Abstract
Objective
p38 inhibitors demonstrate limited benefit in rheumatoid arthritis (RA), perhaps due to anti-inflammatory functions of p38α. We determined if selective deletion of p38α in macrophages affects severity of arthritis. We also determined whether blocking upstream kinases in the p38 pathway, such as MKK3 or MKK6, avoids some limitations of p38α blockade.
Methods
Wild type mice and mice with selective deletion of p38α in macrophages (p38αΔLysM) were injected with K/BxN sera. Antigen-induced arthritis was also induced in these mice. Joint extracts were evaluated by ELISA, qPCR and Western blot analysis. Bone marrow derived macrophages (BMDM) were stimulated with LPS and were evaluated by qPCR and WB. Bone marrow chimeras were generated using mkk3−/− and mkk6−/− mice and K/BxN serum was administered to induced arthritis.
Results
p38αΔLysM mice had increased disease severity and delayed resolution of the arthritis compared to WT mice which correlated with higher expression of synovial inflammatory mediator expression and ERK phosphorylation. In contrast to WT BMDM cultured in the presence of a p38a/β inhibitor, LPS-stimulated MKK6 and MKK3-deficient BMDM had suppressed LPS-mediated IL-6 expression but had normal IL-10 production, DUSP1 expression, and MAPK phosphorylation. WT chimeric mice with MKK6 and MKK3-deficient bone marrow had markedly decreased passive K/BxN arthritis severity.
Conclusions
Inhibiting p38α in a disease that is dominated by macrophage cytokines like RA could paradoxically suppress anti-inflammatory functions and interfere with efficacy. Targeting an upstream kinase that regulates p38 could be more effective by suppressing pro-inflammatory cytokines while preventing decreased IL-10 expression and increased MAPK activation.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease marked by synovial hyperplasia and invasion into cartilage and bone (1). This process is mediated, in part, by cytokines like IL-1 and TNF that activate a broad array of cell signaling mechanisms and leads to release of destructive proteases. The mitogen activated protein kinase (MAPK) family regulates cytokines and metalloproteinase (MMP) production that perpetuates inflammation and tissue injury in RA (2). Several MAPK members, including p38, JNK and ERK, are expressed in the rheumatoid synovium and have been implicated in the pathogenesis of RA (3). Of these signaling pathways, p38 MAPK is especially relevant to human inflammatory disease and is activated in the rheumatoid synovium. Phospho-p38α (p-p38α) is localized to the RA synovial intimal lining, which includes fibroblast-like synoviocytes (FLS) and monocytes that produce IL-6 and a variety of other pro-inflammatory mediators (4). p38 inhibitors are effective in many animal models of arthritis and decrease TNF production by cultured synovial tissue cells, providing ample rationale for testing these compounds in RA (5–7).
Despite data supporting the use of p38 inhibitors in RA, these compounds demonstrate modest or no efficacy (8–10). The reasons for limited benefit are uncertain and alternative approaches that target this pathway are needed (5–7, 11). Recent data in acute skin inflammation suggested that p38a exhibits anti-inflammatory activity, suggesting that traditional inhibitors could paradoxically increase synovitis (12, 13). In the present study, we expand on this concept and show that chronic arthritis severity is significantly increased in mice with selective p38α deficiency in macrophages. The absence of p38a in macrophages leads to suppressed dual specificity protein phosphatase 1 (DUSP1) expression, increased activation of other MAP kinases like ERK and JNK, and decreased expression of the anti-inflammatory cytokine IL-10. We also show that targeting upstream kinases that regulate p38, namely MKK3 and MKK6, avoids some of these issues p38 blockade. Therefore, targeting p38a in a macrophage-dominant disease like RA might have limited benefit, while targeting upstream kinases can have anti-inflammatory effects and avoid the unanticipated pro-inflammatory effects of p38 blockade.
Material and Methods
Mice
KRN T cell receptor (TCR) transgenic mice were a gift from Drs. D. Mathis and C. Benoist (Harvard Medical School, Boston, MA) and Institut de Génétique et de Biologie Moléculaire et Cellulaire (Strasbourg, France) (14). Mice with loxP-flanked Mapk14 alleles and mice expressing Cre under control of the lysozyme M (LysM) have been described (15, 16). LysM-Cre mice were purchased from Jackson Laboratories. p38αΔLysM mice were generated by crossing p38αF/F and LysM-Cre. MKK3- and MKK6-deficient mice have been described elsewhere (17, 18). Mice were on the C57Bl/6 background. Mice used in these experiments were 8–12 weeks old. The mice were bred and maintained under standard conditions in the UC San Diego Animal Facility that is accredited by the American Association for Accreditation of Laboratory Animal Care. All animal protocols received prior approval by the institutional review board.
Reagents
Lipopolysaccharide (LPS) and methylated bovine serum albumin (mBSA) was obtained from Sigma. The p38α/β kinase inhibitor SB203580 was purchased from Calbiochem.
Serum transfer and arthritis scoring
Sera from arthritic adult K/BxN mice were pooled and recipient mice were injected intraperitoneally (i.p.) with 150 μl of K/BxN serum on day 0. In the chronic model, mice were injected with 150 μl of K/BxN serum on day 0 and followed by 100 μl per week. Clinical arthritis scores were evaluated as described (19). Bone marrow chimeras were generated as previously described (20). Adult mice were irradiated with 5.6 Gy twice, two hours apart, using a Mark 1 Cs137 irradiator (J.L Shepherd and Associates, San Fernando, CA). Twenty-four hours later, bone marrow cells were flushed from the femurs and tibias of donor mice with 10K media (RPMI 1640 with L-glutamine (2.05mM), FBS (10%), penicillin (100U/ml), streptomycin (100μg/ml) and 2-mercaptoethanol (0.05mM)). The red blood cells were lysed and the remaining bone marrow cells were washed twice in sterile PBS. The cells were counted and the irradiated recipient mice were injected intravenously with 5 × 106 cells in sterile PBS. The recipient mice were maintained on sulfamethoxazole and trimethoprim supplemented water for two weeks post-irradiation. Confirmation of engraftment was performed 8 weeks after bone marrow transplantation by qPCR on peripheral blood samples (21). Mice were considered chimeric with a ≥ 95% donor cell genotype. The K/BxN passive transfer model was induced in chimeric mice at 8 weeks post bone marrow transplantation by injection of 100ul of K/BxN serum i.p. on day 0 and day 2. Mice were euthanized on day 12 of the model. The experiment was considered successful if the reconstituted WT, mkk3−/− and mkk6−/− control groups performed as previously described in non-irradiated animals with serum-induced arthritis (22, 23).
Antigen-induced arthritis (AIA) induction
Experimental AIA was induced by injection of 100 μg of mBSA emulsified in 100 μl of complete Freund’s adjuvant (CFA) subcutaneously (s.c.) in the flank and then another injection one week later of 100 μg of mBSA/CFA intradermally (i.d.) in the tailbase. Two weeks after these injections, arthritis was induced by intraarticular injection of 60 μg of mBSA in 10 μl of saline into the right knee joint. The left knee was injected with PBS to serve as a control. Disease was assessed 10 days post-intraarticular injection by histological analysis as described below.
Histology and cytokine protein analysis
Joints were fixed in 10% formalin, decalcified in 10% EDTA for 2–3 weeks and paraffin embedded. Sections were prepared from the tissue blocks and stained with hematoxylin and eosin (H&E). A blinded semiquantitative scoring system was used to assess synovial inflammation, extra-articular inflammation, bone erosion and cartilage damage (0–5 scale), as previously described (19). For tissue cytokine assays, snap-frozen joints were homogenized in lysis solution as previously described. Protein concentration was measured by BCA assay (Pierce) and IL-1β and IL-6 were measured by ELISA according to manufacturer’s instructions (R&D systems) (24).
Determination of IFN-γ secretion by splenocytes
Mouse spleen cells were isolated and washed in RPMI 1640 supplemented with 10% fetal calf serum, 10 mM Hepes, 1 mM sodium pyruvate, 50 mM 2-mercaptoethanol, 1% L-glutamine, and 100 units/ml of penicillin/streptomycin. Erythrocytes were lysed and after washing, cells were counted and 2×105 cells were placed in each well of a sterile, U-bottomed microculture plate in medium with 12.5 mBSA. Cultures were maintained at 37°C for 2 days. IFN-γ amounts were measured by DuoSet enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN).
Determination of serum antibodies
Methylated BSA-specific antibodies of various isotypes (IgG1, IgG2a) were measured in sera of individuals by ELISA. Antigen was coated on microtiter plates at a concentration of 10 μg/ml. Antibody titers were assessed by 2-fold serial dilutions of sera, followed by detection of bound mouse Ig with a 1:500 dilution of peroxidase-conjugated rabbit anti-mouse Ig. O- phenylenediamine was used as substrate for the peroxidase reactions.
Bone marrow derived macrophages (BMDM)
To generate BMDMs, bone marrow cells were cultured in DMEM (Invitrogen) with 10% FBS and 20% L929 supernatant containing macrophage-stimulating factor for 6 days and were replated for the assays as indicated. BMDM were stimulated with LPS (100ng/ml) and analyzed by qPCR and WB.
Real-time quantitative PCR (qPCR)
Ankle joints and paws were collected, dissected to remove extra-articular tissue, and snap frozen in liquid nitrogen. For macrophages, cells were collected after BMDM stimulation. Total RNA was extracted with Trizol (Invitrogen) and reverse-transcribed with random hexamers and Superscript II Kit (Invitrogen). qPCR was performed with SYBR Green PCR Master Mix Kit (Applied Biosystems, Foster City, CA). The relative amounts of transcripts were compared to those of 18S mRNA and normalized to untreated samples by the ΔΔCt method (25).
Western blot analysis
BMDM or joints were disrupted in lysis buffer (PhosphoSafe™, Novagen, Gibbstown, NJ) containing a protease inhibitor cocktail. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Blots were probed with antibodies against phospho-ERK1/2, phospho-p38, phospho-JNK, phospho-STAT3, ERK1/2 (Cell Signaling Technology, Danvers, MA), JNK (BD Biosciences), STAT3, p38, MKK3 and MKK6 (Santa Cruz, St Jose, CA). Horseradish peroxidase-conjugated anti-IgG (Santa Cruz Biotechnology Inc, Santa Cruz, CA) was used as secondary antibody. Membranes were developed using a chemiluminescence system (ECL detection reagent: Amersham Life Science, Aylesbury, UK). Densitometry analysis was done by using Quantity One 1-D analysis software (Bio-Rad).
RA synovial cell cultures
RA synovial tissue was digested with 0.5mg/ml collagenase A in RPMI for 2 hours at 37C. The cells were washed twice with 10%FCS/DMEM and filtered using a 0.70μm cell strainer (Falcon). The cells were washed, counted and 3×106 cells were plated in each well of a 6-well plate. After overnight incubation, the cells were treated with 33M of the p38 inhibitor SB203580 for 48h and cytokines in the cell supernatants were quantified by multiplex analysis (Bio-Rad). The data are represented as average percent of untreated control
Statistical analysis
Data are expressed as means ± standard error of the mean (SEM). The analysis used unpaired Student’s t test for comparing two groups and ANOVA for multiple group comparisons. Results were considered significant if P < 0.05.
Results
Characterization of p38α deficient mice
To evaluate the contribution of p38α in macrophages in chronic inflammatory arthritis, mutant mice lacking p38α in macrophages were generated. Deletion of loxP-flanked alleles of the gene encoding p38α was mediated by Cre recombinase expressed under the control of the promoter of the myeloid-specific gene encoding Lysozyme M (p38αΔLysM). The mutant mice were born alive and grew to adulthood without showing discernible anomalies or developing spontaneous disease. p38α was not detectable in macrophages, but was expressed by other myeloid cell lineages, including neutrophils and mast cells (Fig 1A).
Figure 1. Selective deletion of p38α in macrophages increases inflammatory arthritis in mice.
BMDM (M), neutrophils (N) and bone marrow derived mast cells (MC) from p38αF/F and p38α ΔLysM mice were analyzed for p38 expression by WB. Note that p38 was only deleted in macrophages. (B) Clinical arthritis scores in p38αF/F (grey circles, n=6) and p38α ΔLysM (black squares, n=6) injected with 150 μl of K/BxN serum on day 0. Values are means ± SEM. * =p<0.05 vs. p38αF/F. (C) Ankles were prepared for histologic scoring from mice with arthritis on day 10. Representative H&E stained sections of ankles from each strain are shown (Magnification ×100). (D) Histological scores for joint inflammation, erosion and cartilage damage in p38αF/F and p38α ΔLysM mice (6 mice/group) on day 7 after serum transfer. Values are means ± SEM.
Selective deletion of p38α in macrophages increases subacute inflammatory arthritis
Passive K/BxN arthritis was studied in p38αΔLysM and p38αF/F. p38α ΔLysM mice had higher clinical scores from day 5 and a delay in the resolution phase of the arthritis compared with WT mice. The day 8 scores were 7.1 ± 1.2 and 9.7 ± 0.1 (P=0.05) and day 14 scores were 1.1 ± 0.3 and 3.0 ± 0.3 (P=0.01) for p38αF/F and p38α ΔLysM respectively (Fig. 1B). Histopathology showed a trend towards increased inflammatory cell infiltration, joint destruction and cartilage damage in p38α ΔLysM mice (Fig. 1C-D). The overall histological damage score was significantly greater in the p38α ΔLysM mice compared with p38αF/F mice (1.85 ± 0.28 vs 1.12 ± 0.23, p<0.05). Similar results were obtained in the antigen-induced arthritis (AIA) model in the p38αF/F and p38α ΔLysM (Supplementary Table 1), where the overall histology score was modestly increased in the p38 deficient mice. Of interest, the p38αF/F mice had normal adaptive immune response as measured by IFN-γ production in vitro and antibody production in vivo. These data suggest that the pro-inflammatory effect in macrophages is due to an effect on innate, not adaptive responses
To evaluate the influence of p38α deficiency in p38α ΔLysM mice on inflammatory mediators, expression of MMP3 by qPCR and expression of IL-1β and IL-6 by ELISA were determined in joints from a second group of mice on day 5 after KxB/N sera injection (day 5 scores were 6.8 ± 1.2 and 9.7 ± 1.3). MMP3 relative mRNA in paw extracts was higher in p38α ΔLysM mice and DUSP1 mRNA expression was lower (P<0.05, Fig. 2A). However, IL-6 and IL-1β protein levels in p38αΔLysM compared to p38αF/F controls were similar at that time point (Fig. 2B), possibly because they are produced by other cell lineages in the joint or because genetic deficiency of p38α has less effect on IL-6 than chemical inhibition (see below). IL-10 and TNF protein expression were at the limit of assay detection and could not be compared. Western blot (WB) analysis of joint extracts showed increased phosphorylation of ERK in the p38α ΔLysM mice (Fig. 2C).
Figure 2. Selective deletion of p38α in macrophages increases inflammatory mediators.
P38αF/F and p38α ΔLysM mice were injected with 150 μl of K/BxN serum on day 0 and they were sacrificed at day 5. Clinical scores at day 5 were 6.8 ± 1.2 and 9.7 ± 1.3 respectively. (A) Joint RNA from naive and arthritic mice was extracted and MMP3 and DUSP1 mRNA were analyzed by qPCR. (B) Joint protein was extracted and protein content quantified. IL-1β and IL-6 levels were analyzed by ELISA. (C) Joint protein from naive (N) and arthritic mice of p38αF/F (+) and p38α ΔLysM (-) mice was extracted and analyzed by WB for the presence of phospho-ERK.
Selective deletion of p38α in macrophages increases chronic inflammatory arthritis
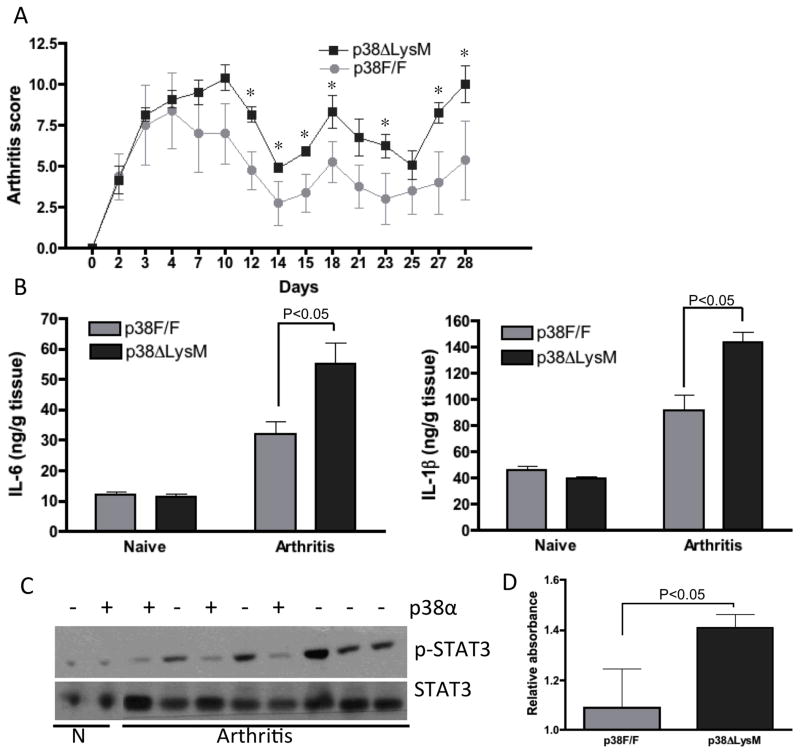
To assess the functional role of p38α deficient macrophages in a long-term model of arthritis, we induced chronic disease by injecting K/BxN serum every 7 days for 4 weeks. As shown in Figure 3A, arthritis scores were significantly higher in p38α ΔLysM mice (day 28 5.2 ± 1.6 and 10.2 ± 1.1, P=0.05, for WT and p38α ΔLysM mice, respectively). In contrast with the shorter term model, IL-6 and IL-1β protein levels were higher in inflamed p38α ΔLysM joints compared with WT joints (P<0.05, Fig 3B). WB analyses also showed increased phosphorylation of STAT3 phosphorylation consistent with the higher IL-6 levels (Fig. 3C and D).
Figure 3. Selective deletion of p38α in macrophages increases chronic inflammatory arthritis.
(A) Clinical arthritis scores and ankle swelling in p38αF/F (grey circles, n=5) and p38α ΔLysM (black squares, n=5) injected with 150 μl of K/BxN serum on day 0 and every 7 days thereafter. Values are means ± SEM. * =p<0.05 vs. p38F/F. (B) Joint protein was extracted on day 28 and protein content quantified. IL-1β and IL-6 levels were analyzed by ELISA. Note higher levels of IL-1 and IL-6 in the p38 deficient mice. (C) Joint protein from naive (N) and arthritic mice (day 28) of p38αF/F (+) and p38α ΔLysM (-) mice was extracted and analyzed by WB for the presence of phospho-STAT3. (D) Quantitative analysis of Western blots (arbitrary densitometry units) after normalizing results to total STAT3. Results are expressed as means of 5 mice/group ± SEM.
Regulation of cytokines and MAPKs by p38 in cultured macrophages
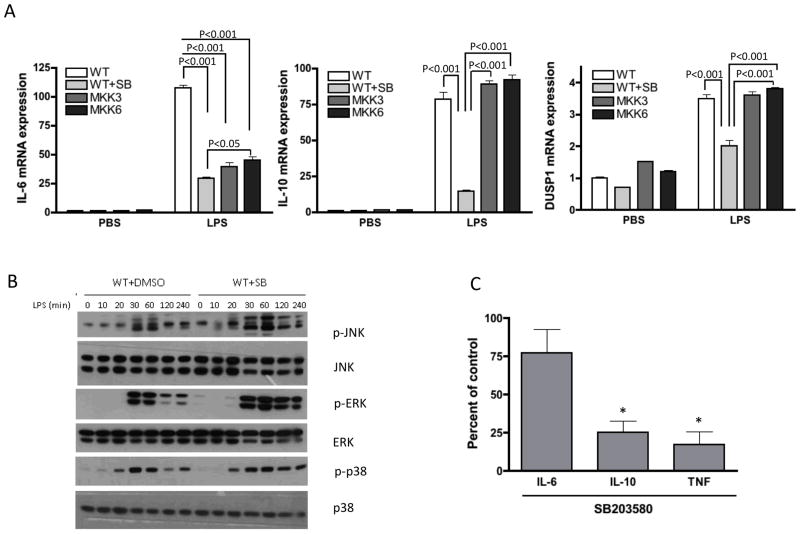
To determine the mechanism increased arthritis severity, WT bone marrow derived macrophages (BMDM) were cultured in the presence or absence of the p38α/β inhibitor SB203580 and stimulated with LPS. LPS-induced IL-10 and IL-6 mRNA were significantly decreased by the inhibitor (Fig. 4A). Of interest, DUSP1 expression was also decreased (P<0.01) (Fig. 4A). ERK and JNK phosphorylation was greater and more prolonged in the presence of the p38 inhibitor, which correlated with the decrease in DUSP1 expression (Fig. 4B). Similar results were observed for p38 deficient macrophages except for IL-6 expression, where no effect was observed on IL-6 expression (Supplementary Figure 1).
Figure 4. Regulation of cytokines and MAPKs by p38 in macrophages and synovial tissue cells.
(A) WT bone marrow derived macrophages (BMDM), in the presence or absence of a p38α/β inhibitor SB203580 were stimulated with LPS (100 ng/ml). After 2 hr BMDM were lysed, RNA extracted and analyzed by qPCR for IL-6, IL-10, and DUSP1. Note that the p38 inhibitor suppressed all three, while only IL-6 was suppressed in the MKK deficient cells. (B) After stimulation with LPS (100 ng/ml), BMDM were lysed and analyzed by WB for the presence of pERK. p-ERK and p-JNK levels were higher and persisted longer than cells cultured without the p38 inhibitor. Representative of 3 different experiments. (C) Human RA synovial cells were digested and 3×106 cells/well were plated in 6-well plates. The cells were treated with 33M of SB203580 for 48h and cytokines in the cell supernatants were quantified by immunoassay (Bio-Rad). The inhibitor decreased the amount of TNF and IL-10 secreted by cultured synovial cells. The effect on IL-6 was less prominent. Data are represented as average percent of untreated control (n=3). *p<0.05.
p38α/β inhibition significantly decreases IL-10 expression in RA synovial tissue cells
Our data indicate that p38α inhibition in macrophages is pro-inflammatory in arthritis and decreases IL-10 production in BMDM. To assess whether a similar phenomenon is observed in RA synovium, rheumatoid synovial tissue cells were cultured in the presence or absence of the p38 inhibitor SB203580. Supernatants were then assayed for TNF, IL-6, and IL-10. The compound substantially decreased TNF and IL-10 production. IL-6 production was only modestly decreased, as previously described by others (26) (Fig. 4C).
Regulation of cytokines and MAPKs in MKK deficient macrophages
Our previous studies suggested that upstream kinases in the p38 pathway might avoid some problems of a direct p38 inhibitor (22). To evaluate the effect of MKK3 or MKK6 deficiency on macrophage function, we cultured mkk3−/− and mkk6−/− BMDM in the presence or absence of LPS. MKK6 and MKK3-deficient BMDM had decreased LPS-mediated IL-6 expression compared with WT cells (P<0.01) (Fig. 4A). Surprisingly, MKK-deficient macrophages had normal IL-10 production, DUSP1 expression, and JNK and ERK phosphorylation (See Figure 5 compared with Figure 4B). As previously described, MKK3, but not MKK6, deficiency decreased p-p38 levels. The lack of effect on p-p38 in the mkk6−/− cells is most likely due to the role of this MKK as a structural protein in the p38 complexes rather than as an active kinase (23).
Figure 5. LPS stimulated MKK6 and MKK3-deficient BMDM had normal JNK and ERK phosphorylation.
MKK6 and MKK3-BMDM were stimulation with LPS (100 ng/ml), lysed and analyzed by WB for the presence of p-ERK and p-JNK. Compared with p38 inhibition, the time course was minimally affected by MKK deficiency. Representative of 3 different experiments.
MKK6 and MKK3-deficient marrow is protective in passive K/BxN arthritis
To assess whether MKK6 or MKK3 deletion in bone marrow cells enhances or protects against passive K/BxN arthritis, bone marrow chimeras were generated by irradiating C57Bl/6 and mkk3−/− or mkk6−/− recipients, and reconstituting with mkk3−/− or mkk6−/− and C57Bl/6 donor bone marrow. After 8 weeks passive K/BxN arthritis was then induced. Arthritis severity was dependent on MKK3 or MKK6 expression in bone marrow-derived cells. Thus WT chimeric mice with MKK6 and MKK3-deficient marrow had markedly decreased passive K/BxN arthritis severity, while WT bone marrow restored arthritis severity in MKK3 and MKK6 deficient mice (Fig. 6).
Figure 6. WT chimeric mice with MKK6 and MKK3-deficient marrow had markedly decreased passive K/BxN arthritis severity.
WT and mkk3−/− (n=5–6/group, (A)) or mkk6−/− mice (n=8–12/group, (B)) were irradiated and reconstituted with mkk3−/−, mkk6−/− or WT bone marrow as indicated. After 8 weeks, the chimeras were injected with 100 μl of pooled K/BxN serum i.p. on day 0 and day 2. Clinical scores and area under the curve expressed as means ± SEM. WT cells rescue disease severity in MKK deficient mice, while MKK deficient cells transfer protection in WT mice.
Discussion
p38α inhibitors demonstrate limited utility in RA despite abundant pre-clinical evidence predicting efficacy. One possible explanation is that p38α has anti-inflammatory functions in addition to its well-defined pro-inflammatory actions. For instance, p38α in macrophages regulates MAPK phosphatases and immunosuppressive cytokines such as IL-10. Genetic deletion of the p38α gene in macrophages increased acute skin edema after toxic exposures like ultraviolet light (12). These data raise the possibility that p38 deficiency in macrophages could increase the severity of chronic inflammation mediated by innate immunity. If so, then it might explain why inhibition of this kinase results in only a limited benefit in diseases dominated by macrophage cytokines like RA.
Macrophages participate in passive K/BxN arthritis evolution (27) and in established RA, where anti-TNF and IL-6 therapy demonstrate clinical efficacy (28). The number of macrophages in rheumatoid synovial biopsies also correlates with joint damage (29), and depletion of macrophages by therapeutic agents is associated with improvement in RA for some therapeutic agents (30). Therefore, a therapy that interferes with macrophages de-activation could increase disease severity even though production of some pathogenic cytokines is suppressed.
Previous studies suggested that p38 blockade or deficiency in macrophages suppresses production of the anti-inflammatory cytokine IL-10 and enhances activation of ERK and JNK (12). Our studies confirmed this observation and led us to explore complex immune-mediated models of inflammation. In vitro experiments focused on chemical p38 inhibition to mimic the situation in human clinical trials, although the results were similar for p38α-deficient macrophages. The only difference observed between genetic and small molecule inhibitors related to IL-6 expression, where genetic deletion appeared to have less effect.
p38α ΔLysM mice demonstrated increased disease severity in a transient model as well as a more persistent month-long chronic model. Cytokine and MMP expression, presumably by from other cell types such as fibroblast like synoviocytes or mast cells, was increased in the joints of p38 deficient mice, as was activation of downstream cytokine signaling molecules like STAT3. Of particular interest, P-ERK levels were also higher in the inflamed joints of p38α ΔLysM mice and correlated with our findings in cultured macrophages. Activation of mast cells and neutrophils are critical initiating events in this model, but the p38α ΔLysM mice have normal p38 expression in these lineages. Thus, the effects observed can be ascribed to macrophages. Similar results in the AIA model in the absence of altered adaptive responses suggest that the pro-inflammatory effect is due to an effect on innate immunity.
The murine data are also consistent with the role of p38 in cultured RA synovial tissue cells, where we confirmed that a p38α inhibitor substantially decreased TNF production from rheumatoid synovial tissue cells (26). We also noted that IL-6 production was only modestly affected by the inhibitor, while IL-10 production was markedly decreased. These data support the notion that p38 regulates both pro- and anti-inflammatory cytokines in RA cells and that could interfere with clinical efficacy.
As an alternative strategy to targeting downstream kinases like p38, we have advocated shifting emphasis to upstream signaling molecules(6). This approach has met with success, and it is especially noteworthy that JAK and Syk inhibitors demonstrate efficacy in RA (31, 32). Our previous studies in the p38 pathway suggest that inhibiting either of its two upstream regulators, namely MKK3 and MKK6, might be more effective than a traditional direct p38 inhibitor. For instance, mice deficient in either MKK have decreased joint inflammation and destruction in the passive K/BxN model and collagen-induced arthritis (22, 33, 34). MKK3 deficiency also mimics p38 inhibitors in a murine model of allodynia (35).
Based on these studies, we evaluated the profile of MKK deficient macrophages in vitro. These studies demonstrated striking dissociation of IL-6 and IL-10 regulation that distinguishes MKK and p38 function. Differential regulation can therefore provide the anti-inflammatory benefit by modulating, rather than blocking, the p38 pathway. We previously showed that p38 and MK2 activities do not necessarily correlate in a linear manner. A threshold level of p38 activation might be necessary for efficient MK2 activation, which is not reached in either mkk3−/− or mkk6−/ cells (23). Increased activation of other MAPKs like ERK and JNK was also substantially less in the MKK deficient cells.
The potential benefit of targeting MKK3 or MKK6 was supported by studies using bone marrow chimeras. MKK deficient marrow protected normal mice from passive K/BxN arthritis, while normal marrow failed to correct the defect in mkk3−/− or mkk6−/− mice. These data suggest that murine macrophages lacking the MKKs are protective, in contrast to the p38α deficient cells. The chimera study design does not precisely mimic the situation in p38α ΔLysM mice because MKK3 or MKK6 is deleted in all bone marrow cell lineages. However, it provides evidence that myeloid cells deficient in the p38 pathway due to targeting upstream kinases has the opposite effect of blocking p38 in macrophages.
Taken together, the in vitro and in vivo studies suggest that selectively blocking an MKK might be beneficial in inflammatory arthritis by sparing p38-regulated functions like IL-10 and DUSP1 expression. This approach could maintain negative feedback loops that are blocked by p38 inhibitors. Therefore, the data provide a rationale for strategies that inhibit upstream MKKs as a therapeutic approach in RA.
Supplementary Material
(A) BMDM from p38αF/F and p38α ΔLysM mice were stimulated with LPS (100 ng/ml). After 2 hr BMDM were lysed, RNA extracted and analyzed by qPCR for IL-6, IL-10, and DUSP1. (B) After stimulation with LPS (100 ng/ml), BMDM were lysed and analyzed by WB for the presence of p-ERK and p-JNK levels.
Acknowledgments
M.G. was supported by the Arthritis Foundation. M.C. was supported by the Arthritis Foundation. Work in M.K. and G.S.F. laboratories was supported by grants from the NIH (R01 AI070555 from NIAID for G.S.F).
This work was supported by grants from the Arthritis Foundation (MG) and the National Institutes of Arthritis and Musculoskeletal and Skin (GSF; AR47825), the National Institute of Allergy and Infectious Diseases (GSF; AI070555) and the National Institute of Health (MK; ES006376)
Footnotes
AUTHOR CONTRIBUTIONS
M.G. designed the project, performed most of the experiments, analyzed the data and wrote the manuscript. D.H. performed rheumatoid synovial experiments. M.C. and M.K. helped to interpret the data and in manuscript preparation, G.F. designed the study, analyzed the data, supervised the overall project and wrote the manuscript.
References
- 1.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–61. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 2.Thalhamer T, McGrath MA, Harnett MM. MAPKs and their relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47(4):409–14. doi: 10.1093/rheumatology/kem297. [DOI] [PubMed] [Google Scholar]
- 3.Schett G, Tohidast-Akrad M, Smolen JS, Schmid BJ, Steiner CW, Bitzan P, et al. Activation, differential localization, and regulation of the stress-activated protein kinases, extracellular signal-regulated kinase, c-JUN N-terminal kinase, and p38 mitogen-activated protein kinase, in synovial tissue and cells in rheumatoid arthritis. Arthritis Rheum. 2000;43(11):2501–12. doi: 10.1002/1529-0131(200011)43:11<2501::AID-ANR18>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Schett G, Zwerina J, Firestein G. The p38 mitogen-activated protein kinase (MAPK) pathway in rheumatoid arthritis. Ann Rheum Dis. 2008;67(7):909–16. doi: 10.1136/ard.2007.074278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sweeney SE, Firestein GS. Mitogen activated protein kinase inhibitors: where are we now and where are we going? Ann Rheum Dis. 2006;65(Suppl 3):iii83–8. doi: 10.1136/ard.2006.058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Ann Rheum Dis. 2010;69 (Suppl 1):i77–82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature reviews Drug discovery. 2003;2(9):717–26. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 8.Cohen SB, Cheng TT, Chindalore V, Damjanov N, Burgos-Vargas R, Delora P, et al. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009;60(2):335–44. doi: 10.1002/art.24266. [DOI] [PubMed] [Google Scholar]
- 9.Genovese MC. Inhibition of p38: has the fat lady sung? Arthritis Rheum. 2009;60(2):317–20. doi: 10.1002/art.24264. [DOI] [PubMed] [Google Scholar]
- 10.Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009;60(5):1232–41. doi: 10.1002/art.24485. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21(2):317–24. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, et al. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9(9):1019–27. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ananieva O, Darragh J, Johansen C, Carr JM, McIlrath J, Park JM, et al. The kinases MSK1 and MSK2 act as negative regulators of Toll-like receptor signaling. Nat Immunol. 2008;9(9):1028–36. doi: 10.1038/ni.1644. [DOI] [PubMed] [Google Scholar]
- 14.Kouskoff V, Korganow AS, Duchatelle V, Degott C, Benoist C, Mathis D. Organ-specific disease provoked by systemic autoimmunity. Cell. 1996;87(5):811–22. doi: 10.1016/s0092-8674(00)81989-3. [DOI] [PubMed] [Google Scholar]
- 15.Nishida K, Yamaguchi O, Hirotani S, Hikoso S, Higuchi Y, Watanabe T, et al. p38alpha mitogen-activated protein kinase plays a critical role in cardiomyocyte survival but not in cardiac hypertrophic growth in response to pressure overload. Mol Cell Biol. 2004;24(24):10611–20. doi: 10.1128/MCB.24.24.10611-10620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999;8(4):265–77. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 17.Wysk M, Yang DD, Lu HT, Flavell RA, Davis RJ. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci U S A. 1999;96(7):3763–8. doi: 10.1073/pnas.96.7.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka N, Kamanaka M, Enslen H, Dong C, Wysk M, Davis RJ, et al. Differential involvement of p38 mitogen-activated protein kinase kinases MKK3 and MKK6 in T-cell apoptosis. EMBO reports. 2002;3(8):785–91. doi: 10.1093/embo-reports/kvf153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60(12):3642–50. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigeoka AA, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J, et al. Nod1 and nod2 are expressed in human and murine renal tubular epithelial cells and participate in renal ischemia reperfusion injury. J Immunol. 2010;184(5):2297–304. doi: 10.4049/jimmunol.0903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Hu LL, Gonzalez-Navajas J, Seo GS, Shen C, Brick J, et al. ERK activation drives intestinal tumorigenesis in Apc(min/+) mice. Nat Med. 2010;16(6):665–70. doi: 10.1038/nm.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue T, Boyle DL, Corr M, Hammaker D, Davis RJ, Flavell RA, et al. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc Natl Acad Sci U S A. 2006;103(14):5484–9. doi: 10.1073/pnas.0509188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshizawa T, Hammaker D, Boyle DL, Corr M, Flavell R, Davis R, et al. Role of MAPK kinase 6 in arthritis: distinct mechanism of action in inflammation and cytokine expression. J Immunol. 2009;183(2):1360–7. doi: 10.4049/jimmunol.0900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosengren S, Firestein GS, Boyle DL. Measurement of inflammatory biomarkers in synovial tissue extracts by enzyme-linked immunosorbent assay. Clinical and diagnostic laboratory immunology. 2003;10(6):1002–10. doi: 10.1128/CDLI.10.6.1002-1010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle DL, Rosengren S, Bugbee W, Kavanaugh A, Firestein GS. Quantitative biomarker analysis of synovial gene expression by real-time PCR. Arthritis Res Ther. 2003;5(6):R352–60. doi: 10.1186/ar1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page TH, Brown A, Timms EM, Foxwell BM, Ray KP. Inhibitors of p38 suppress cytokine production in rheumatoid arthritis synovial membranes: does variable inhibition of interleukin-6 production limit effectiveness in vivo? Arthritis Rheum. 2010;62(11):3221–31. doi: 10.1002/art.27631. [DOI] [PubMed] [Google Scholar]
- 27.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B x N serum-induced arthritis. Eur J Immunol. 2005;35(10):3064–73. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 28.Gierut A, Perlman H, Pope RM. Innate immunity and rheumatoid arthritis. Rheum Dis Clin of North Am. 2010;36(2):271–96. doi: 10.1016/j.rdc.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39(1):115–24. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 30.Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: Implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56(11):3869–71. doi: 10.1002/art.22964. [DOI] [PubMed] [Google Scholar]
- 31.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 32.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58(11):3309–18. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]
- 33.Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J Immunol. 2005;174(7):4301–6. doi: 10.4049/jimmunol.174.7.4301. [DOI] [PubMed] [Google Scholar]
- 34.Hammaker D, Topolewski K, Edgar M, Yoshizawa T, Fukushima A, Boyle DL, et al. Decreased collagen-induced arthritis severity and adaptive immunity in mitogen activated protein kinase kinase 6 -deficient mice. Arthritis Rheum. 2011 doi: 10.1002/art.33359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorkin LS, Boyle DL, Hammaker D, Herman DS, Vail E, Firestein GS. MKK3, an upstream activator of p38, contributes to formalin phase 2 and late allodynia in mice. Neuroscience. 2009;162(2):462–71. doi: 10.1016/j.neuroscience.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) BMDM from p38αF/F and p38α ΔLysM mice were stimulated with LPS (100 ng/ml). After 2 hr BMDM were lysed, RNA extracted and analyzed by qPCR for IL-6, IL-10, and DUSP1. (B) After stimulation with LPS (100 ng/ml), BMDM were lysed and analyzed by WB for the presence of p-ERK and p-JNK levels.