Abstract
INTRODUCTION
The incidence of metastasis of hepatocellular carcinoma (HCC) to the gallbladder is low. Here, we report a case of HCC with metastasis to the gallbladder and discuss the pattern of spread and the treatment.
PRESENTATION OF CASE
A 74-year-old man was diagnosed with advanced hepatocellular carcinoma. Computed tomography and magnetic resonance imaging demonstrated a tumor in the right lobe of the liver with a thrombus in the bifurcation of the portal vein. Because intraoperative ultrasonography showed portal vein tumor thrombosis from the main tumor reaching the umbilical portion, we performed only a cholecystectomy for the elimination of postoperative cholecystitis. Pathological examination showed gallbladder vein tumor thrombosis from poorly differentiated hepatocellular carcinoma.
DISCUSSION
A preoperative diagnosis of metastatic HCC to the gallbladder is difficult because there are no specific findings in the imaging tests. Cancer cells in the liver were thought to migrate to the gallbladder via the connection between the portal system and the cholecystic veins, and grow in the lumen of the veins in our case. The survival rate, in all reported cases including the present case, was increased in patients who underwent radical resection, compared to patients who underwent palliative surgery.
CONCLUSION
The resection of metastatic HCC to the gallbladder might appear to prolong survival.
Keywords: Hepatocellular carcinoma, Gallbladder metastasis
1. Introduction
Hepatocellular carcinoma (HCC) commonly shows intrahepatic spread, compared to extrahepatic metastasis, even in advanced-stage tumors. The majority of extrahepatic metastasis lesions are found in the lungs, lymph nodes, and bones.1–3 The incidence of metastasis of HCC to the gallbladder is low. There have been some clinical reports of cases with metastatic tumors of the gallbladder detected while the patient is alive. Here, we report a case of HCC with metastasis to the gallbladder and discuss the pattern of spread and the treatment.
2. Presentation of case
A 74-year-old man was referred to our hospital because his laboratory profile showed elevated levels of transaminase in April 2011. He had no symptoms. His laboratory profile showed elevated levels of aspartate aminotransferase (51 U/L), alanine transferase (47 U/L). He had a total bilirubin level of 0.7 mg/dL. The indocyanine green retention rate at 15 min was 12.9%. The level of protein induced by vitamin K absence or antagonist II (PIVKA-II) and serum α-fetoprotein were elevated: 33,553 mAU/mL for PIVKA-II and 48,860 ng/mL for serum α-fetoprotein. Hepatitis B surface antigen and hepatitis C virus antibody were negative. Computed tomography demonstrated a tumor measuring 88 mm × 70 mm in diameter in S1/5/6/7/8 of the liver (Fig. 1a). This tumor showed contrast enhancement in the hepatic arterial phase and then became less dense than the liver parenchyma in the portal phase. Multiple satellite nodules were also seen around the main tumor. Tumor thrombus was seen in the bifurcation between the right and the left branches of the portal vein (Fig. 1b). Magnetic resonance imaging using gadoxetate acid demonstrated that these tumors showed a pattern of early enhancement and washout. An angiographic examination showed that the satellite nodules were fed by the proper hepatic artery. The right branch of the portal vein was not demonstrated in the portal phase. The gallbladder was intact in these imaging tests (Fig. 1c). Therefore, the diagnosis was HCC (T3N0M0 Stage IIIA according to the International Union Against Cancer classification) in the right lobe, with tumor thrombus reaching the bifurcation of the portal vein. Following transarterial chemoembolization, we intended to perform right and caudate lobectomies of the liver and a cholecystectomy in August 2011. During surgery, no peritoneal dissemination, ascites, or lymph node metastasis was observed, but intraoperative ultrasonography showed that the portal vein tumor thrombosis from the main tumor reached the umbilical portion of the left lobe of the liver (Fig. 2). We only conducted a cholecystectomy for the elimination of postoperative cholecystitis, because the patient's liver reserve was not enough for an extended right hepatectomy. Macroscopically, no tumor was found on the surface of the gallbladder (Fig. 3a). Pathological examination showed that the tumor cells consisted of alveolar or trabecular cells with eosinophilic cytoplasm and diffuse invasion of the veins in the mucosal and submucosal layers of the gallbladder as gallbladder tumor thrombosis (Fig. 3b and c). The final diagnosis was HCC with gallbladder metastasis.
Fig. 1.
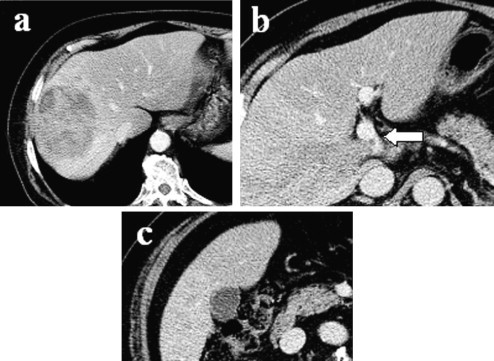
(a) Computed tomography scan showing a 88-mm × 70-mm tumor in the right lobe of the liver. (b) A tumor thrombus is seen in the bifurcation of the portal vein (arrow). (c) The gallbladder appears normal.
Fig. 2.
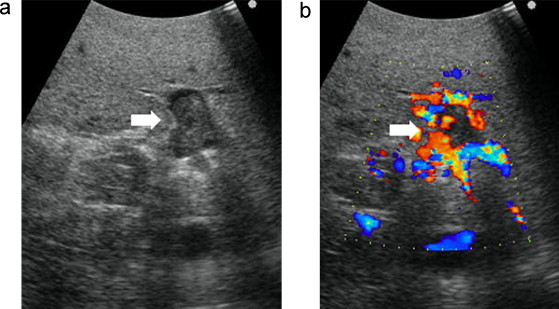
Intraoperative ultrasonography showing that a portal vein tumor thrombus from the main tumor reached the umbilical portion of the left lobe of the liver.
Fig. 3.
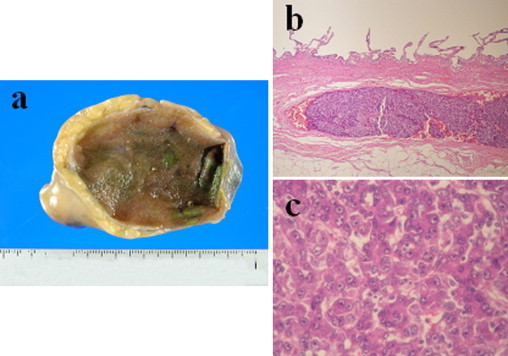
(a) Macroscopically, no tumorous lesion is seen in the mucosa or wall of the gallbladder. (b) Histopathological staining showing a representative tumor thrombus in the gallbladder veins (hematoxylin and eosin, 10×). (c) Pathological examination showing that the tumor cells consisted of alveolar or trabecular cells with eosinophilic cytoplasm and diffuse invasion of the veins in the mucosal and submucosal layers of the gallbladder as a tumor thrombosis (hematoxylin and eosin, 100×).
The patient made an uneventful recovery. Sorafenib was introduced for the treatment of HCC, but his condition gradually deteriorated and he died in October 2011, 2 months after surgery.
3. Discussion
A metastatic tumor of the gallbladder is rare. An autopsy series represented the incidence of metastatic tumor of the gallbladder in 1000 cases to be 5.8%.4 The most common primary carcinoma leading to a metastatic tumor of the gallbladder is malignant melanoma. Das Gupta and Brasfield found a 15% incidence of gallbladder metastasis in patients dying from malignant melanoma.5 Extrahepatic metastasis of HCC is rather common. Recent clinical follow-up studies found the incidence of extrahepatic metastasis of HCC to be 13.5–36.7%.1–3 Uchino3 reported that the distribution of the metastatic sites of HCC was lungs in 135 patients (39.5%), lymph nodes in 117 patients (34.2%), bones in 87 patients (25.4%), adrenal glands in 30 patients (8.8%), brain in 4 patients (1.2%), spleen in 2 patients (0.6%), and breast in 1 patient (0.3%), for a total of 376 extrahepatic occurrences in 342 patients. The incidence of metastasis of HCC to the gallbladder is 2.8–5.8% of extrahepatic metastasis in an autopsy series.6,7 However, there have been a few clinical reports of cases with metastatic tumor of the gallbladder detected while the patient was alive. There have been 16 reported cases of metastatic HCC to the gallbladder. The resected cases of metastatic HCC to the gallbladder are shown in Tables 1 and 2.8–15
Table 1.
Clinical characteristics of metastatic hepatocellular carcinoma to the gallbladder.
| Case | First author | Year | Age/sex | Background | Preoperative image of gall bladder | Evidence of cholecystitis | HCC |
Synchronous (S) or metachronous (M) | PVTT | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Size (cm) | Number | |||||||||
| 1 | Maruo8 | 1994 | 73/M | Non B non C | np | − | S4 | 48 | St | S | − |
| 2 | Nishida9 | 1997 | 48/M | HBV | GB dilation | + | S4/5 | NA | Mt | S | + |
| 3 | Lane10 | 2002 | 78/M | NA | GB dilation | + | Right lobe | NA | Mt | S | NA |
| 4 | Terashima11 | 2007 | 49/M | HBV | Well demarcated, enhanced polypoid mass | − | S5/6/7/8 | 10.7 | Mt | M | + |
| 5 | Ando12 | 2009 | 75/M | HCV | Well demarcated, enhanced tumor | − | NA | NA | Mt | S | NA |
| 6 | Murakami13 | 2010 | 53/M | HBV Alc | GS | − | S7/8 | 14 | Mt | S | + |
| 7 | 61/M | HCV | np | − | S5/8 | 9.5 | Mt | S | + | ||
| 8 | 79/M | Alc | Wall thickness | − | S2/3/4 | 13 | Mt | S | + | ||
| 9 | 47/M | HBV | GB dilation | − | S4 | 6.5 | Mt | S | + | ||
| 10 | 47/M | HBV | np | − | S2/3/4 | 13 | Mt | S | + | ||
| 11 | 32/M | HBV Alc | np | − | S5/6/7/8 | 15 | Mt | S | + | ||
| 12 | 74/M | HCV | Wall thickness | − | S5/6 | 5 | St | S | + | ||
| 13 | 66/M | Alc | np | − | S5/8 | 3.5 | Mt | S | + | ||
| 14 | Monden14 | 2011 | 66/M | HCV | Polypoid mass | − | S5/8 | NA | St | M | − |
| 15 | Kanzaki15 | 2011 | 48/F | Non B non C | Round-shaped tumor | − | S5 | 1.3 | St | S | − |
| 16 | Our case | 2012 | 74/M | HCV | np | − | S1/5/6/7/8 | 88 | Mt | S | + |
HBV, hepatitis B virus; HCV, hepatitis C virus; Alc, alcoholic hepatitis; np, nothing particular; GB, gallbladder; GS, gallstones; Mt, multiple tumors; St, single tumor; NA, not available.
Table 2.
Treatment, histopathological characteristics, and outcome.
| Case | Preoperative treatment | Operation | Residual tumor | Postoperative therapy | Morphologic type | Histological type | Prognosis |
|---|---|---|---|---|---|---|---|
| 1 | None | Left + chole | 0 | Omentectomy TAE | Elevated | Mod | 32 mo alive |
| 2 | None | Chole | 2 | NA | Diffuse invasion to the muscularis propria and submucosal layer | Mod | NA |
| 3 | None | Chole | 2 | NA | GBTT | Well | Died of pneumonia |
| 4 | IFN TAI |
Chole | 0 | Hepatectomy TAI |
Polypoid mass | Mod | 13 mo alive |
| 5 | TACE RFA | Chole | NA | NA | Pedunculated tumor | NA | NA |
| 6 | None | Right + chole | 0 | FAIT | GBTT | Poor | 63 mo alive |
| 7 | None | E-Right + chole | 0 | FAIT | GBTT | Poor | 4 mo alive |
| 8 | None | E-Left + chole | 2 | FAIT | GBTT | Mod | 6 mo dead |
| 9 | None | Left + chole | 0 | FAIT | NA (mp) | Poor | 54 mo dead |
| 10 | TACE | E-Left + chole | 2 | FAIT | NA (mp) | Poor | 9 mo dead |
| 11 | None | E-right + chole | 2 | FAIT | NA (mp) | Poor | 3 mo dead |
| 12 | TACE | Right + chole | 0 | None | NA (sm) | Poor | 5 mo dead |
| 13 | TACE | Ant + chole | 2 | None | Protruding (m-mp) | Poor | 6 mo dead |
| 14 | TAE RFA HIMAC | Chole | 0 | None | Elevated + diffuse | Mod | 10 mo alive |
| 15 | En bloc resection of gallbladder and the liver surrounding the gallbladder | Chole | 0 | None | Mass located below serosa | Mod | 24 mo alive |
| 16 | TACE | Chole | 2 | Sorafenib | GBTT | Poor | 2 mo dead |
IFN, interferon; TAI, transcatheter hepatic arterial infusion; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; TAE, transcatheter hepatic arterial embolization HIMAC, heavy-ion medical accelerator complex; Left, left hepatectomy; chole, cholecystectomy; Right, right hepatectomy; E-, extended; Ant, anterior segmentectomy; FAIT, intraarterial infusion of 5-FU and subcutaneous interferon-alpha injection therapy; GBTT, gallbladder vein tumor thrombus; Mod, moderate; NA, not available: mo, month.
A preoperative diagnosis of metastatic HCC to the gallbladder is difficult. Two patients required cholecystectomy due to cholecystitis.9,10 Although imaging showed wall thickening or tumorous lesions of the gallbladder in some cases, there are no specific findings in the imaging tests of metastatic HCC to the gallbladder. In our case, the gallbladder metastasis was identified only by histopathological examination after the cholecystectomy because the surface of the gallbladder macroscopically appeared almost normal.
The location of the primary tumor was at or near the center of the gallbladder bed. Thirteen of the 16 reported cases had a primary tumor in S4 or 5 and 11 of 14 patients had portal vein tumor thrombosis. Five cases, including our case, showed only gallbladder tumor thrombosis without an apparent tumor mass in the mucosa or wall. Nakashima et al.7 proposed four possible routes by which HCC can involve the gallbladder: (i) the hematogenous route via the portal venous system, usually with portal vein tumor thrombosis, (ii) the lymphogenous route, (iii) the route of direct invasion of HCC from the liver, and (iv) metastasis to the gallbladder with peritoneal dissemination of HCC. They reported that tumor growth was found in the portal trunk or in the major portal branch at the hilum in 150 (64.7%) of 232 cases and that the majority of the gallbladder metastasis of HCC occurred by the hematogenous route via the portal venous system. Some cases with only tumor thrombosis in the cholecystic veins without any invasion to the gallbladder wall were also seen in their series. Anatomically, all cholecystic veins enter the right anterior branch of the portal vein system through the liver bed of the gallbladder through Calot's triangle.16 In our case, cancer cells in the liver were thought to migrate to the gallbladder via the connection between the portal system and the cholecystic veins, and grow in the lumen of the veins.
In general, the indications for resection of the extrahepatic metastasis of HCC are limited because of the poor survival rate.17 Uchino et al.3 reported that the cumulative survival rates after the diagnosis of extrahepatic metastasis were 39.3% at 1 year, 15.3% at 2 years, 7.4% at 3 years, and 4% at 5 years and that the median survival was 8.1 months (range, from 1 day to 108.7 months). They concluded that the major cause of death in patients with HCC who have extrahepatic metastasis is progression of the intrahepatic HCC lesion and that the extrahepatic metastasis is not the direct cause of death in the majority of affected patients. A pulmonary metastasectomy is associated with good outcomes in selected patients.18 There are few reports of the resection of metastatic HCC in sites other than lung, but a metastasectomy appears to prolong survival.19
The survival rate, in all reported cases including the present case, was increased in patients who underwent radical resection, compared to patients who underwent palliative surgery, which suggests that radical resection might be the more useful method for curability.15 Although strong evidence is lacking to support an aggressive approach to resection, it seems reasonable to perform radical resections for patients with potentially curable disease and to produce a satisfactory local response. More experience and long-term follow-up is necessary to justify the risks of surgical resection.
Conflict of interest
The authors declare no potential conflict of interest.
Funding
None.
Ethical approval
Written informed consent was obtained from the patients for the information to be included in our manuscript. His information has been de-identified to the best of our ability to protect his privacy.
Authors’ contribution
Each author participated in writing the manuscript and all agreed to accept equal responsibility for the accuracy of the content of the paper.
References
- 1.Katyal S., Oliver J.H., 3rd., Peterson M.S., Ferris J.V., Carr B.S., Baron R.L. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 2.Natsuizaka M., Omura T., Akaike T., Kuwata Y., Yamazaki K., Sato T. Clinical features of hepatocellular carcinoma with extrahepatic metastases. Journal of Gastroenterology and Hepatology. 2005;20:1781–1786. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 3.Uchino K., Tateishi R., Shiina S., Kanda M., Masuzaki R., Kondo Y. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 4.Abrams H.L., Spiro R., Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Das Gupta T., Brasfield R. Metastatic melanoma: a clinicopathological study. Cancer. 1964;17:1323–1339. doi: 10.1002/1097-0142(196410)17:10<1323::aid-cncr2820171015>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 6.Edmondson H.A., Steiner P.E. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::aid-cncr2820070308>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Nakashima T., Okuda K., Kojiro M., Jimi A., Yamaguchi R., Sakamoto K. Pathology of hepatocellular carcinoma in Japan. 232 consecutive cases autopsied in ten years. Cancer. 1983;51:863–877. doi: 10.1002/1097-0142(19830301)51:5<863::aid-cncr2820510520>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Maruo H., Watahiki Y., Ohsaku M., Kosaka A., Morii I. A case of hepatocellurar carcinoma with metastasis to the gallbladder and the omentum. Gastroenterology Surgery. 1994;17:1379–1383. (in Japanese) [Google Scholar]
- 9.Nishida J., Tanaka M., Suto K., Sasaki Y., Yamagata K., Aizawa T. A case of metastatic gallbladder tumor derived from hepatocellular carcinoma. Journal of Gastroenterology. 1997;94:857–860. (in Japanese) [PubMed] [Google Scholar]
- 10.Lane J.E., Walker A.N. Metastatic hepatocellular carcinoma of the gallbladder. Digestive Surgery. 2002;19:267–268. doi: 10.1159/000064573. [DOI] [PubMed] [Google Scholar]
- 11.Terashima T., Yamashita T., Arai K., Kakinoki K., Kagaya T., Sakai Y. A case metastatic gallbladder tumor of the hepatocellular carcinoma. Kanzo. 2007;48:363–369. (in Japanese) [Google Scholar]
- 12.Ando K., Sakamoto Y. A case of gallbladder metastasis from hepatocellular carcinoma. Japanese Journal of Clinical Oncology. 2009;39:540. doi: 10.1093/jjco/hyp092. [DOI] [PubMed] [Google Scholar]
- 13.Murakami M., Kobayashi S., Marubashi S., Eguchi H., Takeda Y., Tanemura M. Isolated metastasis to the gallbladder from hepatocellular carcinoma. Hepatology Research. 2010;40:793–798. doi: 10.1111/j.1872-034X.2010.00689.x. [DOI] [PubMed] [Google Scholar]
- 14.Monden K., Nakagohri T., Kojima M., Kato Y., Gotohda N., Takahashi S. A case of gallbladder metastasis from hepatocellular carcinoma. The Japanese Journal of Gastroenterological Surgery. 2011;44:259–265. (in Japanese) [Google Scholar]
- 15.Kanzaki R., Yamada T., Gotoh K., Takahashi H., Murata M., Tomita Y. Surgical resection for hepatocellular carcinoma with metastasis to the gallbladder: report of a case. Surgery Today. 2011;41:285–291. doi: 10.1007/s00595-010-4223-2. [DOI] [PubMed] [Google Scholar]
- 16.Sugita M., Ryu M., Satake M., Kinoshita T., Konishi M., Inoue K. Intrahepatic inflow areas of the drainage vein of the gallbladder: analysis by angio-CT. Surgery. 2000;128:417–421. doi: 10.1067/msy.2000.107380. [DOI] [PubMed] [Google Scholar]
- 17.Okusaka T., Okada S., Ishii H., Nose H., Nagahama H., Nakasuka H. Prognosis of hepatocellular carcinoma patients with extrahepatic metastases. Hepato-Gastroenterology. 1997;44:251–257. [PubMed] [Google Scholar]
- 18.Tomimaru Y., Sasaki Y., Yamada T., Eguchi H., Takami K., Ohigashi H. The significance of surgical resection for pulmonary metastasis from hepatocellular carcinoma. American Journal of Surgery. 2006;192:46–51. doi: 10.1016/j.amjsurg.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Lo C.M., Lai E.C., Fan S.T., Choi T.K., Wong J. Resection for extrahepatic recurrence of hepatocellular carcinoma. British Journal of Surgery. 1994;81:1019–1021. doi: 10.1002/bjs.1800810730. [DOI] [PubMed] [Google Scholar]


