Abstract
Breast cancer remains one of the most prevalent and lethal malignancies in women. The inability to diagnose small volume metastases early has limited effective treatment of stage 4 breast cancer. Here we report the rational development and use of a multifunctional superparamagnetic iron oxide nanoparticle (SPION) for targeting metastatic breast cancer in a transgenic mouse model and imaging with magnetic resonance (MR). SPIONs coated with a copolymer of chitosan and polyethylene glycol (PEG) were labeled with a fluorescent dye for optical detection and conjugated with a monoclonal antibody against the neu receptor (NP-neu). SPIONs labeled with mouse IgG were used as a non-targeting control (NP-IgG). These SPIONs had desirable physiochemical properties for in vivo applications such as near neutral zeta potential and hydrodynamic size around 40 nm, and were highly stable in serum containing medium. Only NP-neu showed high uptake in neu expressing mouse mammary carcinoma (MMC) cells which was reversed by competing free neu antibody, indicating their specificity to the neu antigen. In vivo, NP-neu was able to tag primary breast tumors and significantly, only NP-neu bound to spontaneous liver, lung, and bone marrow metastases in a transgenic mouse model of metastatic breast cancer, highlighting the necessity of targeting for delivery to metastatic disease. The SPIONs provided significant contrast enhancement in MR images of primary breast tumors; thus, they have the potential for MRI detection of micrometastases, and provide an excellent platform for further development of an efficient metastatic breast cancer therapy.
Keywords: iron oxide, nanoparticle, genetically engineered mouse model, HER2/neu, theranostics, breast cancer, metastases
With 207,090 new cases and 39,840 resultant deaths estimated in 2010, breast cancer is the second most common malignancy and the second leading cause of cancer-related deaths among women in the U.S.1 Despite better understanding of the molecular basis of cancer and advances in treatments, the number of breast cancer deaths per year has shown only a modest decrease over the past 20 years (43,391 breast cancer deaths in 1990 to 39,840 in 2010).2 The greatest challenge has been the early detection and treatment of metastases, as metastatic disease accounts for most of the cancer mortality.3–5 Current treatment consists of surgical resection and radiotherapy for local treatment of the primary cancer and nodal disease, and liberal administration of chemotherapy for locoregional and distant metastatic disease. Unfortunately, more than 80% of breast cancer patients are treated with toxic chemotherapies, whereas only 40% of them have metastatic disease.3 This over-treatment decreases quality of life of patients and imposes added strain to the health care system, however, is still employed given the inability to effectively detect early-stage metastatic disease.
Nanotechnology provides a unique opportunity to generate more effective and less invasive diagnostic and treatment strategies through synthesis of multifunctional nanoparticles (NPs) that provides molecularly targeted therapy.6 Furthermore, the nanoscale imparts unique physical properties on materials used for synthesis such as optical properties on noble metal NPs, fluorescence properties on semiconductor NPs, and superparamagnetic properties on metal oxide NPs that can be exploited for imaging purposes. Of the nanomaterials studied, superparamagnetic iron oxide nanoparticles (SPIONs) have garnered significant attention owing to their biodegradability and inherent contrast enhancement in magnetic resonance imaging (MRI).7, 8 Ideally, a SPION used for breast cancer detection should have: (1) sufficient functional groups on the surface of the SPION for attachment of targeting agents; (2) hydrodynamic size between 10–100 nm to prevent elimination from the blood through the kidneys and liver; (3) near neutral zeta-potential to minimize non-specific interaction with blood components and off-target cells; and (4) high stability in physiologically relevant media. Furthermore, the targeting ligand attached to the surface of the SPION must have a high affinity to breast cancer cell receptors to maximize uptake in target cells while minimizing non-specific uptake in off-target cells.
Neu (HER2/neu in humans or ErbB2) is a proto-oncogene which is over-expressed in up to 30% of breast cancers. Patients harboring HER2/neu positive breast cancers generally have a poorer prognosis because of the highly aggressiveness and chemoresistance of the disease.9, 10 Neu targeting is a promising strategy for detecting aggressive metastatic breast cancer,11 and trastuzumab, a HER2/neu antibody, is currently used for clinical treatment.12 A number of different SPION formulations have been developed to target neu over-expressing breast cancers for imaging and therapies13–16 indicating neu is a good tumor-specific ligand for breast cancer cells. However, clinical translation of SPIONs for breast cancer detection has been slow due to the challenges in meeting all the requirements of SPIONs as contrast agents, including unfavorable pharmacokinetic properties with accumulation of significant amounts of particles in non-target tissues and insignificant amounts in tumor tissues, and more importantly to insufficient pre-clinical testing using artificial xenograft models. Transgenic mouse models more faithfully mimic human disease than xenograft models and offer an improved pre-clinical model for SPION testing.17 Tumors develop in the tissue of interest and recapitulate the evolution of the microenvironment observed in the human disease. Furthermore, unlike the nude and SCID mice used for xenografts, transgenic mice have an intact immune system. Importantly, the development and metastatic progression of the disease mimic what would be seen in a clinical setting. Pre-clinical testing in transgenic mice is expected to accelerate translation since only more robust SPIONs will make it to clinical testing and be less likely to fail in these later, more costly stages.17
Here we report the development of a SPION-based formulation with the physiochemical properties required for targeting breast cancers over-expressing neu receptors, and with the required properties for in vivo imaging as demonstrated in a neu transgenic mouse model. The nanoparticle formulation is made of a SPION core coated with a co-polymer of chitosan-grafted PEG (namely NPCP) and conjugated with neu antibody. Chitosan is a biodegradable natural polymer consisting of multiple functional groups that provide anchoring for drugs, imaging agents, and targeting moieties. PEG is a commonly used polymer that provides steric stabilization for increased colloidal stability and decreased immune recognition. We test the ability of this SPION to specifically recognize breast cancer cells and tag breast tumors in transgenic mice for detection in MRI. Furthermore, we investigate the extent of micrometastases labeling in the lungs, livers, and bone marrow from these transgenic mice.
METHODS
NP Synthesis
SPIONs (Fe3O4) coated with a copolymer of chitosan-g-PEG were synthesized via a co-precipitation method as previously described.25 Briefly here, chitosan oligosaccharide (5 kDa) was PEGylated with aldehyde-activated methoxy PEG (2 kDa), and monolabeled chitosan-g-PEG (CP) was purified using ion exchange chromatography. Pure CP (150 mg) was mixed with iron chlorides (9.3 mg Fe2+ and 16 mg Fe3+) in 2.2 mL of degassed deionized water. A 15 % ammonium hydroxide solution (1.2 mL) was titrated in slowly at 40°C until a final pH of 10 was reached to ensure complete nucleation of NPs. NPs were purified through size exclusion chromatography in S-200 resin (GE Healthcare, Piscataway, NJ) into thiolation buffer (100mM sodium bicarbonate buffer, pH 8.0 containing 5 mM EDTA). Synthesized NPs contained approximately 150 CPs per iron core which provided free amine groups for subsequent conjugations as determined by the fluorescamine assay.
NP Conjugations
Monoclonal antibody specific to the transgenic rat neu (7.16.4) expressed by the MMC cells and FVB/N transgenic mouse model used in this study was purchased from the UCSF Monoclonal Antibody Core. Mouse IgG (Invitrogen, Carlsbad, CA) was used as a control. Antibodies (2.5 mg/mL in thiolation buffer) were thiolated with Traut’s reagent (100 µg/mL in thiolation buffer) by mixing 874 µL antibody with 25 µL Traut’s reagent for 1.5 hr in the dark at room temperature. Unreacted Traut’s reagent was removed through Zeba spin columns (Thermo Fisher Scientific, Rockford, IL). Concurrently, NPCP were labeled with Alexa Fluor 647 (AF647, Invitrogen, Carlsbad, CA). NPCP (1.1 mg in 1 mL thiolation buffer) were reacted with 0.5 mg of AF647 in 100 µL DMSO for 1 hr at room temperature protected from light with gentle rocking. For in vitro confocal imaging experiments, NPCP were labeled with Oregon Green 488 (1.1 mg NP in 1 mL thiolation buffer, 0.25 mg Oregon Green 488 in 100 µL DMSO). Unreacted fluorophore was removed using S-200 resin and pure NPCP-fluorophore was collected. NPCP-fluorophore was reacted with 9.5 µL of 2.5 mM NHS-PEG24-maleimide in the dark at room temperature with gentle rocking for 15 min before removing unreacted PEG through PD-10 desalting columns (GE Healthcare, Piscataway, NJ). The thiolated antibodies were mixed with thiol-reactive NPs (2 mg antibody per 1 mg NPs) and allowed to react for 4 hr in the dark at room temperature with gentle rocking. Unreacted antibody was removed from NP conjugated antibodies through size exclusion chromatography in S-200 resin to have pure control NP-IgG and targeted NP-neu.
NP-Antibody Characterizations
The size and zeta potential of NP-IgG and NP-neu were determined using a DTS Zetasizer Nano (Malvern Instruments, Worcestershire, UK) by measuring dynamic light scattering of a 100 µg/mL suspension of NPs at pH 7.4. The stability of NPs were determined at 100 µg/mL in DMEM containing 10% FBS and 1% antibiotic-antimycotic. AF647 labeling of NPCP was determined through absorbance measurement at 650 nm using unlabeled NPCP as a background. Molar concentration of AF647 was calculated following the manufacturer’s protocol, and the number of AF647 per NPCP was calculated assuming a NP core diameter of 8 nm (see Supplementary Methods for detailed description). Antibody loading on NPs was determined through reducing SDS-PAGE and quantifying the amount of light chain released from NPs (see Supplementary Methods for detailed description). The number of antibodies per NP was calculated assuming an antibody molecular weight of 150 kDa and a NP core diameter of 8 nm (see Supplementary Methods for detailed description).
In Vitro Targeting
Mouse mammary carcinoma (MMC) cells that express rat transgenic neu were maintained at 37°C in 95%/5% humidified air/CO2 in DMEM containing 10% FBS and 1% antibiotic-antimycotic. For targeting experiments, 50,000 cells were plated in 24-well plates the day before NP treatment. NP treatments were performed in fully supplemented culture medium at 50 µg/mL NPs. For the competition treatment, cells were treated with 50-fold excess free neu antibody for 15 minutes before adding NP-neu. After a 2 hr treatment, cells were washed thrice before preparation for detection of NP labeling. For fluorescence detection, cells were detached using Versene (Invitrogen, Carlsbad, CA) and collected in FACS buffer (2% FBS in PBS). Cell uptake of NPs was determined by flow cytometry detection of AF647 fluorescence of at least 10,000 cells on a BD FACSCanto flow cytometer (Beckton Dickinson, Franklin Lakes, NJ). Data was analyzed using the FlowJo software package (Tree Star, Ashland, OR). For iron detection, the ferrozine assay was used as previously described40 and iron content was normalized to protein content using Bradford (see Supplementary Methods for a more detailed description of the assay).
Confocal Imaging
MMC cells (500,000) were plated on 22 × 22 mm glass cover slips in 6-well plates the day before NP treatment. Cells were treated with NPs as described above before fixing in 4% methanol-free formaldehyde (Polysciences Inc., Warrington, PA) for 15 min. Cell membranes were stained with wheat germ agglutinin, Alexa Fluor conjugates (Invitrogen, Carlsbad, CA) following the manufacturer’s protocol. Cover slips were then mounted on microscope slides using Prolong Gold anti-fade solution (Invitrogen, Carlsbad, CA) containing DAPI for cell nuclei staining. Cell images were obtained on a LSM 510 Meta confocal fluorescence microscope (Carl Zeiss Inc., Peabody, MA) with the appropriate filters (see Supplementary Information for detailed procedures).
In Vivo MRI
All animal experiments were performed in accordance with the University of Washington Internal Animal Care and Use Committee (IACUC). Transgenic mice (FVB/N/Tg) which are homozygous for the mouse mammary tumor virus (MMTVneu) rat transgene and spontaneously form mammary tumors were purchased from The Jackson Laboratory (Sacramento, CA) and bred in-house. Mice were used for experiments after palpable tumors developed. For pre-contrast MR imaging, mice were anesthetized with isofluorane and placed into a 4-mouse holder fit for a knee-coil and maintained at 35°C with heated air. Imaging was conducted on a Philips Achieva 3T Whole Body Scanner (Philips Medical Systems, Andover, MA). A series of T2 weighted axial images were generated by using a single echo multi-slice pulse sequence with a TR of 8004 ms and variable TE values from 40 to 420 ms in 20 ms increments. The spatial resolution parameters were as follows: acquisition matrix of 512 × 512, field of view of 75 × 75 mm, slice number of 15, slice thickness of 2 mm, gap thickness of 2 mm, and 2 averages for T2 weighted images. Mice were then injected with a single bolus containing 200 µL of 522 µg/mL NP-IgG or NP-neu intravenously through the tail vein using a 27-gage needle. Post-contrast MR imaging was then performed 48 hrs after injection for uninjected (n = 2), NP-IgG injected (n = 3), and NP-neu injected (n = 3) mice. Colorized T2 maps were generated using the OsiriX open-source software package and the manually selected tumor region was overlaid onto anatomical images. Average T2 values over the manually selected tumor regions were used to calculate ΔT2 (average pre-contrast T2 – average post-contrast T2). The volumes of the manually selected regions for tumors from uninjected, NP-IgG treated, and NP-neu treated mice were 120 ± 74 mm3, 75 ± 27 mm3, and 91 ± 15 mm3, respectively.
Ex Vivo Imaging
Anesthetized mice were euthanized through cervical dislocation 48 hrs post-injection. Mice were dissected to remove tumors, livers, kidneys, spleens, hearts, and lungs which were placed into 10% Formalin. Fluorescence from each organ was imaged using the IVIS Spectrum imaging system (Caliper Life Sciences, Hopkinton, MA) with the Living Image software package. Imaging conditions consisted of a high lamp level, excitation and emission wavelengths of 640 nm and 680 nm, respectively, f-stop at f4, and 1 s exposure time.
Histology
Formalin fixed tissues were placed into 30% sucrose at 4°C until tissues sank. Tissues were then embedded in OCT compound and frozen. Frozen 4 µm sections were stained with Prussian blue for iron detection and counterstained with nuclear fast red. Stained sections were imaged using an upright Nikon Eclipse E600 microscope (Nikon Instruments, Melville, NY) and QImaging Retigia EX cooled CCD camera (QImaging, Surrey, BC, Canada).
In Vivo Flow Cytometry
Anesthetized mice were euthanized through cervical dislocation 48 hrs post-injection. Mice were dissected to remove tumors, livers, brains, and femurs. Organs were placed into a collagenase solution (1% collagenase in PBS) on ice for 30 min and femurs were cleaned of all muscle and bone marrow aspirated with serum free DMEM medium. Organs were disaggregated through gentle pipetting with a wide pore pipette tip and tissue aggregates were removed through filtration with a 70 µm pore cell strainer (BD Biosciences, Franklin Lakes, NJ). Single cell suspensions of organs and bone marrow were then centrifuged at 300× g and the cell pellets resuspended into ACK buffer (150 mM ammonium chloride, 10 mM potassium bicarbonate, 0.1 mM EDTA) to lyse erythrocytes. Cells were washed thrice in ACK buffer until erythrocytes were completely removed. Cells were then stained with FITC conjugated anti-CD44 and PE conjugated anti-integrin alpha 6 (Abcam, Cambridge, MA) to identify the tumor cell population.37, 38 Cells were then analyzed on a BD FACSCanto flow cytometer (Beckton Dickinson, Franklin Lakes, NJ). Data was analyzed using the FlowJo software package (Tree Star, Ashland, OR).
Statistical Analysis
All acquired data are expressed as mean ± SD. Statistical significance was determined using Student’s t-test. A p-value less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
NP Synthesis and Characterization
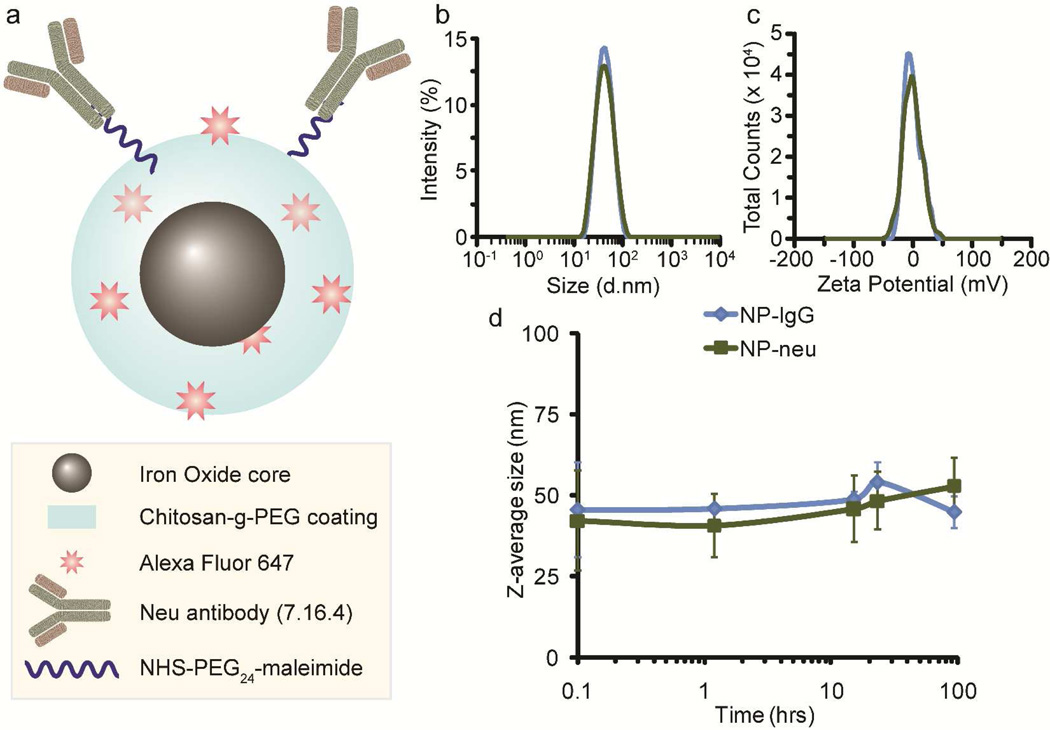
Iron oxide (Fe3O4) nanoparticles coated with a copolymer of chitosan and PEG (NP) were used as the base NP in this study owing to their proven stability and biocompatibility. Amine functional NPs were tagged with Alexa Fluor 647 (AF647) for optical detection in in vivo experiments or Oregon Green 488 for in vitro detection. These optically detectible NPs were then activated with thiolated neu antibody18 through a heterobifunctional PEG linker to display neu away from the surface of the NP, generating NP-neu. The structure of NP-neu is shown in Figure 1a. Mouse IgG was attached to NP to form NP-IgG as a control. The numbers of AF647 and antibodies per NP were calculated assuming a NP core size of 8 nm (Suppl. Figure 1) and are provided in Table 1.
Figure 1.
NP architecture and characterizations. a) Illustration of the architecture of the fluorescently labeled and neu antibody activated NP. b) Hydrodynamic size distributions of NP-IgG and NP-neu as determined by DLS. c) Zeta potential distributions of NP-IgG and NP-neu as determined by DLS. d) SPION stability in biological fluid (DMEM containing 10% FBS).
Table 1.
NP characterizations.
| Sample | AF-647 per NPa | Ab per NPb | Z-Average (d.nm)c | PdId | Zeta Potential (mV) |
|---|---|---|---|---|---|
| NP | 0 | 0 | 47 ± 12 | 0.19 ± 0.03 | −1.4 ± 1.5 |
| NP-IgG | 25 ± 3 | 8 ± 0. 5 | 44 ± 6 | 0.18 ± 0.08 | −1.3 ± 1.3 |
| NP-neu | 25 ± 3 | 6 ± 2 | 40 ± 1 | 0.15 ± 0.06 | −0.4 ± 0.8 |
All NP characterizations are provided as the average and standard deviation of five batches of NPs.
The number of AF-647 per NP was determined by absorbance at 650 nm and calculated according to the manufacturer’s (Invitrogen, Carlsbad, CA) protocol assuming a NP core diameter of 8 nm.
The number of antibodies per NP was determined by reducing SDS-PAGE and calculated assuming an antibody molecular weight of 150 kDa and NP core diameter of 8 nm.
Hydrodynamic Z-average diameter in nm.
Polydispersity index.
The size and zeta potential of NPs have a dramatic effect on non-specific uptake by off-target cells in the body. After conjugation of antibodies, NPs slightly decreased in size which may be due to the additional purifications using size exclusion chromatography that could slowly remove the largest, and smallest, NPs. NP-IgG had a Z-average size of 44 ± 6 and NP-neu had a Z-average size of 40 ± 1 (Table 1), with small PdI values (Table 1) and narrow size distributions (Figure 1b) indicating monodispersity. The slight decrease in PdI values further suggests the decrease in the average hydrodynamic size of the NPs was due to additional purifications that removed the largest, and smallest, NPs. NPs between the sizes of 10–100 nm in diameter are desirable since they are too large to be filtered out by the kidneys but small enough to not be recognized by the reticuloendothelial system for elimination.8, 19 Furthermore, NPs smaller than 60 nm are expected to have better penetration away from blood vessels and into the tumor.20
The zeta potential, a measure of the surface charge on the NP, also plays a significant role in non-specific uptake and tumor targeting. After antibody attachment, NP-IgG and NP-neu had zeta potentials of −1.3 ± 1.3 mV and −0.4 ± 0.8 mV, respectively (Figure 1c and Table 1), similar to the zeta potential of unmodified NPs (Table 1). Although chitosan alone is slightly cationic (pKa around 6.5), PEGylation and coating onto iron oxide NPs creates a near neutral NP. These near neutral zeta potentials are ideal for a targeted NP so that antibody-antigen binding will not be confounded by non-specific electrostatic interactions. Highly cationic NPs are preferentially taken up by the liver,21 and show poor penetration into the tumor.22 Slightly anionic NPs show the best tumor uptake and penetration.21, 22 However, highly anionic NPs can still non-specifically interact with positively charged membrane-bound proteins. Therefore, neutral NPs are expected to provide the best targeting ability since non-specific electrostatic interactions between the NP and off-target cells will be minimal. Furthermore, the similar sizes and zeta potentials of NP-neu and NP-IgG ensures any targeting role is solely due to the presence of the targeting antibody and not to the differences in physiochemical properties shown in Table 1.
NP stability is important to ensure NP targeting functionality. Aggregation of NPs can reduce targeting ability and increase uptake by the RES and the risk of embolism. The stability of NP-IgG and NP-neu were tested in DMEM containing 10% FBS (Figure 1d). Both NPs were stable for at least five days in culture medium and did not induce aggregation of erythrocytes (Suppl. Figure 2) suggesting they should function well in targeting experiments and not risk embolism in vivo. Furthermore, previous experiments in mice showed high stability of these chitosan-grafted PEG coated NPs in whole blood as evidenced by a long serum half-life of 7–8 hrs.23 Furthermore, these NPs have proven non-toxicity24, 25 (Suppl. Figure 3 and Supplementary Results and Discussion).
In Vitro Targeting
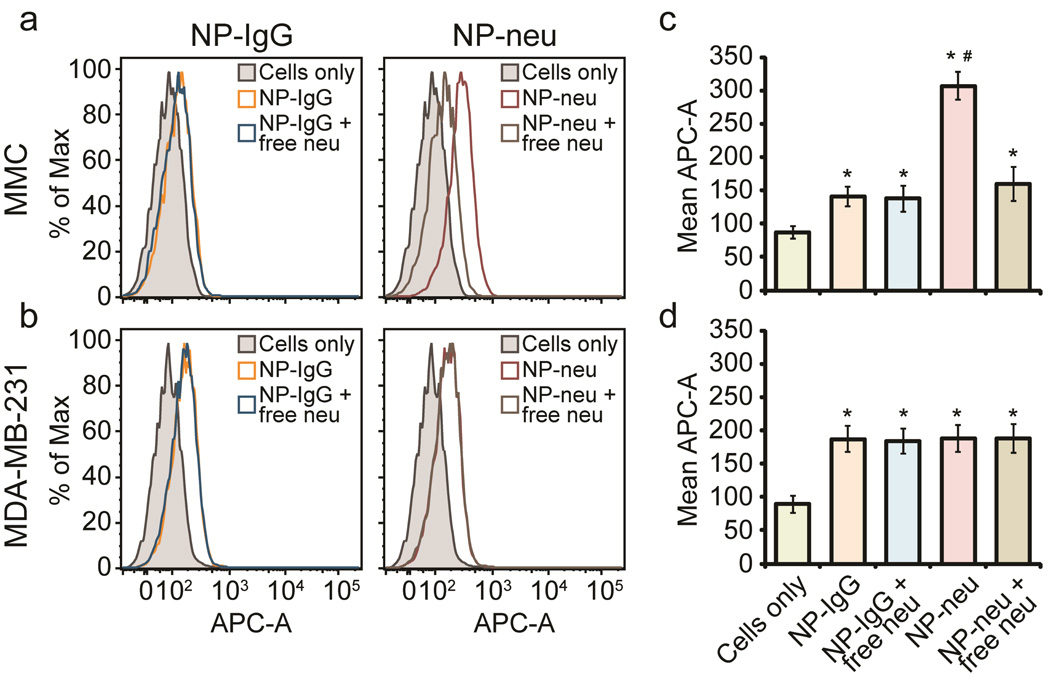
To test the specific binding of NP-neu to the neu antigen on the surface of MMC cells, both target MMC cells and control MDA-MB-231 cells were treated with various AF647 labeled NP formulations including 1) NP-neu, 2) NP-IgG, and 3) either NP-neu or NP-IgG with an excess of free competing neu antibody. In the third situation, the free neu antibody competes for surface binding sites with NP-neu, so an excess of free neu antibody ensures most binding sites are not available for NP-neu binding. Since neu is an internalized antibody (Suppl. Figure 4), NP-IgG was treated with excess free neu antibody to ensure the free antibody in solution did not promote non-specific uptake of control NPs through increased endocytosis. NP labeling of target MMC and control MDA-MB-231 cells was analyzed by flow cytometry (Figure 2). NP-IgG showed little binding to MMC cells which was not affected by free neu in solution, whereas NP-neu provided significantly increased binding to MMC cells which was abrogated to background uptake when free neu was used (Figure 2 a, c). This approximately 4-fold increase in cell labeling suggests that NP-neu selectively recognized the neu antigen on the surface of MMC cells. On the other hand, both NP-IgG and NP-neu provided similar cell labeling in control MDA-MB-231 cells which was not affected by free neu in solution (Figure 2 b, d). These data show the ability of NP-neu to selectively label target MMC cells through direct binding to the neu antigen on the surface of these cells.
Figure 2.
Flow cytometry analysis of NP labeling of breast cancer cells. a) Histograms of AF647 fluorescence in target MMC cells treated with control NP-IgG and targeted NP-neu in the presence or absence of free neu. b) Histograms of AF647 fluorescence in control MDA-MB-231 cells treated with control NP-IgG and targeted NP-neu in the presence or absence of free neu. c) Quantification of AF647 fluorescence in target MMC cells treated with control NP-IgG and targeted NP-neu. d) Quantification of AF647 fluorescence in control MDA-MB-231 cells treated with control NP-IgG and targeted NP-neu. * indicates a statistical significance as compared to untreated cells, # indicates a statistical significance as compared to NP-IgG control, n = 3 for each condition.
To confirm NP labeling of target MMC cells, the iron uptake in treated cells was determined using the ferrozine assay (Figure 3). Untreated cells had little iron and there was a small amount of non-specific uptake of NP-IgG by MMC cells. The cells treated with NP-neu had significantly greater iron content than those treated with NP-IgG, which was reduced to below background uptake through addition of free neu antibody in a competition assay. This 3-fold improvement, after accounting for baseline intracellular iron content, in NP uptake combined with free antibody competition tests further suggests NP-neu is able to specifically target neu on the surface of MMC cells.
Figure 3.
In vitro targeting of MMC cells. Iron in cells treated with NP-control, NP-neu, and NP-neu plus free neu was determined using the ferrozine assay (n = 3 for each condition). * indicates statistical significance as compared to cells only. # indicates statistical significance as compared to NP-control.
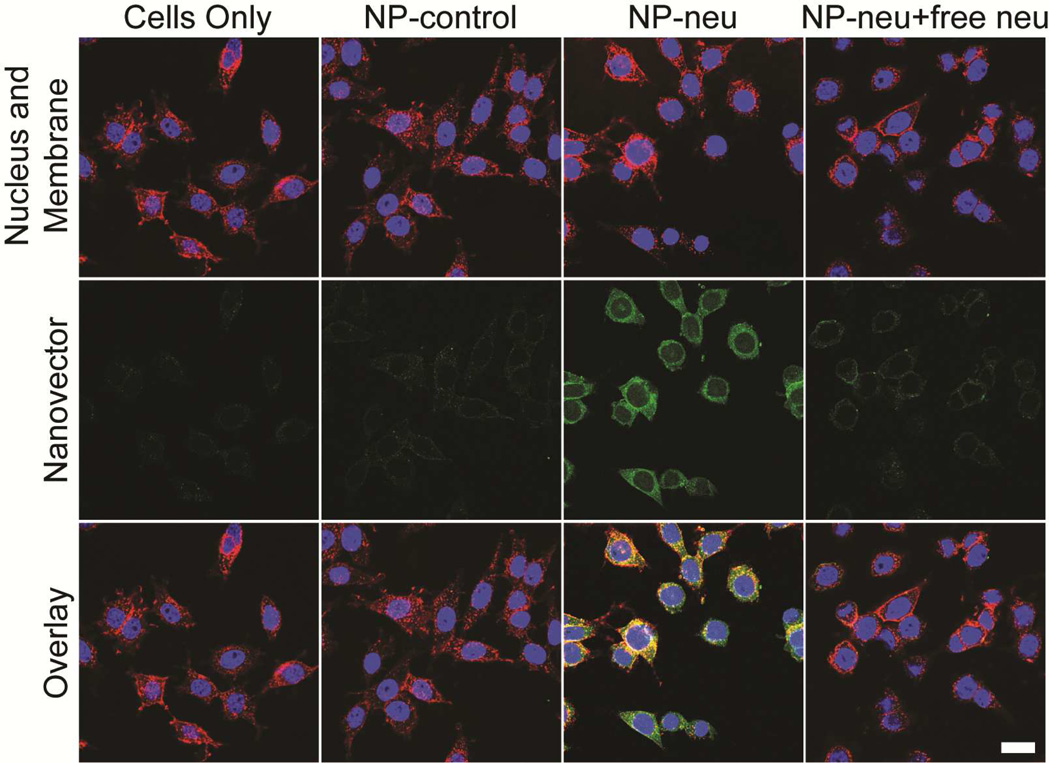
To further confirm the targeting and determine cellular localization of NP-neu, treated cells were imaged using confocal microscopy (Figure 4). Untreated cells showed no fluorescence as expected. Cells treated with NP-IgG showed minimal fluorescence as a result of the low non-specific uptake of these NPs. Cells treated with NP-neu showed high labeling efficiency which was blocked by free neu if present, a result of the specific interaction of NP-neu with the neu receptor on these cells. The fluorescent signal from NP-neu treated cells was both colocalized with the cell membrane (yellow color) and in the perinuclear region (green color around the nucleus), which suggested receptor-mediated endocytosis.26 The specific targeting and intracellular accumulation of neu (Suppl. Figure 4) shows this neu labeled NP should function well as a breast cancer targeting agent for in vivo imaging, if labeled with an appropriated near-infrared fluorophore, and drug delivery, through conjugation of drugs to available amine groups on the surface of the NP. The internalization of NP-neu by target cells increases the likelihood of the delivered therapy reaching its intracellular site of action. The near neutral charge of the NP along with the biocompatible chitosan-g-PEG coating provides a highly stable NP that shows minimal non-specific interaction with cells. These properties are ideal for a targeting NP where the specific interaction between the targeting agent and cell surface receptor must far outweigh any non-specific electrostatic or hydrophobic interactions or surface energy induced aggregation or binding.
Figure 4.
Confocal imaging of NP treated MMC cells. Cells were treated with non-targeted NP-control, targeted NP-neu, or targeted NP-neu with excess free neu for 2 hrs in fully supplemented culture media. The images of untreated cells (first column) are provided as a reference. Bright green fluorescence is only seen in the NP-neu treated cells indicating these NPs are able to target the neu receptor on neu expressing cells. Image acquisition times were identical for all the samples. Scale bar corresponds to 20 µm.
MRI of neu Transgenic Mice
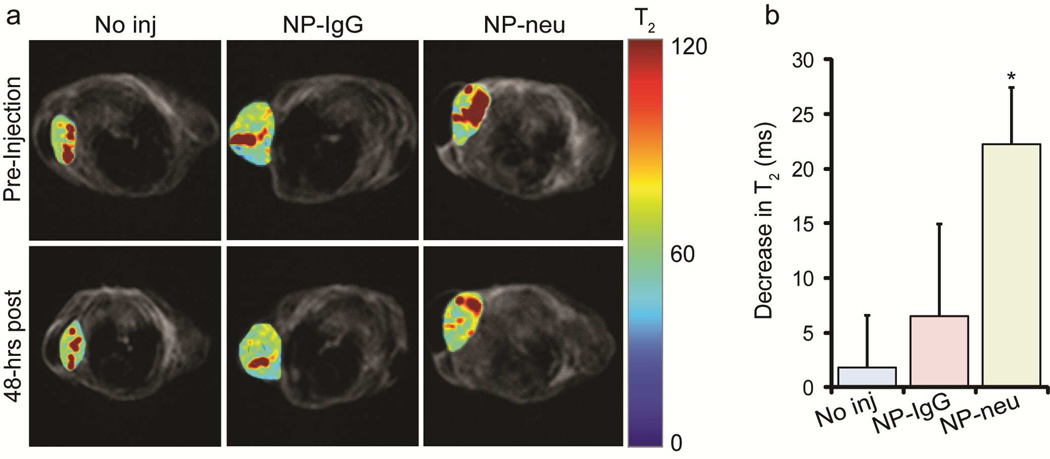
The ability of NP-neu to target metastatic breast cancer and be detected in MRI was tested in transgenic mice. T2 maps were generated for mice injected with NPs and and the tumor region overlaid on anatomical images (Figure 5a). The dark regions in the anatomical images correspond to the lungs as the early developing tumors were located in the anterior mammary tissue, near the front legs. Uninjected mice showed no change in T2 relaxation time in the tumor region between pre-injection and 48 hrs post-injection. Mice injected with non-targeted NP-IgG showed a small shortening in T2 relaxation time 48 hrs after injection as a result of non-specific uptake into the tumor through the enhanced permeability and retention (EPR) effect. The tumors from mice injected with targeted NP-neu showed a significant shortening in T2 relaxation time which reveals the ability of NP-neu provide contrast enhancement in MRI for disease detection or monitoring of drug delivery. Furthermore, the contrast enhancement 48 hrs after injection reveals the ability of NP-neu to persist in the tumor at a detectable level for a significant period of time which is useful for response monitoring. The decreases in T2 relaxation time from the pre-injection to 48 hrs post-injection were quantified for all mice and are provided in Figure 5b. This quantification shows the insignificant change in T2 relaxation time over 48 hrs in uninjected mice (n = 2), and provides a detection limit for the SPIONs since SPION concentrations that shorten T2 by less than approximately 6 ms would not be detected over background in these mice. This corresponds to a tumor concentration of approximately 6 µg/mL assuming a relaxivity of 100 s−1mM−1 at 3T. In the clinic, the minimal SPION concentration would have to be determined from pre-contrast MRI images and based on the noise between images. T2 values in tumors from NP-IgG injected mice (n = 3) decreased slightly, and decreased significantly in tumors from NP-neu treated mice (n = 3). The slight, but insignificant, contrast enhancement in NP-IgG injected mice is likely due to the non-specific uptake through the EPR effect which has not yet cleared out. On the other hand, NP-neu specifically binds to breast cancer cells and thus remains in the tumor to provide significant contrast enhancement as compared to uninjected mice. Similar results have been observed using xenograft mouse models where the targeting moiety on the surface of the NP promotes cell uptake and distribution throughout the tumor.27–31
Figure 5.
MRI of NP treated transgenic breast cancer mice. a) Colorized T2 maps of the tumor site were overlaid onto anatomical images. Non-injected and control NP-IgG injected mice showed no significant T2 changes in their tumors 48 hrs post-injection. Targeted NP-neu treated mice showed shortened T2 relaxation times in tumors 48 hrs post-injection indicating there was a significant uptake of NPs. b) Decrease in T2 relaxation times 48 hrs after injection. A much larger decrease in T2 relaxation time was observed in tumors of NP-neu treated mice (n = 3) as compared to no injection (n = 2) than NP-IgG (n = 3) suggesting a targeting role of NP-neu. * indicates a statistical difference as compared to no injection.
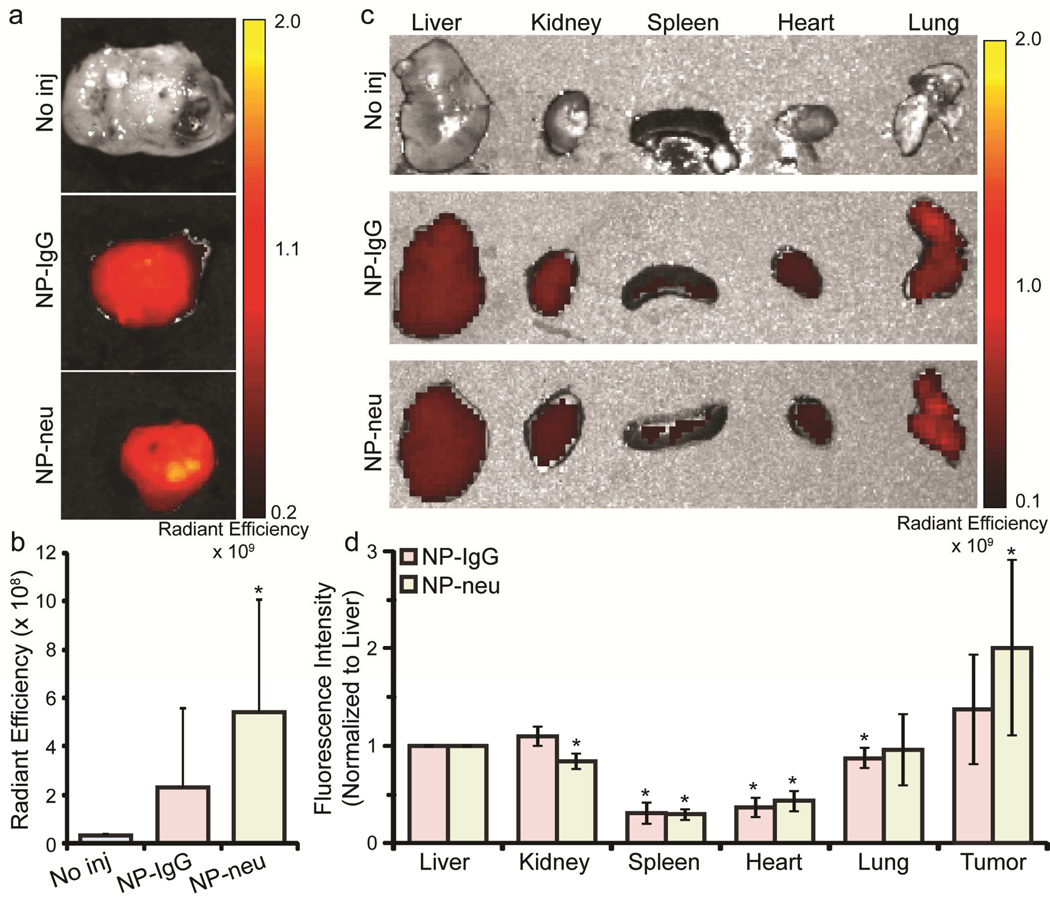
Ex Vivo Fluorescence Imaging
Mice were sacrificed and dissected to remove tumors, livers, spleens, kidneys, lungs, and hearts 48 hrs after NP injections. Organs were then imaged using an IVIS Xenogen imaging system which detects the AF647 tag on the NPs. Figure 6a shows representative tumors from mice receiving no injection, NP-IgG injection, and NP-neu injection. Mice receiving no injection showed no fluorescence as expected. Tumors from mice treated with NP-IgG and NP-neu showed fluorescent signal with NP-neu treated tumors appearing brighter than NP-IgG treated tumors. Similarly to MRI analysis, tumors from NP-IgG treated mice did not show a statistically significant increase in fluorescence as compared to uninjected mice (p = 0.22) whereas tumors from NP-neu treated mice showed a statistically significant increase in fluorescence as compared to uninjected mice (p = 0.04). Fluorescence intensity (average radiant efficiency) was quantified in tumors from NP-IgG and NP-neu injected mice and normalized to liver intensity to account for any confounding factors between animals. Figure 6b shows the liver normalized fluorescence intensity where tumors from NP-neu treated mice were consistently brighter than tumors from NP-IgG treated mice. This further suggests a targeting role of NP-neu in breast cancer even though improvement in NP-neu uptake in vivo was not as significant as in vitro. This is not unexpected and is consistent with current literature indicating the targeting agent attached to NPs does not significantly increase accumulation in the tumor but improves uptake and retention in tumor cells.27–31 The EPR effect promotes the accumulation of both targeted and non-targeted NPs into the tumor site, but only targeted NPs selectively bind to, or are taken up by, tumor cells.
Figure 6.
Xenogen imaging of harvested tumors and organs from transgenic breast cancer mice. a) Higher fluorescence intensity can be observed in tumors from NP-neu (targeted) treated mice than from NP-IgG (control) treated mice. b) Fluorescence intensities (photons/sec/cm2/steradian)/(µW/cm2) of tumors from mice left untreated (n = 5) or treated with NP-IgG (n = 5 tumors) or NP-neu (n = 7 tumors). * indicates a statistical difference as compared to no injection. c) Xenogen images of livers, kidneys, spleens, hearts, and lungs from uninjected, NP-IgG injected (control, n = 3), and NP-neu injected (targeted, n = 5) mice. d) Liver normalized fluorescence quantification of organs indicating non-specific uptake was similar for both NP-IgG and NP-neu, and uptake in tumors was higher than in off-target organs. * indicates a statistical difference from liver uptake.
To examine non-specific binding of NPs, the livers, kidneys, spleens, lungs, and hearts from treated mice were imaged using the Xenogen imaging system. Figure 6c shows representative images of organs, which shows that the non-specific uptake of NPs was the same for both control NP-IgG and targeted NP-neu. Figure 6d shows the liver normalized fluorescent signal (original liver fluorescence signal was 1.7 × 108 ± 1.65 × 108 (photons/sec/cm2/steradian)/(µW/cm2) for NP-IgG and 2.24 × 108 ± 1.36 × 108 (photons/sec/cm2/steradian)/(µW/cm2) for NP-neu) from the organs confirming no statistical different in off-target uptake between control NP-IgG and targeted NP-neu. The hearts and spleens of treated animals had significantly lower NP uptake than the liver and were not affected by targeting. The kidneys from NP-neu treated mice and lungs from NP-IgG treated mice had slightly lower uptake of NPs than liver. These off-target results indicate the improved tumor uptake achieved by NP-neu was specific and not due higher non-specific interaction with cells. Furthermore, the uptake in the tumors was higher than in off-target organs including the liver, and almost two-fold higher for NP-neu. This is likely a result of the long serum half-life of these chitosan-grafted PEG coated NPs,23 and indicates their optimal size and neutral charge to avoid elimination by the kidneys and liver and sufficient passivation to prevent immune recognition.
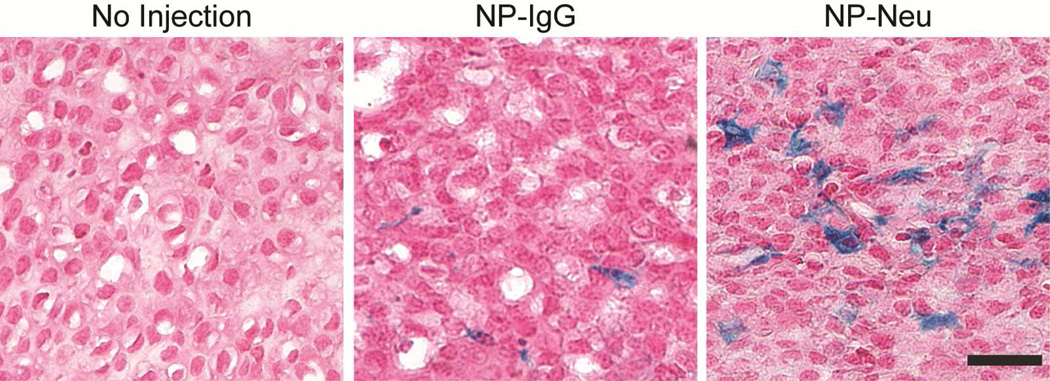
Tumor Histology
To observe distribution of NPs within the tumor, tumor sections were stained with Prussian blue to detect iron from NPs and counterstained with nuclear fast red (Figure 7). Tumors from mice treated with NP-neu showed significant iron staining distributed throughout the tumor whereas those from mice treated with NP-IgG showed little, isolated iron staining. Tumors from untreated mice showed no positive iron staining. This further suggests the targeting ability of NP-neu towards the breast cancer cells. Furthermore, this highlights the importance of a targeting agent for breast cancer diagnosis and therapy, as a larger proportion of cells are exposed to the injected NP-neu as a result of targeting, increasing the efficiency of an attached therapeutic agent.
Figure 7.
Histology images of tumors from NP treated transgenic breast cancer mice 48 hrs post-injection. Iron was stained using Prussian blue, and nuclei were counterstained with nuclear fast red. NP uptake in tumors from NP-neu (targeted) treated mice was much more pronounced and broadly distributed than the uptake in tumors of NP-IgG (control) treated mice. Scale bar corresponds to 20 µm.
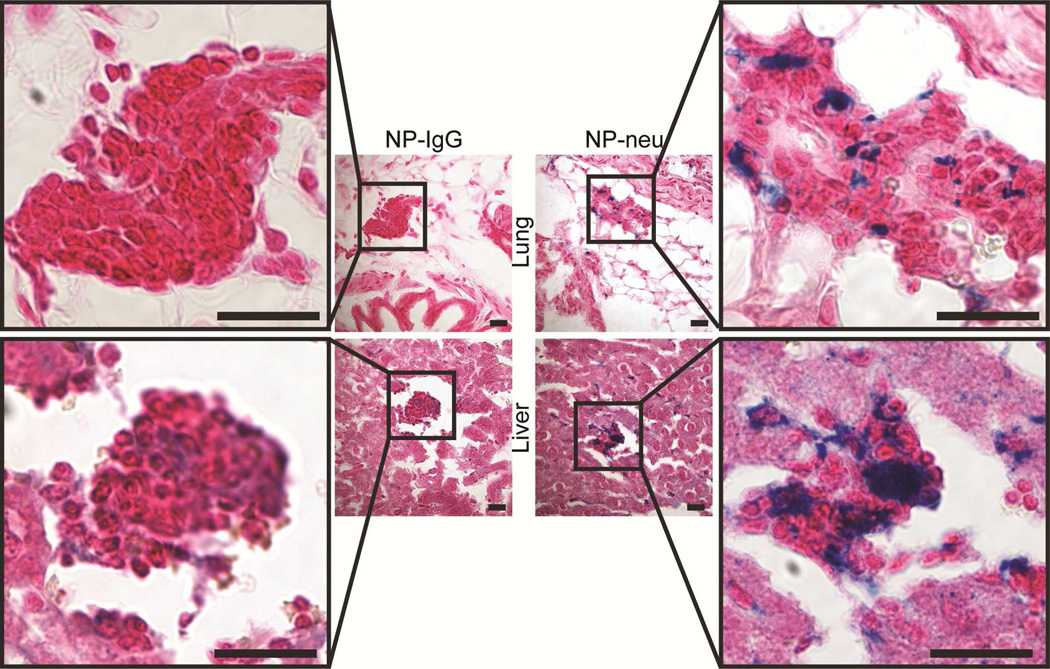
Targeting Micrometastases
It is important to detect and treat metastatic disease as this is the most common cause of cancer mortality. In fact, 90% of cancer-related mortality is caused by metastases.3–5 The transgenic mice used in this study are known to develop lung, liver, and bone marrow metastases within 8 months and thus are an excellent model for targeting metastases.32–34 To observe targeting of breast cancer metastases, lungs and livers from treated mice were sectioned and stained identically to the primary tumors (Figure 8). Positive Prussian blue staining in the lung and liver metastases indicates the presence of NPs.35, 36 Micrometastases in lungs and livers from mice treated with control NP-IgG showed no positive iron staining. On the other hand, lung and liver micrometastases from NP-neu treated mice showed significant positive iron staining. This targeting effect on micrometastases by NP-neu was further confirmed using flow cytometry analysis of NP fluorescence in CD44-Integrin-α6 (CD49f) double positive metastatic cells37, 38 (Figure 9a, Suppl. Table 1). Little to no labeling of metastatic cells in the lungs and livers from NP-IgG treated mice was observed, whereas there was dramatically higher NP fluorescence in NP-neu treated mice. Furthermore, metastatic cancer cells in aspirated bone marrow were successfully labeled with NP-neu as compared to NP-IgG (p = 0.05, n = 3). This demonstrates the ability of these NPs to selectively detect and label early stage micrometastases, but imaging was only possible through histology or flow cytometry and not MRI since the micrometastases were beyond the spatial resolution of 3T MRI. Flow cytometry analysis also reveals the non-specific uptake of NPs in non-cancerous cells (Figure 9b). Again, we see no significant difference between the non-specific uptake of NP-IgG and NP-neu in off-target cells showing the specificity of NP-neu. Also, the NP uptake in the tumor cells is higher than NP uptake in non-cancerous liver cells as seen in Figure 6d. Furthermore, there is much lower uptake of NP-neu in non-cancerous cells as compared to metastatic cells in these tissues, whereas uptake of NP-IgG is similar between cancerous and non-cancerous cells.
Figure 8.
Prussian blue stained histology images of micrometastases in lungs and livers from NP-IgG (control) and NP-neu (targeted) treated transgenic breast cancer mice. Scale bars correspond to 20 µm.
Figure 9.
Flow cytometry analysis of NP uptake in metastatic cancer cells and organs. a) NP fluorescence in the cancer cell population from the primary tumor, liver, lung, and bone marrow of NP-IgG and NP-neu treated mice. Higher fluorescence was observed in double positive metastatic cancer cells in all tested organs from mice treated with NP-neu as compared to NP-IgG. b) NP fluorescence in the non-cancer cell population from the primary tumor, liver, lung, and bone marrow of NP-IgG and NP-neu treated mice. Similar fluorescence levels were observed between NP-neu and NP-IgG treated animals. Fluorescence intensities were normalized by subtracting the background fluorescence of cells from untreated mice to allow for direct comparison between treatment groups and cell types. * indicates a statistical significance as compared to NP-IgG.
Targeting of micrometastases was much more pronounced than primary tumor targeting when comparing targeted (NP-neu) and non-targeted (NP-IgG) NPs, which is likely due to the lack of the EPR effect in early stage micrometastases. Large tumors contain leaky vasculature allowing large influx of blood including nutrients and oxygen, and have insufficient lymph vessels to remove waste. This leads to the EPR effect which can be used for passive targeting of tumors with NPs. However, micrometastases, which can currently only be identified histologically, are still small enough where diffusion provides adequate oxygen,39 so tumor angiogenesis is not yet induced by anoxic conditions. Unlike the large tumors, lymphatic drainage from the lungs and liver, and hepatic ducts of the liver, are adequate to remove waste. Therefore, these metastases do not have the enhanced retention associated with large tumors so cannot be passively targeted. This explains why a more significant targeting effect was seen in these metastases by targeted delivery; NP-neu attached to breast cancer cells through specific interaction with neu expressed on the cell surface, and NP-IgG was likely removed through lymphatic or hepatic drainage. This further confirms the targeting role of NP-neu and highlights the critical need for targeted delivery especially for metastatic disease.
CONCLUSIONS
Rationally designed superparamagnetic iron oxide NPs activated with an antibody against the neu receptor were successfully prepared. These NPs were able to specifically target neu expressing mouse mammary carcinoma cells in vitro and in vivo in a transgenic mouse model and were detected with MRI. Furthermore, these NPs were able to recognize and tag spontaneous micrometastases in the lungs, livers, and bone marrow of these mice indicating the potential for MRI detection of micrometastases. Control NPs showed no labeling of metastatic cells highlighting the importance of targeting for delivery to metastatic disease. Given that these NPs have a significant number of functional groups, therapeutic payloads such as small molecule chemotherapy drugs, DNA, or siRNA could be attached for targeted therapy. The favorable properties displayed by this SPION system in a transgenic mouse model warrants its further development as a diagnostic tool for metastatic breast cancer, and as a therapeutic agent to significantly improve the prognosis of patients afflicted with metastatic breast cancer.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported in part by NIH grants R01CA134213 and R01EB006043. FK and OV acknowledge support from NIH training grant T32CA138312. We thank the Diagnostic Imaging Sciences Center, Animal Bioimaging Center, and Keck Microscopy Imaging Facility at the University of Washington for use of resources and equipment. We acknowledge the assistance of E. Gad and L. Rastetter for maintaining and providing transgenic mice. We also thank J. Phillips and D. Cheung for laboratory assistance.
Footnotes
Supporting information available: TEM images of NP cores, erythrocyte aggregation assay, z-stack confocal imaging, and detailed procedures. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, et al. Seer Cancer Statistics Review, 1975–2007. National Cancer Institute. 2010 [Google Scholar]
- 3.Weigelt B, Peterse JL, van 't Veer LJ. Breast Cancer Metastasis: Markers and Models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 4.Mehlen P, Puisieux A. Metastasis: A Question of Life or Death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, Teruya-Feldstein J, Bell GW, Weinberg RA. Therapeutic Silencing of Mir-10b Inhibits Metastasis in a Mouse Mammary Tumor Model. Nat Biotechnol. 2010;28:341–347. doi: 10.1038/nbt.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kievit FM, Zhang M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery across Biological Barriers. Adv Mater. 2011;23:H217–H247. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao R, Xing R, Xu Z, Hou Y, Gao S, Sun S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv Mater. 2010;22:2729–2742. doi: 10.1002/adma.201000260. [DOI] [PubMed] [Google Scholar]
- 8.Veiseh O, Gunn JW, Zhang M. Design and Fabrication of Magnetic Nanoparticles for Targeted Drug Delivery and Imaging. Adv Drug Deliv Rev. 2010;62:284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P, et al. Breast Cancer Molecular Subtypes Respond Differently to Preoperative Chemotherapy. Clin Cancer Res. 2005;11:5678–5685. doi: 10.1158/1078-0432.CCR-04-2421. [DOI] [PubMed] [Google Scholar]
- 10.Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, Davies S, Fauron C, He X, Hu Z, et al. Supervised Risk Predictor of Breast Cancer Based on Intrinsic Subtypes. J Clin Oncol. 2009;27:1160–1167. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo M, Corsi F, Foschi D, Mazzantini E, Mazzucchelli S, Morasso C, Occhipinti E, Polito L, Prosperi D, Ronchi S, et al. Her2 Targeting as a Two-Sided Strategy for Breast Cancer Diagnosis and Treatment: Outlook and Recent Implications in Nanomedical Approaches. Pharmacol Res. 2010;62:150–165. doi: 10.1016/j.phrs.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al. Use of Chemotherapy Plus a Monoclonal Antibody against Her2 for Metastatic Breast Cancer That Overexpresses Her2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 13.Mazzucchelli S, Colombo M, De Palma C, Salvade A, Verderio P, Coghi MD, Clementi E, Tortora P, Corsi F, Prosperi D. Single-Domain Protein a-Engineered Magnetic Nanoparticles: Toward a Universal Strategy to Site-Specific Labeling of Antibodies for Targeted Detection of Tumor Cells. ACS Nano. 2010;4:5693–5702. doi: 10.1021/nn101307r. [DOI] [PubMed] [Google Scholar]
- 14.Xu C, Wang B, Sun S. Dumbbell-Like Au-Fe3o4 Nanoparticles for Target-Specific Platin Delivery. J Am Chem Soc. 2009;131:4216–4217. doi: 10.1021/ja900790v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual Drug Loaded Superparamagnetic Iron Oxide Nanoparticles for Targeted Cancer Therapy. Biomaterials. 2010;31:3694–3706. doi: 10.1016/j.biomaterials.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 16.Singh A, Dilnawaz F, Mewar S, Sharma U, Jagannathan NR, Sahoo SK. Composite Polymeric Magnetic Nanoparticles for Co-Delivery of Hydrophobic and Hydrophilic Anticancer Drugs and Mri Imaging for Cancer Therapy. ACS Applied Materials & Interfaces. 2011;3:842–856. doi: 10.1021/am101196v. [DOI] [PubMed] [Google Scholar]
- 17.Sharpless NE, Depinho RA. The Mighty Mouse: Genetically Engineered Mouse Models in Cancer Drug Development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 18.Steinhauser I, Spankuch B, Strebhardt K, Langer K. Trastuzumab-Modified Nanoparticles: Optimisation of Preparation and Uptake in Cancer Cells. Biomaterials. 2006;27:4975–4983. doi: 10.1016/j.biomaterials.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol Pharm. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Popovic Z, Liu W, Chauhan VP, Lee J, Wong C, Greytak AB, Insin N, Nocera DG, Fukumura D, Jain RK, et al. A Nanoparticle Size Series for in Vivo Fluorescence Imaging. Angew Chem Int Ed Engl. 2010;49:8649–8652. doi: 10.1002/anie.201003142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao K, Li Y, Luo J, Lee JS, Xiao W, Gonik AM, Agarwal RG, Lam KS. The Effect of Surface Charge on in Vivo Biodistribution of Peg-Oligocholic Acid Based Micellar Nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS. Tuning Payload Delivery in Tumour Cylindroids Using Gold Nanoparticles. Nat Nanotechnol. 2010;5:465–472. doi: 10.1038/nnano.2010.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee MJ, Veiseh O, Bhattarai N, Sun C, Hansen SJ, Ditzler S, Knoblaugh S, Lee D, Ellenbogen R, Zhang M, et al. Rapid Pharmacokinetic and Biodistribution Studies Using Cholorotoxin-Conjugated Iron Oxide Nanoparticles: A Novel Non-Radioactive Method. PLoS ONE. 2010;5:e9536. doi: 10.1371/journal.pone.0009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, Ellenbogen RG, Olson JM, Zhang MQ. Pei-Peg-Chitosan-Copolymer-Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, Complexation, and Transfection. Advanced Functional Materials. 2009;19:2244–2251. doi: 10.1002/adfm.200801844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veiseh O, Sun C, Fang C, Bhattarai N, Gunn J, Kievit F, Du K, Pullar B, Lee D, Ellenbogen RG, et al. Specific Targeting of Brain Tumors with an Optical/Magnetic Resonance Imaging Nanoprobe across the Blood-Brain Barrier. Cancer Res. 2009;69:6200–6207. doi: 10.1158/0008-5472.CAN-09-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veiseh O, Sun C, Gunn J, Kohler N, Gabikian P, Lee D, Bhattarai N, Ellenbogen R, Sze R, Hallahan A, et al. Optical and Mri Multifunctional Nanoprobe for Targeting Gliomas. Nano Lett. 2005;5:1003–1008. doi: 10.1021/nl0502569. [DOI] [PubMed] [Google Scholar]
- 27.Kirpotin DB, Drummond DC, Shao Y, Shalaby MR, Hong K, Nielsen UB, Marks JD, Benz CC, Park JW. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization but Does Increase Internalization in Animal Models. Cancer Res. 2006;66:6732–6740. doi: 10.1158/0008-5472.CAN-05-4199. [DOI] [PubMed] [Google Scholar]
- 28.Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of Tumor-Specific Targeting on the Biodistribution and Efficacy of Sirna Nanoparticles Measured by Multimodality in Vivo Imaging. Proc Natl Acad Sci U S A. 2007;104:15549–15554. doi: 10.1073/pnas.0707461104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Li J, Wang Y, Cho KJ, Kim G, Gjyrezi A, Koenig L, Giannakakou P, Shin HJ, Tighiouart M, et al. Hft-T, a Targeting Nanoparticle, Enhances Specific Delivery of Paclitaxel to Folate Receptor-Positive Tumors. ACS Nano. 2009;3:3165–3174. doi: 10.1021/nn900649v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi CH, Alabi CA, Webster P, Davis ME. Mechanism of Active Targeting in Solid Tumors with Transferrin-Containing Gold Nanoparticles. Proc Natl Acad Sci U S A. 2010;107:1235–1240. doi: 10.1073/pnas.0914140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, Zhang M. Chlorotoxin Labeled Magnetic Nanovectors for Targeted Gene Delivery to Glioma. ACS Nano. 2010;4:4587–4594. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guy CT, Cardiff RD, Muller WJ. Induction of Mammary Tumors by Expression of Polyomavirus Middle T Oncogene: A Transgenic Mouse Model for Metastatic Disease. Mol Cell Biol. 1992;12:954–961. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakur ML, Devadhas D, Zhang KJ, Pestell RG, Wang CG, McCue P, Wickstrom E. Imaging Spontaneous Mmtvneu Transgenic Murine Mammary Tumors: Targeting Metabolic Activity Versus Genetic Products. J. Nucl. Med. 2010;51:106–111. doi: 10.2967/jnumed.109.069542. [DOI] [PubMed] [Google Scholar]
- 34.Knutson KL, Lu HL, Stone B, Reiman JM, Behrens MD, Prosperi CM, Gad EA, Smorlesi A, Disis ML. Immunoediting of Cancers May Lead to Epithelial to Mesenchymal Transition. J. Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 35.Leuschner C, Kumar CS, Hansel W, Soboyejo W, Zhou J, Hormes J. Lhrh-Conjugated Magnetic Iron Oxide Nanoparticles for Detection of Breast Cancer Metastases. Breast Cancer Res Treat. 2006;99:163–176. doi: 10.1007/s10549-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 36.Branca RT, Cleveland ZI, Fubara B, Kumar CS, Maronpot RR, Leuschner C, Warren WS, Driehuys B. Molecular Mri for Sensitive and Specific Detection of Lung Metastases. Proc Natl Acad Sci U S A. 2010;107:3693–3697. doi: 10.1073/pnas.1000386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JC, Deng T, Lehal RS, Kim J, Zacksenhaus E. Identification of Tumorsphere- and Tumor-Initiating Cells in Her2/Neu-Induced Mammary Tumors. Cancer Res. 2007;67:8671–8681. doi: 10.1158/0008-5472.CAN-07-1486. [DOI] [PubMed] [Google Scholar]
- 38.Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. Tgfbeta/Tnf(Alpha)-Mediated Epithelial-Mesenchymal Transition Generates Breast Cancer Stem Cells with a Claudin-Low Phenotype. Cancer Res. 2011;71:4707–4719. doi: 10.1158/0008-5472.CAN-10-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial Ph and Po2 Gradients in Solid Tumors in Vivo: High-Resolution Measurements Reveal a Lack of Correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 40.Veiseh O, Gunn JW, Kievit FM, Sun C, Fang C, Lee JS, Zhang M. Inhibition of Tumor-Cell Invasion with Chlorotoxin-Bound Superparamagnetic Nanoparticles. Small. 2009;5:256–264. doi: 10.1002/smll.200800646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.