax translocated from cytosol to mitochondria, indicating the activation of Bax (Fig. 2B, right panel). However, when cells were overexpressed with YFP-Hsp70, UV-induced Bax translocation to mitochondria was markedly delayed (Fig. 2C). Detailed time courses of the mitochondrial CFP-Bax fluorescence intensity after different treatments are shown in Fig. S2 (Supplementary Information). Quantitative analyses show that Bax translocation was time-dependent after UV treatment and overexpression of Hsp70 could delay the translocation. Taken together, these results suggest that Hsp70 can inhibit translocation of Bax in UV-induced apoptosis.
Fig. 2.
Prevention of Bax mitochondrial translocation by Hsp70 in UV-induced apoptosis. ASTC-a-1 cells were transiently transfected with CFP-Bax alone or co-transfected with YFP-Hsp70. MitoTracker was used to label mitochondria. (A) Bax translocated to mitochondria after UV irradiation. β-actin and CoxIV were used as control markers for the cytosolic and mitochondrial fractions, respectively. (B) Control cells without Bax translocation (left panel) and time-lapse images of CFP-Bax redistribution after UV irradiation (right panel). Bar, 10 μm. (C) Delay of Bax mitochondrial translocation after UV irradiation in Hsp70 overexpressed cells. Bar, 10 μm.
3.3. Hsp70 prevents UV-induced Bax activation by inhibiting JNK/Bim signaling pathway
Our results show that Hsp70 can inhibit the redistribution of Bax after UV irradiation. However, how it does this remains unknown. We hypothesize that Hsp70 prevents Bax activation through inhibition of JNK in UV-induced apoptosis. In order to test this hypothesis, western blotting was performed to detect the level of JNK phosphorylation. The results show that JNK was activated after UV irradiation (Fig. S3, Supplementary Information), and overexpression of Hsp70 decreased the level of phosphorylated JNK (Fig. 3A). To further determine the role of Hsp70 in inactivating JNK, we detected the level of JNK phosphorylation after knocking down Hsp70. The results show that depletion of Hsp70 resulted in a high level of activated JNK (Fig. 3A). These results demonstrate that Hsp70 could inhibit JNK activation in UV-induced apoptosis.
Fig. 3.
Inhibition of JNK/Bim pathway by Hsp70 to prevent Bax activation in UV-induced apoptosis. (A) Levels of Hsp70, p-JNK, and activated Bax after different treatments. Western blotting shows that Hsp70 inhibits JNK and Bax activation after UV treatment. β-actin served as a control of Hsp70 and Bax. JNK served as a control of p-JNK. (B) Inhibition of Bim and JNK protects cells from apoptosis as determined by flow cytometry. Numbers represent the percent of cells with positive staining. (C) ASTC-a-1 cells were transiently co-transfected with GFP-BimL and DsRed-Mit. Control cells without BimL translocation (left panel). GFP-BimL redistributed after UV irradiation (middle panel) and the mitochondrial translocation of GFP-BimL was inhibited in the presence of SP600125 (right panel). Bar, 10 μm. (D) GFP-BimL translocation was prevented in the Hsp70 overexpressed cells after UV irradiation. Bar, 10 μm.
To determine the role of JNK in promoting Bax activation after UV irradiation, cells were pretreated with 20 μM SP600125 for 1 h before UV irradiation. In the presence of SP600125, Bax mitochondrial translocation was markedly delayed compared to UV-only treatment (Figs. S4 and S5, Supplementary Information). Further, our data show that the level of activated Bax decreased in parallel with that of phosphorylated JNK when Hsp70 was overexpressed (Fig. 3A). In contrast, the amount of activated Bax increased when Hsp70 was depleted by shRNA (Fig. 3A). The above results suggest that Hsp70 can prevent Bax activation via inhibition of JNK in UV-induced apoptosis.
Kui Lei et al. reported that JNK was the upstream signal of Bim [5]. Moreover, our previous studies have demonstrated that BimL, one important isoform of Bim, can promote Bax activation via directly neutralizing Bcl-xL [17,21]. Herein, we ask whether Hsp70 could inhibit JNK/Bim signaling pathway to prevent Bax activation. The role of Bim in UV-induced apoptosis was determined by flow cytometry after silencing of Bim using RNA interference approach. The data show that depletion of Bim as well as inhibition of JNK by SP600125 decreased apoptotic cells compared to UV-only treatment (Fig. 3B). Statistical results of apoptotic cells under different treatments are given in Fig. S6 (Supplementary Information). Furthermore, western blotting was performed to confirm Bim knockdown, and shRNA NC was used as control. (Fig. S6).
The effect of Hsp70 on JNK/Bim pathway was detected using real-time single-cell analysis. Cells were transfected with GFP-BimL to follow BimL migration with fluorescence imaging, and DsRed-Mit was transfected to label the mitochondria. GFP-BimL had a diffuse distribution throughout the cytoplasm in non-apoptotic control cells (Fig. 3C, left panel). As shown in Fig. 3C (middle panel), BimL clearly translocated to mitochondria after UV treatment. In the presence of SP600125, BimL largely remained in the cytoplasm throughout the observation period after UV irradiation (Fig. 3C, right panel), indicating that JNK activation was required for Bim mitochondrial translocation. Cells were transiently co-transfected with GFP-BimL and YFP-Hsp70. As shown in Fig. 3D, Hsp70 overexpression inhibited BimL mitochondrial translocation as effectively as inhibition of JNK with SP600125 after UV irradiation. Detailed time courses of the mitochondrial GFP-BimL fluorescence intensity after different treatments are given in Fig. S7 (Supplementary Information). Together with the above results, we conclude that Hsp70 can prevent Bax activation by inhibiting the JNK/Bim signaling pathway in UV-induced apoptosis.
3.4. Direct interaction between Hsp70 and Bax increases after UV irradiation
Direct visual proof of FRET in living cells can be obtained by bleaching a certain region of the acceptor and imaging the corresponding increase in fluorescence of the donor in that region. This occurs because the energy of the donor is no longer transferred in the place where the acceptor has been effectively destroyed. To determine whether Hsp70 interacts with Bax in ASTC-a-1 cells, FRET acceptor photo-bleaching experiments were carried out. Cells were transiently co-transfected with CFP-Bax and YFP-Hsp70. As shown in Fig. 4A, after photo-bleaching of YFP-Hsp70 in the indicated area (identified by a rectangle) both in the control cells and in UV treated cells, the fluorescence of YFP-Hsp70 in YFP-channel and in FRET-channel decreased but that of CFP-Bax in CFP-channel increased, indicating that there was direct interaction between Hsp70 and Bax. To further confirm the above results, co-immunoprecipitation was utilized. The data show that the amount of Hsp70 binding to Bax increased after UV irradiation (Fig. 4B). These results demonstrate that Hsp70 can prevent Bax activation not only by inhibiting JNK/Bim signaling pathway but also by directly interacting with Bax in UV-induced apoptosis. A model of Hsp70 preventing Bax mitochondrial translocation in UV-induced apoptosis is shown in Fig. S8 (Supplementary Information).
Fig. 4.
Direct interaction between Hsp70 and Bax. (A) CFP-Bax and YFP-Hsp70 were transiently cotransfected into ASTC-a-1 cells. Fluorescent images of the indicated area (identified by a rectangle) in control cells (left panel) and in UV treated cells (right panel) before and immediate after photo-bleaching in CFP-channel, FRET-channel and YFP-channel respectively. Bar, 10 μm. (B) Interaction between Hsp70 and Bax after UV irradiation. Co-immunoprecipitation with anti-Bax antibody was used to pull down total Bax, and western blotting was performed to detect Hsp70. The amount of Hsp70 binding to Bax increased after UV treatment.
4. Discussion
Hsp70 has been proposed to be a decisive negative regulator of the mitochondrial pathway of apoptosis and it can prevent apoptosis at different levels: at the premitochondrial stage by inhibiting stress-inducing signaling, at the mitochondrial stage by preventing mitochondrial membrane permeabilization through inhibition of Bax activation, at the postmitochondrial level by interacting with AIF and Apaf-1 [11]. Previous studies showed that Hsp70 could directly bind to Apaf-1, thereby preventing the recruitment of procaspase-9 to the apoptosome [22]. Others showed that Hsp70 interacted with procaspase-3 and procaspase-7 and prevented their maturation [23]. Additionally, Hsp70 could interact with AIF directly, leading to inhibition of AIF-induced chromatin condensation [24]. These reports clearly established the anti-apoptotic function of Hsp70 downstream of mitochondria. However, the mechanisms of how Hsp70 inhibits Bax activation to prevent apoptosis at the mitochondrial stage are not clear. Previous reports showed that Hsp70 could inhibit JNK activation to prevent apoptotic signals upstream of mitochondria in heat-induced apoptosis [13]. Fei Guo et al. reported that Hsp70 could increase the level of Bcl-xL to improve its antiapoptotic activity via upregulation of STAT5 in Bcr-Abl–expressing leukemia cells [15]. Additionally, it has been shown that Hsp70 regulates the activity of Bcl-2 via interaction with Bag-1 [25]. Therefore, the machinery of how Hsp70 prevents apoptotic signals upstream of mitochondria is complex; it may depend on the experimental model. In this study, we investigated the cytoprotective function of Hsp70 in UV-induced apoptosis, with a specific focus on how Hsp70 prevented Bax activation. The results show that UV irradiation induced JNK phosphorylation, leading to Bim translocation to mitochondria, and resulted in Bax activation on mitochondria subsequently (Figs. 2-3); knockdown of Hsp70 resulted in high levels of JNK phosphorylation and Bax activation, while overexpression of Hsp70 inhibited these processes (Fig. 3A). These findings demonstrate that Hsp70 prevented Bax activation via inhibiting JNK/Bim pathway during UV-induced apoptosis.
The role of Bim activation in UV-induced apoptosis was investigated by knocking down Bim using RNA interference approach. Our data show that depletion of Bim reduced cell apoptosis (Fig. 3B and Fig. S6). However, the reduction in apoptosis by silencing Bim was less than by inhibiting JNK (Fig. 3B and Fig. S6). These results suggest that Bim activation is not entirely responsible for induction of apoptosis and other mechanisms are involved. Previous studies have shown that Bmf, a member of the BH3-only subgroup of Bcl2-related proteins, can be phosphorylated by JNK and plays a role in promoting Bax activation [5]. Other studies have demonstrated that phosphorylation of 14-3-3 by JNK releases proapoptotic Bad. As a consequence, Bad is dephosphorylated and translocates to the mitochondria, exerting its proapoptotic functions [26]. Therefore, Bim activation is not entirely responsible for induction of apoptosis; other mechanisms are also involved, such as Bmf-mediated apoptotic pathway.
Phosphorylation by JNK activates both BimL and BimEL and increases their apoptotic activity via engaging the mitochondrial apoptotic pathway [5,27]. In this study, we focused on BimL because our previous studies have proved that BimL can promote Bax activation by directly neutralizing Bcl-xL [17,21]. Since BimEL can also be phosphorylated by JNK and promote apoptosis, we will conduct future study on the effects of BimEL.
It has been reported that activated Bax undergoes a conformational change and exerts its proapoptotic activity [4,6]. Recent studies reported that Hsp70 could directly interact with Bax, preventing Bax from changing into the proapoptotic conformation and thus inhibiting apoptosis [12,14,15]. However, the interaction between Hsp70 and Bax was not detected in human acute lymphoblastic T cell line during heat-induced apoptosis [13]. It is probably that it is the differences between the cell lines that lead to the different results. In the present study, FRET technique, a powerful tool for revealing the dynamic activity of protein-protein interaction [20,28,29], was utilized to detect the relationship between Hsp70 and Bax. The results show that there was direct interaction between Hsp70 and Bax (Fig. 4A). Co-immunoprecipitation experiments also confirmed such an interaction and the increased binding of Hsp70 to Bax was detected (Fig. 4B). Since high expression of Hsp70 in cancer has been correlated with poor patient outcome [30], it would be helpful for cancer therapy if some inhibitors could block the activity of Hsp70 effectively.
In conclusion, the present study demonstrates that Hsp70 can prevent Bax activation both by inhibiting the JNK/Bim pathway and by interacting with Bax in UV-induced apoptosis. Considering that Hsp70 is abundantly expressed in most cancer cells [31], it may thus be a therapeutic target for prevention and treatment of cancer.
Supplementary Material
Acknowledgements
This research is supported by the National Basic Research Program of China (2010CB732602), the Program for Changjiang Scholars and Innovative Research Team in University (IRT0829), the National Natural Science Foundation of China (30870676; 30870658), and by the US National Institutes of Health (P20 RR016478 from the INBRE Program of the National Center for Research Resources). We thank Dr. Morimoto for providing the YFP-Hsp70 plasmid and Dr. Gotoh for gifting the pDsRed-Mit plasmid, and we also thank Dr. Tolkovsky for providing the Hsp70 shRNA plasmids.
Abbreviations
- LCSM
laser confocal scanning microscopy
- JNK
c-Jun N-terminal kinase
- CCK-8
Cell Counting Kit-8
- CFP, YFP, DsRed and GFP
cyan, yellow, red and green fluorescent protein
- FRET
fluorescence resonance energy transfer
- Hsp70
heat shock protein 70
- UV
Ultraviolet
- shRNA
short hairpin RNA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rosette C, Karin M. Ultraviolet light and osmotic stress: activation of the JNK cascade through multiple growth factor and cytokine receptors. Science. 1996;274:1194–7. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 4.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA. 2003;100:2432–7. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newmeyer DD, Ferguson-Miller S. Mitochondria: releasing power for life and unleashing the machineries of death. Cell. 2003;112:481–90. doi: 10.1016/s0092-8674(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 7.Young JC, Agashe VR, Siegers K, Hartl FU. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol. 2004;5:781–91. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- 8.Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267:21987–90. [PubMed] [Google Scholar]
- 9.Milarski KL, Morimoto RI. Mutational analysis of the human HSP70 protein: distinct domains for nucleolar localization and adenosine triphosphate binding. J Cell Biol. 1989;109:1947–62. doi: 10.1083/jcb.109.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998;3:228–36. doi: 10.1379/1466-1268(1998)003<0228:hspros>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitt E, Gehrmann M, Brunet M, Multhoff G, Garrido C. Intracellular and extracellular functions of heat shock proteins: repercussions in cancer therapy. J Leukoc Biol. 2007;81:15–27. doi: 10.1189/jlb.0306167. [DOI] [PubMed] [Google Scholar]
- 12.Guo F, et al. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res. 2005;65:10536–44. doi: 10.1158/0008-5472.CAN-05-1799. [DOI] [PubMed] [Google Scholar]
- 13.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–39. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 14.Gotoh T, Terada K, Oyadomari S, Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- 15.Guo F, et al. Mechanistic role of heat shock protein 70 in Bcr-Abl-mediated resistance to apoptosis in human acute leukemia cells. Blood. 2005;105:1246–55. doi: 10.1182/blood-2004-05-2041. [DOI] [PubMed] [Google Scholar]
- 16.King MA, Goemans CG, Hafiz F, Prehn JH, Wyttenbach A, Tolkovsky AM. Cytoplasmic inclusions of Htt exon1 containing an expanded polyglutamine tract suppress execution of apoptosis in sympathetic neurons. J Neurosci. 2008;28:14401–15. doi: 10.1523/JNEUROSCI.4751-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Xing D, Liu L, Chen WR. BimL directly neutralizes Bcl-xL to promote Bax activation during UV-induced apoptosis. FEBS Lett. 2009;583:1873–9. doi: 10.1016/j.febslet.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Xing D, Chen T, Zhang L. BimL involvement in Bax activation during UV irradiation-induced apoptosis. Biochem Biophys Res Commun. 2007;358:559–65. doi: 10.1016/j.bbrc.2007.04.167. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Xing D, Liu L. PUMA promotes Bax translocation by both directly interacting with Bax and by competitive binding to Bcl-XL during UV-induced apoptosis. Mol Biol Cell. 2009;20:3077–87. doi: 10.1091/mbc.E08-11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pei Y, Xing D, Gao X, Liu L, Chen T. Real-time monitoring full length bid interacting with Bax during TNF-alpha-induced apoptosis. Apoptosis. 2007;12:1681–90. doi: 10.1007/s10495-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L, Xing D, Chen M. Bim(L) displacing Bcl-x(L) promotes Bax translocation during TNFα-induced apoptosis. Apoptosis. 2008;13:950–8. doi: 10.1007/s10495-008-0226-5. [DOI] [PubMed] [Google Scholar]
- 22.Beere HM, et al. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469–75. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- 23.Komarova EY, Afanasyeva EA, Bulatova MM, Cheetham ME, Margulis BA, Guzhova IV. Downstream caspases are novel targets for the antiapoptotic activity of the molecular chaperone hsp70. Cell Stress Chaperones. 2004;9:265–75. doi: 10.1379/CSC-27R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruchalski K, et al. Distinct hsp70 domains mediate apoptosis-inducing factor release and nuclear accumulation. J Biol Chem. 2006;281:7873–80. doi: 10.1074/jbc.M513728200. [DOI] [PubMed] [Google Scholar]
- 25.Song J, Takeda M, Morimoto RI. Bag1-Hsp70 mediates a physiological stress signalling pathway that regulates Raf-1/ERK and cell growth. Nat Cell Biol. 2001;3:276–82. doi: 10.1038/35060068. [DOI] [PubMed] [Google Scholar]
- 26.Sunayama J, Tsuruta F, Masuyama N, Gotoh Y. JNK antagonizes Akt-mediated survival signals by phosphorylating 14-3-3. J Cell Biol. 2005;170:295–304. doi: 10.1083/jcb.200409117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hübner A, Barrett T, Flavell RA, Davis RJ. Multisite Phosphorylation Regulates Bim Stability and Apoptotic Activity. Mol. Cell. 2008;30:415–25. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Xing D, Chen WR, Chen T, Pei Y, Gao X. Calpain-mediated pathway dominates cisplatin-induced apoptosis in human lung adenocarcinoma cells as determined by real-time single cell analysis. Int J Cancer. 2008;122:2210–22. doi: 10.1002/ijc.23378. [DOI] [PubMed] [Google Scholar]
- 29.Sekar RB, Periasamy A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol. 2003;160:629–33. doi: 10.1083/jcb.200210140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brondani Da Rocha A, et al. Radio resistance is associated to increased Hsp70 content in human glioblastoma cell lines. Int J Oncol. 2004;25:777–85. [PubMed] [Google Scholar]
- 31.Creagh EM, Sheehan D, Cotter TG. Heat shock proteins--modulators of apoptosis in tumour cells. Leukemia. 2000;14:1161–73. doi: 10.1038/sj.leu.2401841. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.