Abstract
Imiquimod (IMQ), a Toll-like receptor (TLR) 7/8 agonist, has been used to treat various skin neoplasms, including genital warts, actinic keratoses and superficial basal cell carcinomas. Although IMQ has been recognized to activate both innate and adaptive immunity, the underlying mechanism(s) by which IMQ exerts its anti-tumor activity in vivo remains largely unknown. In this study, we took advantage of skin cancer-prone mice to characterize the effects of IMQ on ultraviolet irradiation (UV)-induced de novo carcinogenesis. Transgenic mice with keratinocytes expressing constitutively activated Stat3 (K5.Stat3C mice) developed squamous cell carcinomas (SCC in situ) as early as after 14 weeks of UVB irradiation, while wild-type mice required much higher doses of UVB with more than 25 weeks of UVB irradiation to produce SCC. Topical treatment of K5.Stat3C mice with IMQ attenuated UVB-induced epidermal dysplasia (SCC in situ). In addition, SCC growth due to increased total irradiation doses was significantly attenuated by IMQ treatment. Topical IMQ treatment induced T cell and plasmacytoid dendritic cell infiltrates at the tumor sites, where levels of IL-12/23p40, IL-12p35, IL-23p19, IL-17A and IFN-γ mRNAs were up-regulated. Immunohistochemistry revealed T cell infiltrates consisting of T1, Th17 and CD8+ T cells. We speculate that topical IMQ treatment attenuates the de novo growth of UVB-induced SCC through activation of Th17/Th1 cells and cytotoxic T lymphocytes.
Keywords: TLR agonist, UVB-induced skin cancer, Th1/Th17
INTRODUCTION
Imiquimod (IMQ) is an immune response modifier that is used topically to treat various neoplasms such as external genital warts, actinic keratoses (AK) [1] [2] [3] and superficial basal cell carcinomas (BCCs) [4] [5] [6]. IMQ exerts its anti-cancer effects by activating innate and adaptive immunity through stimulation of Toll-like receptors (TLR) 7/8, through which the activation of central transcription factors, in particular NF-κB, takes place [7]. Because of their prominent expression of TLR7 and TLR8, plasmacytoid DCs (pDCs) and myeloid DCs (mDCs) are likely candidates for the initiation of IMQ-induced host defense reactions [8]. NF-κB transduces the activation of downstream genes, including those encoding a number of cytokines and chemokines [9]. It has been demonstrated that TLR7/8 agonists induce the expression of proinflammatory cytokines, including interferon (IFN)-α, IFN- γ, tumor necrosis factor (TNF)- α, interleukin (IL-2), −6, −8, −12, G-CSF and GM-CSF, as well as chemokines such as CCL3, CCL4 and CCL2 [7] [10]. Therefore, topical application of IMQ is capable of recruiting a variety of inflammatory cells, such as dendritic cells, macrophages and T cells, all of which confer cellular immune responses against cancer cells. Antitumor immunity can be enhanced by IMQ administration after immunization with cancer antigens or DC vaccination, by which tumor antigen-specific cytotoxic T lymphocytes (CTLs) are successfully induced [11] [12]. Previous studies have demonstrated that IMQ stimulation induces the activation of pDCs through TLR7 triggering, leading to a burst of IFN-α production, which contributes to anti-tumor as well as anti-viral immunity [13] [8]. IFN responses by pDCs also link innate and adaptive immunity by facilitating the activities of mDCs, T cells and natural killer (NK) cells [14]. pDC-derived IFNs polarize naïve T cells into Th1 cells [15] [16] as well as Th17 cells [17].
Although previous studies have demonstrated that IMQ administration attenuates transplanted cancers in combination with vaccination of tumor-specific epitopes, very few animal models have been used to address the efficacy of IMQ on de novo cancers as seen in the clinic, such as actinic keratoses.
We have established a transgenic mouse with keratinocytes expressing the Stat3C transgene, which is a constitutively active form of Stat3 [18] [19]. Those transgenic mice, termed K5.Stat3C mice, demonstrate a trait to be cancer-prone when subjected to the two stage carcinogenesis protocol, using 9,10-dimethyl-1,2-benzanthracene (DMBA) as an initiator and 12-O-tetradecanoylphorbol 13-acetate (TPA) as a promoter. That protocol induces skin squamous cell carcinomas (SCCs) with a short latency and increased multiplicity in K5.Stat3C mice compared to wild-type mice [20]. Further, K5.Stat3C mice develop ultraviolet B (UVB)-induced cancers as early as after 14 weeks of exposure, whereas more than 25 weeks of UVB exposure are required for tumor development in wild-type mice [21]. Whereas wild-type mice have been used in a number of previous studies for UV-induced carcinogenesis, the advantage of K5.Stat3C mice in carcinogenesis experiment depends not only on the readiness of cancer development but also on their aggressive nature of SCC, which resembles human SCC in situ (actinic keratosis) and progressive SCC [20, 21]. In this study, we demonstrate that topical IMQ attenuates SCC in this mouse model and speculate that this anti-tumor effect is associated with activation of Th17 and Th1 cells.
MATERIALS AND METHODS
Mice
K5.Stat3C transgenic mice, in which keratinocytes express a constitutively active form of Stat3, have been described earlier [22]. Transgenic mice and non-transgenic mice were maintained on an FBV/N background. Female mice at 8–9 weeks of age were used in the study, which strictly adhered to institutional guidelines for minimizing distress to animals.
UVB skin carcinogenesis
Dermaray M-DMR-1 lamp bulbs (Dermaray, Perth, Australia) with a peak emission at 311 nm were used. Mice were irradiated at a dose of 200 mJ/cm2 3 times weekly after shaving to assess carcinogenesis on their dorsum. Ear skins were left unshaven before irradiation because hair on the ear is negligible and the mice developed skin cancers at the same time as on the dorsal skins. Therefore, we assessed the effects of IMQ on cancers on the ear.
Treatment with IMQ
IMQ cream (Beselna Cream, 5% IMQ) was kindly provided by the Mochida Pharmaceutical Co. (Tokyo, Japan). We topically applied approximately 1.25 mg IMQ on the right ear on each mouse 3 times weekly during the indicated periods. Three protocols were conducted to assess the efficacy of IMQ on skin carcinogenesis. 1. Efficacy of IMQ on the initiation phase of UVB-induced cancers: K5.Stat3C mice were topically treated with IMQ on the right ears and were left untreated on the left ears after each irradiation for 14 weeks. 2. Efficacy of IMQ on carcinomas in situ: K5.Stat3C mice were irradiated for 13 weeks (when epidermal dysplasia appeared) then were topically treated with IMQ on the right ears and were left untreated on the left ears for 6 weeks. 3. Efficacy of IMQ on established carcinomas: K5.Stat3C mice were irradiated for 16 weeks by which time SCCs had developed, and then were topically treated with IMQ on the right ears and were left untreated on the left ears for 4 weeks.
Immunohistochemistry
For immunohistochemical staining, antibodies used included anti-CD3ε (sc-1127, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-mouse pDC (120G8, Dendritics, Lyon, France) and anti-Ki67 (Dako, Glostrup, Denmark). Deparaffinized skin specimens were incubated in 10 mmol/L sodium citrate for 5 min using a microwave oven, then were treated with H2O2 and washed with PBS. Slides were treated with a blocking reagent (Protein Block Serum-Free, Dako) for 1 hour at room temperature, then stained with antibodies, followed by treatment with secondary antibodies conjugated with HRP (Dako), and visualized with diaminobenzidine. Apoptotic cells were detected using the TdT-mediated dUTP biotin nick-end labeling (TUNEL) method, according to the manufacturer’s protocol (Roche, Basel, Switzerland). Cells positive for anti-CD3, -pDC, -Ki67 and TUNEL were counted in three nonoverlapped fields per mice. For immunofluorescence staining, snap-frozen sections were treated with a blocking reagent for 1 hour at room temperature, and then were treated overnight at 4°C with monoclonal antibodies: goat anti-mouse CD3ε (sc-1127, Santa Cruz), rat anti-mouse CD4 mAb (H129.19, BD Pharmingen, San Diego, CA, USA), rat anti-mouse CD8α mAb (53–6.7, R&D Systems, Minneapolis, MN, USA), rabbit anti-mouse IL-17A polyclonal Ab (H-132, Santa Cruz Biotechnology), or rat anti-mouse IFN-γ (XMG1.2, Thermo Scientific, Rockford, IL, USA), followed by treatment with secondary antibodies: anti-goat IgG-Alexa 488, anti-rat IgG-Alexa 488/594, or anti-rabbit IgG-Alexa 594 (Invitrogen, Carlsbad, CA). DAPI was used for nuclear staining (Vectashield DAPI, Vector Laboratories, Burlingame, CA, USA).
Quantitative RT-PCR
Total RNAs from mouse skin lesions were extracted using an RNA isolation kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol, and were reverse-transcribed using M-MLV reverse transcriptase (Invitrogen) with random oligonucleotide hexamers (Invitrogen). PCR reactions were performed using PowerSYBER GreenPCR Master Mix (Applied Biosystems, Foster City, CA, USA) and amplification conditions were: 50°C for 2 min, 90°C for 10 min for 1 cycle, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The primers used were as previously reported [23] except for; (sense 5′-3′, anti-sense 5′-3′), IFN-α, CATTCTGCAATGACCTCCAC, TCAGGGGAAATTCCTGCAC; and Bcl-x, TGACCACCTAGAGCCTTGGA, ACAAGGGGCGTGGTTCTTA. The quantity of each transcript was analyzed using the 7300 Fast System Software (Applied Biosystems), and was normalized to HPRT mRNA according to the ΔΔCt method.
Statistics
Statistical analysis of significance was calculated using the Mann-Whitney U-test or Student’s t-test. p<0.05 is considered significant, and all data are shown as means ± SD.
RESULTS
K5.Stat3C mice are sensitive to UVB-induced carcinogenesis
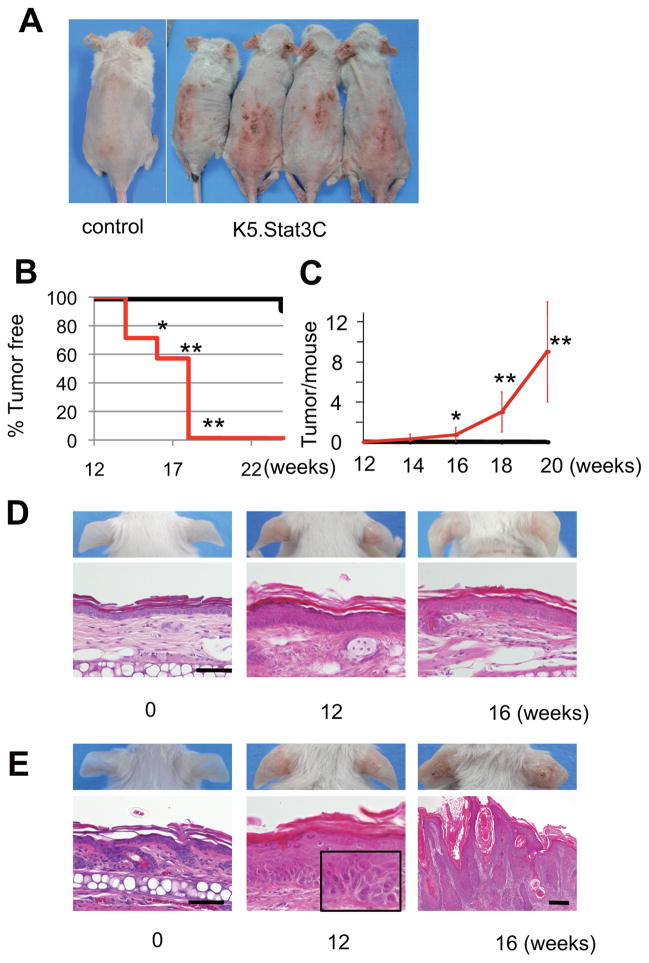
Constitutive activation of Stat3 enhanced the susceptibility to UVB-induced skin carcinogenesis, as previously reported [21]. All K5.Stat3C mice developed tumors on their ears and dorsal skins by 18 weeks of the UVB carcinogenesis regimen described in the Materials and Methods (Figure 1A, B). In contrast, only 8% (1 out of 12) of wild-type mice developed tumors after 24 weeks of UVB irradiation (Figure 1B). An average of 8 tumors developed in K5.Stat3C mice after 20 weeks of UVB irradiation (Figure 1C). Histological examination of the ear skins of K5.Stat3C mice at 12 weeks of UVB exposure revealed precancerous lesions with atypical epidermal cells with hyperchromatic nuclei (Figure 1E, bottom middle panel), although no visible tumors were seen at that time (Figure 1E, top middle panel). This indicates that K5.Stat3C mice are highly sensitive to UVB to produce cellular malignancies. At 16 weeks of irradiation, K5.Stat3C mice developed tumors with histological features of SCCs (Figure 1E, right panels). In contrast, wild-type mice were resistant to UVB exposure over this same time period, and did not generate atypical cells in the epidermis except for a mild epidermal thickening (Figure 1D, bottom panels). Thus, this skin cancer-prone trait of K5.Stat3C mice facilitates the investigation of the effects of topical IMQ treatment on UVB-induced carcinogenesis.
Figure 1.
K5.Stat3C transgenic mice are prone to UVB-induced skin cancers. (A) Development of skin cancers in K5.Stat3C mice at 18 weeks of UVB irradiation. At this time point, SCCs are present on the dorsal and ear skin of all K5.Stat3C mice tested, while no tumors are observed in non-transgenic control mice. (B, C) Percentage of mice without skin tumors (B) and the average number of skin tumors per mouse (C; mean ± SD) over time with UVB irradiation. black lines, non-transgenic mice (n = 12); red lines, K5.Stat3C mice (n = 7). *, p<0.05; **, p<0.01; Mann-Whitney U-test. (D, E) Representative images and corresponding histology of ear skins from control mice (D) and from K5.Stat3C mice (E) at 0, 12 and 16 weeks of UVB irradiation. K5.Stat3C mice show atypical cells harboring nuclear hyperchromatin (E, inset) at 12 weeks, and progressive SCC at 16 weeks. No cell atypicality or cancer in the epidermis is observed in control mice over this same time period, except for mild acanthosis. Scale bars in D, E, 50μm; except for that in the right bottom panel of E, indicating 200μm. Inset, an enlarged magnification of the epidermis showing atypical keratinocytes.
IMQ treatment does not prevent the initiation of UVB-induced skin cancers in K5.Stat3C mice
To examine whether topical treatment with IMQ affects the initiation of UVB-induced carcinogenesis, K5.Stat3C mice were subjected to topical IMQ treatment from the beginning of the UVB carcinogenesis regimen. At 14 weeks of UVB irradiation, the epidermis demonstrated hyperplasia with cell atypicality, including mitotic and hyperchromatic nuclei both in the IMQ-treated and in the untreated ears (data not shown). Therefore, this result suggests that topical IMQ treatment does not prevent the initiation of UVB-induced carcinogenesis. In some mice, however, inflammatory cell infiltrates were more pronounced in IMQ-treated skins compared to untreated controls (data not shown).
IMQ treatment inhibits the progression of UVB-induced SCCs in situ
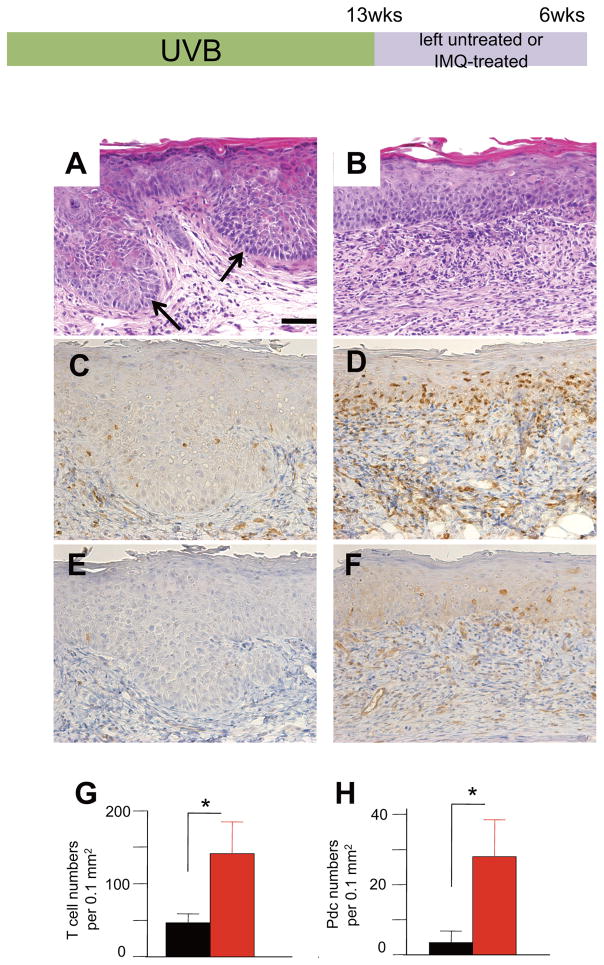
UVB irradiation for 12 weeks generated histological epidermal dysplasia in K5.Stat3C mice (Figure 1E). Six weeks after the discontinuation of the 13-week UVB irradiation, the precancerous lesions spontaneously progressed to intraepidermal SCCs (Figure 2A), namely SCC in situ, which resemble human actinic keratoses. The SCCs in situ showed downward proliferation, and epidermal cells exhibited disarrangement of polarity with pleomorphic and atypical nuclei (Figure 2A, arrows). In contrast, repeated IMQ treatment of the ears after the discontinuation of UVB irradiation inhibited the progression towards SCCs (Figure 2B). IMQ-treated skins demonstrated less epidermal nuclear atypicality and mitoses. Furthermore, in IMQ-treated mice, greater numbers of inflammatory cell infiltrates were found than in untreated skins (Figure 2B). Immunohistochemical studies revealed that T cells were increased in the epidermis and in the dermis of IMQ-treated mice (Figure 2D, G) compared with controls (Figure 2C, G). Furthermore, appreciable numbers of pDCs were also found in the dermis of IMQ-treated skins (Figure 2F, H), although there were very few pDCs in the untreated controls (Figure 2E, H). These results suggest that topical IMQ stimulation facilitates the recruitment of T cells and pDCs, which might contribute to anti-tumor immunity.
Figure 2.
IMQ prevents the progression of UVB-induced SCCs in situ. Mice are UVB irradiated for 13 weeks followed by no treatment (A, C, E) on the left ears or by topical treatment with IMQ on the right ears (B, D, F) for 6 weeks. Representative results of hematoxylin-eosin staining (A, B), immunohistochemical staining with anti-CD3 (C, D) and with anti-pDC (E, F). There is downward growth of the epidermis, in which disorientated atypical keratinocytes were observed in the left ear (A, arrows) at 6 weeks after the discontinuation of the 13 weeks of UVB irradiation. Note that IMQ attenuated the cell atypicality in the epidermis with underlying cell infiltrates (B). Anti-CD3 staining reveals a number of T cells in the epidermis and in the dermis of the IMQ-treated right ear skin (D), in contrast to the left ear skin, where the growing tumor is infiltrated with very few T cells (C). (G) Number of T cells (mean numbers ± SD per 0.1 mm2, n=4). Black bar, untreated; red bar, IMQ-treated. p<0.05. Student’s t-test. Anti-pDC staining reveals an increased number of pDCs at the epidermal-dermal border and in the upper dermis of IMQ-treated ear skin (F), whereas very few pDCs are present in tumors on the left ear (E). (H) Number of pDCs (mean numbers ± SD per 0.1 mm2). Black bar, n = 4 mice, untreated; red bar, n = 4 mice, IMQ-treated. p<0.05. Student’s t-test. Scale bar, 50 μm in A to F.
IMQ treatment attenuates cell proliferation and induces apoptosis in the dysplastic epidermis
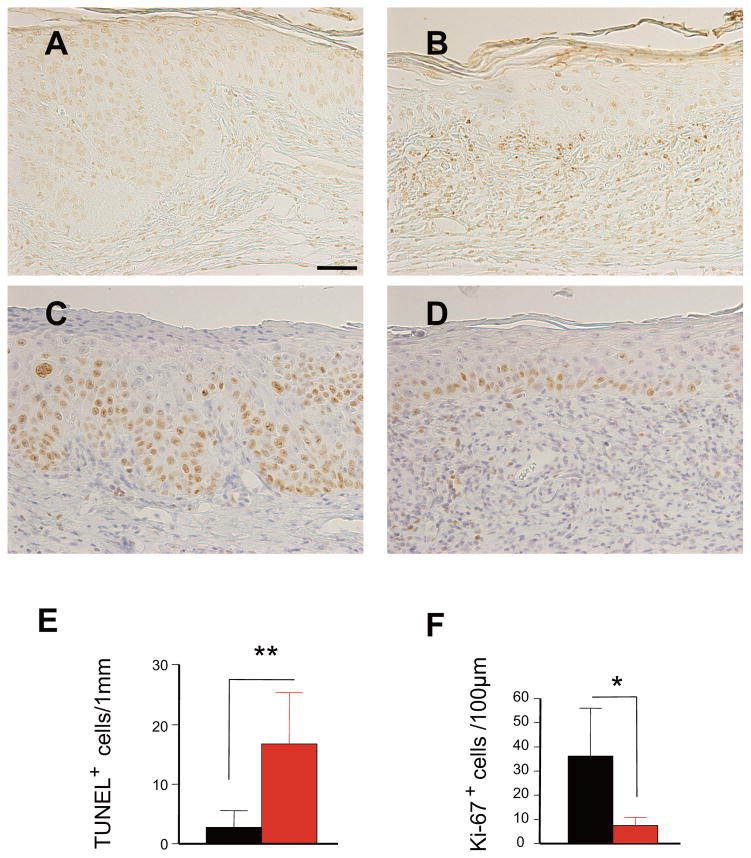
In the same experimental setting, cell proliferation and apoptosis were evaluated by Ki-67 staining and TUNEL staining, respectively. IMQ treatment increased cell apoptosis (Figure 3A, B, E) at the epidermal-dermal border and significantly reduced epidermal cell proliferation (Figure 3C, D, F), which resulted in the attenuation of cancer growth. These results suggest that the anti-tumoral activity of IMQ is mediated by immunocytes and/or a direct pro-apoptotic effect on cancer cells.
Figure 3.
IMQ induces apoptosis and attenuates the proliferation of epidermal cells in UVB-irradiated skin. TUNEL staining shows an increased number of apoptotic cells at the epidermal-dermal border in the right ear at 6 weeks of IMQ treatment after UVB irradiation for 13 weeks (B), whereas very few apoptotic cells are found in SCCs in situ in the untreated ear skin (A,). Staining with anti-Ki-67 reveals that cell proliferation is reduced in IMQ-treated skin (D), while a number of proliferating cells were observed in SCCs on the untreated right ear (C). Quantitative evaluation of apoptotic cells (E, mean numbers of TUNEL+ cells ± SD per 1mm) and cell proliferation (F, mean numbers of Ki67+ cells ± SD per 100μm of the epidermis). Black bar, n = 4 mice, untreated; red bar, n = 4 mice, IMQ-treated. **p<0.01, *p<0.05. Student’s t-test. Scale bars, 50μm in A to D.
IMQ treatment attenuates the growth of UVB-induced SCCs
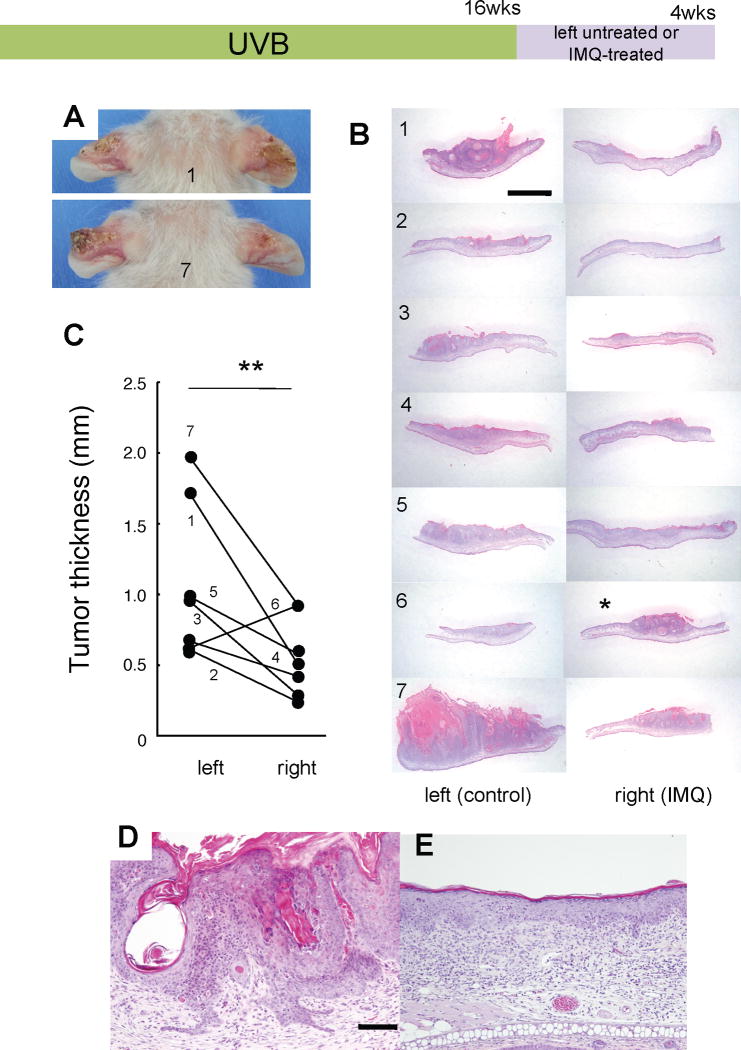
The UVB carcinogenesis regimen for 16 weeks resulted in the development of full-fledged SCCs in all K5.Stat3C mice. After discontinuation of the UVB irradiation, mice were then treated for 4 weeks with IMQ or were untreated in the right or left ears, respectively. SCCs in the IMQ-untreated ears increased in size continuously. However, the growth of SCC appeared to be inhibited by IMQ treatment. Figure 4A shows two representative mice (mouse #1 and #7). They developed SCC tumors in their left ears, but only flattened scaly lesions were observed in their IMQ-treated right ears. SCCs in all mice, except for 1 mouse (#6), underwent regression following topical IMQ treatment (Figure 4B). Quantitative analysis of tumor thickness of 7 mice revealed a significant difference between untreated and IMQ-treated SCCs (p<0.01, Student’s t-test). Four weeks after the discontinuation of UVB irradiation, SCC progressed even further, including cell atypia, hyperkeratosis, pseudo horncysts and decreased proliferation (Figure 4D). In contrast, 4 weeks of IMQ treatment markedly attenuated the malignant features of SCCs with underlying inflammatory cell infiltrates (Figure 4E).
Figure 4.
IMQ attenuates the progression of established SCCs. Full-fledged SCCs induced by UVB-irradiation for 16 weeks are subsequently treated for 4 weeks with IMQ on the right ears or were untreated on the left ears. (A) Macroscopic views of mice #1 (top) and #7 (bottom), in which tumor masses are present on the right ears whereas only flattened, scaly lesions are observed on the left ears. (B) Histological views of untreated SCCs on the left ears (left panels) and the IMQ-treated right ears (right panels) from 7 mice as individually numbered. IMQ-treated SCCs appear to be decreased in size (right panels) compared with those on the left ears (left panels) except for mouse #6 (asterisk). Scale bar, 2 mm. (C) Tumor thickness of untreated SCCs (left) compared with IMQ-treated SCCs (right) from individual mice as numbered. All mice but #6 demonstrated decreased thickness of SCCs following IMQ treatment. **, p<0.01, Student’s t-test. (D, E) Close-up histologic views of representative SCCs on the ears (from mouse #2) treated (E) or untreated (D) with IMQ. Four weeks after the discontinuation of 16 weeks of UVB irradiation, SCCs became more progressive including cell atypia, hyperkeratosis, pseudo horncysts and downward invasion (D), whereas IMQ treatment greatly attenuated that malignant nature, but instead band-like inflammatory cell infiltrates were noted (E). Scale bars, 100 μm (E, same magnification as D).
Involvement of Th1/Th17 cells in regressing SCCs with IMQ treatment
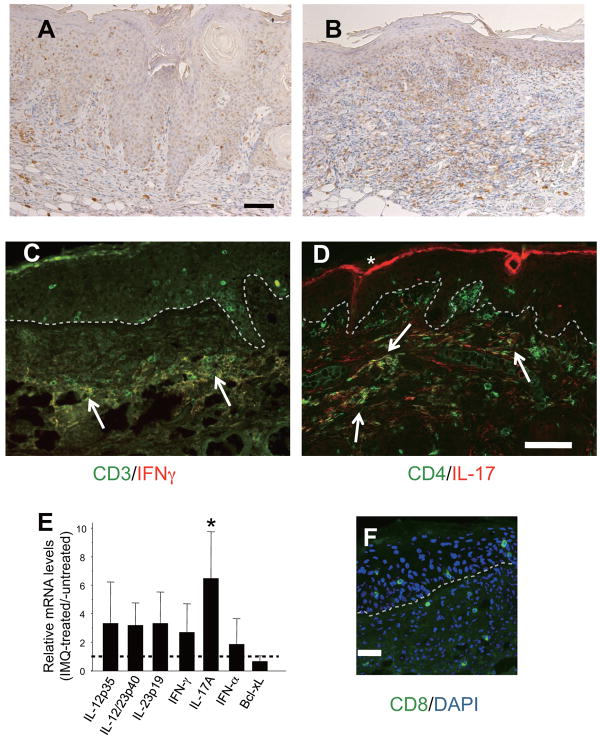
Immunohistochemical staining for CD3 revealed a number of T cell infiltrates in the underlying dermis of the regressing SCCs with IMQ treatment (Figure 5B), in contrast to the untreated controls, in which very few T cells were noted (Figure 5A). Quantitative RT-PCR of the SCC lesions demonstrated that IL-17A gene expression was significantly up-regulated in the IMQ-treated SCCs compared with the untreated SCCs (Figure 5E). Although not statistically significant, IFN-γ, IL-12p35, IL-23p19 and IL-12/23p40 mRNA transcript levels were also up-regulated in the IMQ-treated lesions. Despite not definitively, this result might suggest the roles for IL-12/Th1 and IL-23/Th17 axes in IMQ-induced attenuation of SCCs. Immunohistology of IMQ-treated skins revealed a number of IFN-γ+ CD3+ T cells in the lesions (Figure 5C), in which Th1/Tc1 (T1) cell activation and accumulation might occur following IMQ treatment. In addition, there were Th17 cell infiltrates in the dermis (Figure 5D). Furthermore, CD8+ T cells were found in the epidermis and in the dermis (Figure 5F), which suggests that CTLs are infiltrating the tumor sites. Taken collectively, we speculate that the regression of SCC induced by topical IMQ treatment is mediated by anti-tumoral activities associated with Th1/Th17 cells and CTLs.
Figure 5.
IMQ-induced regression of SCCs is associated with Th1/Th17 cell infiltrates. Immunohistochemistry reveals dense T cell infiltrates in the regressing SCCs (B), whereas very few T cells are observed in untreated SCCs (A). Scale bar, 100μm in A, B. (C) Immunofluorescence staining of an IMQ-treated regressing tumor with anti-IFN-γ (red) and anti-CD3 (green); a merged image is shown. Arrows indicate IFN-γ+CD3+ cell infiltrates, namely Tc1/Th1 cells, in the dermis. (D) Staining with anti-IL-17A (red) and anti-CD4 (green); a merged image is shown. Arrows indicate Th17 cells. *, unspecific staining of the cornified layer. (E) Quantitative RT-PCR SCC lesions from IMQ-treated versus untreated controls. Expression of each gene was normalized to HPRT mRNA and relative mRNA levels from IMQ-treated lesions (mean ± SD) relative to those from untreated SCCs are shown. The horizontal dotted line is the basal level of each gene expression from untreated SCC samples being set to 1. IL-12, IL-23, IFN-γ and IL-17A mRNAs were up-regulated over 2-fold. n = 4 mice, p<0.05, Mann-Whitney U-test. (F) CD8+ cells infiltrating the epidermis and the dermis. Dotted lines, epidermal-dermal borders. Scale bars, 100μm in A to D; 50μm in F.
DISCUSSION
SCCs are one of the most common cancers. Actinic keratoses represent SCCs in situ at sun-exposed areas through genomic perturbation due to UV radiation, and may develop progressively and even to metastatic SCCs. To date, topical treatment with IMQ, a TLR7/8 agonist, has been used to treat actinic keratoses, although the underlying mechanism remains undetermined [24] [25] [26]. In this study, we took advantage of skin cancer-prone K5.Stat3C transgenic mice in order to investigate the therapeutic mechanism involved in the topical IMQ treatment of UV-induced SCCs.
Similar to the effect on human actinic keratoses, IMQ successfully attenuated UVB-induced SCCs in this mouse model. Following IMQ treatment, a number of cell infiltrates was noted in the dermis. It has been demonstrated that following the application of IMQ, pDCs migrate into the skin and produce large amounts of IFN-α [27]. Similar to human skin, pDC infiltrates were found beneath the mouse SCCs following IMQ treatment. In addition, it has been demonstrated that IMQ induces the production of other proinflammatory cytokines, such as TNFα and IL-12, which facilitate a weighted-Th1 activation rather than Th2 [7]. As a result, anti-tumoral immune responses of NK cells and/or antigen-specific CTL activation might occur. IMQ-treated regressing tumor involved TUNEL+ cells at the epidermal-dermal border. They might represent apoptotic cancer cells, which were attacked by cytotoxic cells, although there might be apoptotic inflammatory cells if any. In this study, since IMQ-treated lesions demonstrate increased levels of mRNAs encoding IL-12 and IFN-γ, which confer the IL-12/Th1 axis, it is likely that CTL activation is induced. Since IMQ was applied on SCCs on the right ears whereas SCCs on the left ears were left untreated, it should be noted that even though tumor antigen-specific CTLs, if any, might have proliferated systemically, they were not able to attenuate SCCs on the untreated ears. Although the reason for this is unclear, any systemic anti-tumoral immunity elicited by IMQ on the right ears might be local, and systemic immunity was overcome by the progression of SCCs on the untreated ears. It is also possible that tumor-specific antigen(s) might be exposed exclusively at the IMQ-treated sites, since locally produced IFNs could enhance the expression of tumor antigens in association with MHC class I [28].
Notably, we also found that an appreciable number of Th17 cells accumulated at the regressing tumor sites following IMQ treatment, suggesting an anti-tumoral role of Th17. Previous studies have demonstrated that topical application of IMQ aggravates psoriasis in humans [29], and induces de novo psoriasis-like skin lesions in mice through activation of the IL-23/Th17 axis [17]. Our recent studies have demonstrated that K5.Stat3C mice, in which Stat3 is constitutively activated in epidermal keratinocytes, develop psoriasis-like skin lesions following wound healing or topical treatment with a phorbol ester, TPA [22] [19]. The development of psoriasis-like lesions in K5.Stat3C mice is dependent on the IL-23/Th17 axis, which closely resembles human psoriasis [23]. Interestingly, psoriasis-like lesions in K5.Stat3C mice are somehow resistant to the generation of SCCs through the two-stage carcinogenesis regimen [19]. This might correspond to the long-known paradox claimed by a British surgeon, Dr. Hutchinson, who concluded that anatomical sites in which arsenic keratoses and SCCs develop in psoriatic patients who had been treated with potassium arsenite did not include those at which psoriatic plaques developed [30] [31]. It has also been pointed out that PUVA-induced skin abnormalities generally spare psoriatic plaques and a recent study by others showed that psoriasis confers protection against actinic keratoses [32]. Given that the IL-23/Th17 axis is involved in psoriasis, it may be protective against the development of SCC. Therefore, IMQ could exert its anti-tumor effect by the activation of Th17 as well as Th1. It has been demonstrated that tumor-specific Th17 cells eradicate mouse melanomas in vivo in an IFN-γ-dependent manner [33]. However, the role of IL-17 and Th17 in the tumor microenvironment is still controversial [34] [35]. Since the present study did not provide direct evidence showing that the IL-23/Th17 and/or the IL-12/Th1 axis contributed to anti-tumoral immunity, their roles will be further validated by experiments using anti-IL-12/23p40 antibody, IL-17-deficient or IFN-γ-deficient mice.
We could not demonstrate that IMQ treatment prevents the de novo development of UVB-induced cancers, which suggests that IMQ does not inhibit the initiation of UVB-induced carcinogenesis. In contrast, IMQ treatment inhibits the early progression of SCCs (SCC in situ) and attenuates full-fledged SCCs. Thus, using this cancer-prone mouse model not only recapitulates the clinical efficacy of IMQ against actinic keratoses but also postulates a novel mechanistic interaction between pDCs and T cells in the anti-tumor immunity.
Acknowledgments
We thank Shogo Takamura, Kousei Kawamura, Saori Miyamoto and Tomoko Nagayama for technical assistance, and Naoko Kumagai for kind suggestions on the statistical analyses. This study was supported in part by grants from the Ministry of Education, Science, Sports and Culture of Japan, from the Japan Science and Technology Agency, from The Kochi University President’s Discretionary Funds, and from an NIH grant CA076520 (to JD).
Abbreviations
- AK
actinic keratosis
- BCC
basal cell carcinoma
- CTL
cytotoxic T lymphocyte
- DMBA
7,12-dimethylbenz[a]anthracene
- IFN
interferon
- IL
interleukin
- IMQ
imiquimod
- mDC
myeloid dendritic cell
- NK
natural killer
- pDC
plasmacytoid dendritic cell
- SCC
squamous cell carcinoma
- TLR
Toll-like receptor
- TPA
12-O-tetradecanoylphorbol-13-acetate
- TNF
tumor necrosis factor
- TUNEL
TdT-mediated dUTP biotin nick-end labeling
- UVB
ultraviolet B
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- 1.Stockfleth E, Meyer T, Benninghoff B, Christophers E. Successful treatment of actinic keratosis with imiquimod cream 5%: a report of six cases. Br J Dermatol. 2001;144:1050–1053. doi: 10.1046/j.1365-2133.2001.04197.x. [DOI] [PubMed] [Google Scholar]
- 2.Persaud AN, Shamuelova E, Sherer D, et al. Clinical effect of imiquimod 5% cream in the treatment of actinic keratosis. J Am Acad Dermatol. 2002;47:553–556. doi: 10.1067/mjd.2002.123492. [DOI] [PubMed] [Google Scholar]
- 3.Szeimies RM, Gerritsen MJ, Gupta G, et al. Imiquimod 5% cream for the treatment of actinic keratosis: results from a phase III, randomized, double-blind, vehicle-controlled, clinical trial with histology. J Am Acad Dermatol. 2004;51:547–555. doi: 10.1016/j.jaad.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Marks R, Gebauer K, Shumack S, et al. Imiquimod 5% cream in the treatment of superficial basal cell carcinoma: results of a multicenter 6-week dose-response trial. J Am Acad Dermatol. 2001;44:807–813. doi: 10.1067/mjd.2001.113689. [DOI] [PubMed] [Google Scholar]
- 5.Sterry W, Ruzicka T, Herrera E, et al. Imiquimod 5% cream for the treatment of superficial and nodular basal cell carcinoma: randomized studies comparing low-frequency dosing with and without occlusion. Br J Dermatol. 2002;147:1227–1236. doi: 10.1046/j.1365-2133.2002.05069.x. [DOI] [PubMed] [Google Scholar]
- 6.Stockfleth E, Trefzer U, Garcia-Bartels C, Wegner T, Schmook T, Sterry W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: an overview. Br J Dermatol. 2003;149 (Suppl 66):53–56. doi: 10.1046/j.0366-077x.2003.05626.x. [DOI] [PubMed] [Google Scholar]
- 7.Schon MP, Schon M. TLR7 and TLR8 as targets in cancer therapy. Oncogene. 2008;27:190–199. doi: 10.1038/sj.onc.1210913. [DOI] [PubMed] [Google Scholar]
- 8.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 10.Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174:1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- 11.Rechtsteiner G, Warger T, Osterloh P, Schild H, Radsak MP. Cutting edge: priming of CTL by transcutaneous peptide immunization with imiquimod. J Immunol. 2005;174:2476–2480. doi: 10.4049/jimmunol.174.5.2476. [DOI] [PubMed] [Google Scholar]
- 12.Prins RM, Craft N, Bruhn KW, et al. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 13.Palamara F, Meindl S, Holcmann M, Luhrs P, Stingl G, Sibilia M. Identification and characterization of pDC-like cells in normal mouse skin and melanomas treated with imiquimod. J Immunol. 2004;173:3051–3061. doi: 10.4049/jimmunol.173.5.3051. [DOI] [PubMed] [Google Scholar]
- 14.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 15.Wagner TL, Ahonen CL, Couture AM, et al. Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol. 1999;191:10–19. doi: 10.1006/cimm.1998.1406. [DOI] [PubMed] [Google Scholar]
- 16.Conrad C, Meller S, Gilliet M. Plasmacytoid dendritic cells in the skin: to sense or not to sense nucleic acids. Semin Immunol. 2009;21:101–109. doi: 10.1016/j.smim.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 17.van der Fits L, Mourits S, Voerman JS, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 18.Sano S, Chan KS, Kira M, et al. Signal transducer and activator of transcription 3 is a key regulator of keratinocyte survival and proliferation following UV irradiation. Cancer Res. 2005;65:5720–5729. doi: 10.1158/0008-5472.CAN-04-4359. [DOI] [PubMed] [Google Scholar]
- 19.Sano S, Chan KS, DiGiovanni J. Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. J Dermatol Sci. 2008;50:1–14. doi: 10.1016/j.jdermsci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Chan KS, Sano S, Kataoka K, et al. Forced expression of a constitutively active form of Stat3 in mouse epidermis enhances malignant progression of skin tumors induced by two-stage carcinogenesis. Oncogene. 2008;27:1087–1094. doi: 10.1038/sj.onc.1210726. [DOI] [PubMed] [Google Scholar]
- 21.Kim DJ, Angel JM, Sano S, DiGiovanni J. Constitutive activation and targeted disruption of signal transducer and activator of transcription 3 (Stat3) in mouse epidermis reveal its critical role in UVB-induced skin carcinogenesis. Oncogene. 2009;28:950–960. doi: 10.1038/onc.2008.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sano S, Chan KS, Carbajal S, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11:43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, Kanda T, Takaishi M, et al. Distinct roles of IL-23 and IL-17 in the development of psoriasis-like lesions in a mouse model. J Immunol. 2011;186:4481–4489. doi: 10.4049/jimmunol.1000148. [DOI] [PubMed] [Google Scholar]
- 24.Hadley G, Derry S, Moore RA. Imiquimod for actinic keratosis: systematic review and meta-analysis. J Invest Dermatol. 2006;126:1251–1255. doi: 10.1038/sj.jid.5700264. [DOI] [PubMed] [Google Scholar]
- 25.Lysa B, Tartler U, Wolf R, et al. Gene expression in actinic keratoses: pharmacological modulation by imiquimod. Br J Dermatol. 2004;151:1150–1159. doi: 10.1111/j.1365-2133.2004.06236.x. [DOI] [PubMed] [Google Scholar]
- 26.Ooi T, Barnetson RS, Zhuang L, et al. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. Br J Dermatol. 2006;154:72–78. doi: 10.1111/j.1365-2133.2005.06932.x. [DOI] [PubMed] [Google Scholar]
- 27.Urosevic M, Dummer R, Conrad C, et al. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97:1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- 28.Bohm W, Thoma S, Leithauser F, Moller P, Schirmbeck R, Reimann J. T cell-mediated, IFN-gamma-facilitated rejection of murine B16 melanomas. J Immunol. 1998;161:897–908. [PubMed] [Google Scholar]
- 29.Gilliet M, Conrad C, Geiges M, et al. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140:1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 30.Nickoloff BJ. Creation of psoriatic plaques: the ultimate tumor suppressor pathway. A new model for an ancient T-cell-mediated skin disease. Viewpoint J Cutan Pathol. 2001;28:57–64. doi: 10.1034/j.1600-0560.2001.280201.x. [DOI] [PubMed] [Google Scholar]
- 31.Nickoloff BJ. The skin cancer paradox of psoriasis: a matter of life and death decisions in the epidermis. Arch Dermatol. 2004;140:873–875. doi: 10.1001/archderm.140.7.873. [DOI] [PubMed] [Google Scholar]
- 32.Paltiel O, Adler B, Herschko K, Tsukrov B, David M. Are patients with psoriasis susceptible to the classic risk factors for actinic keratoses? Arch Dermatol. 2004;140:805–810. doi: 10.1001/archderm.140.7.805. [DOI] [PubMed] [Google Scholar]
- 33.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maniati E, Soper R, Hagemann T. Up for Mischief? IL-17/Th17 in the tumour microenvironment. Oncogene. 2010;29:5653–5662. doi: 10.1038/onc.2010.367. [DOI] [PMC free article] [PubMed] [Google Scholar]