Abstract
INTRODUCTION
Primary melanoma of the bile duct is extremely rare with only nine cases of primary melanoma of the bile duct reported in the literature.
PRESENTATION OF CASE
A 55-year-old previously healthy gentleman developed increasing jaundice over several months and subsequently underwent an ERCP with stone extraction. Cytology brushings in an area of a distal stricture in the bile duct were concerning for cholangiocarcinoma. The patient was referred to our institution and underwent a pancreaticoduodenectomy. The surgical specimen showed a single 4.5 cm polypoid lesion located in the bile duct. A diagnosis of melanoma was rendered after immunohistochemical studies on the tumor demonstrated positivity for melanoma markers. Follow-up of the patient with skin, ocular, and lymph node exams showed no evidence of melanoma. A PET scan 4 and 10 months post-surgery failed to reveal either a primary skin lesion or other sites of metastases.
DISCUSSION
The vast majority of melanomas of the bile duct represent metastases from a cutaneous source and tend to present as multiple flat pigmented lesions. Conversely, cases of primary bile duct melanoma are characterized by a distinct gross morphology consisting of a solitary intraluminal polypoid lesion attached by a pedicle with no other identifiable primary lesion. Other supporting criteria include absence of other involved sites and presence of an in situ junctional component.
CONCLUSION
Given the clinical history, gross findings, and lack of a primary cutaneous site or other demonstrable metastases, this patient likely represents the tenth reported case of primary biliary tract melanoma.
Keywords: Melanoma, Bile duct, Gastrointestinal tumors
1. Introduction
The vast majority of melanomas of the bile duct represent metastases from a cutaneous source and tend to present as multiple, flat pigmented lesions.1 Nonetheless, nine cases of primary bile duct melanoma have been reported in the literature (Table 1).2–11 These cases have shared several characteristics including no identifiable primary lesion, a solitary, polypoid lesion on gross examination, and a junctional in situ component. We present the case of a malignant melanoma found in the common bile duct with characteristics suggestive of a primary origin and summarize the previous reported cases of primary biliary tract melanoma in the literature.
Table 1.
Summary of prior reported cases of primary bile duct melanoma.
| Source | Age/sex | Clinical presentation | Tumor description | Therapy | Survival |
|---|---|---|---|---|---|
| Zaide2 | 47/male | Jaundice | Friable, black, polypoid mass | Cholecystectomy and resection of common bile duct | In good health 6 months following surgery |
| Carstens et al.3 and Wright et al.4 | 30/male | Indigestion, abdominal pain, and jaundice | 1.3 cm brown, polypoid mass | Whipple resection | Metastatic disease with death 6 months following onset of symptoms |
| Deugnier et al.5 | 34/female | Abdominal pain, nausea, vomiting, slight jaundice | 4.5 cm intraluminal tumor | Left hepatectomy with partial right hepatic and common bile duct resection | Widespread involvement of lymph nodes with death approximately 1.5 years after presentation |
| Zhang et al.12 | 58/male | Jaundice | Fungating, polypoid, 1.0 cm black mass | Whipple resection | Disease-free 2 years after presentation |
| Washburn et al.6 and Gates et al.7 | 45/male | Month of itching and dark urine | 1.5 cm black mass | Whipple resection | Alive and well 6 years after presentation |
| Washburn et al.6 and Gates et al.7 | 43/male | Lethargy, itching, and dark urine | 2.2 cm mass | Right hepatic lobectomy and cholecystectomy | Alive 11 months after presentation |
| Wagner et al.8 | 48/male | Right upper quadrant pain and jaundice | Fungating, polypoid 4.0 cm mass | Whipple resection | Found to have brain metastases 3 months after presentation; died 9 months after diagnosis |
| Medina et al.9 and Bejarano Gonzalez et al.10 | 47/male | One month of progressive jaundice, dark urine | Obstructive intraluminal mass in bile duct seen on imaging (no surgical specimen) | No surgery performed due to metastatic disease | Died 2 years after presentation |
| Hoshi et al.11 | 55/male | Jaundice | 3 cm black, polypoid mass | Bile duct resection | Died 4 months after presentation |
2. Presentation of case
A 55-year-old previously healthy gentleman developed diffuse pruritis with mild jaundice. He underwent a magnetic resonance cholangiopancreatography (MRCP) procedure that was concerning for a distal common bile duct mass or stone. An endoscopic retrograde cholangiopancreatography (ERCP) revealed a stone in the common bile duct that was successfully extracted. There was also a question of a mass/obstruction and an endo-biliary stent was placed (Fig. 1). Endoscopic cytology brushings in an area of a distal stricture in the bile duct were concerning for cholangiocarcinoma. A repeat CT scan was obtained that showed a persistent mass in the common bile duct. The patient was thus referred to our institution and underwent a pancreaticoduodenectomy with an uneventful post-operative course.
Fig. 1.
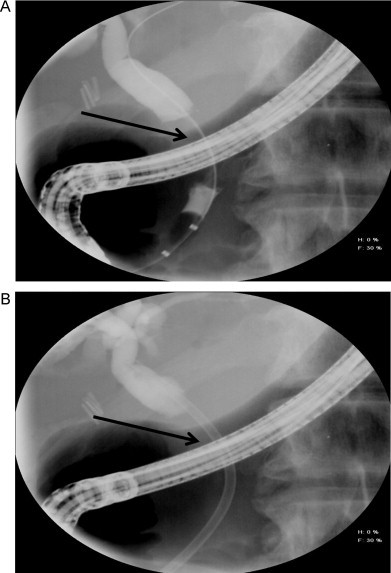
Cholangiogram from the ERCP depicting. (A) The area of obstruction (arrow) and (B) the subsequently placed endo-biliary stent (arrow).
Gross examination of the surgical specimen revealed a solitary 4.5 cm firm, tan-red, pedunculated lesion with surface ulceration and central necrosis arising from the bile duct (Fig. 2). Microscopically, the lesion consisted of cells with moderate amounts of eosinophilic cytoplasm and large, hyperchromatic, pleomorphic nuclei with prominent nucleoli and intranuclear inclusions. Scattered pigmented cells and cellular discohesion were also noted (Figs. 3 and 4). The diagnosis of melanoma was confirmed after immunohistochemical studies demonstrated positivity for S-100, HMB-45, and Melan-A and negativity for AE1/AE3, CK7, CK20, CDX2, and chromogranin. Thorough skin, ocular, and lymph node examination showed no masses or evidence of melanoma. Additionally, a positron emission tomography (PET) scan 4 and 10 months post-resection failed to reveal either a primary skin lesion or other sites of metastases. The patient remains disease-free a year following surgery.
Fig. 2.
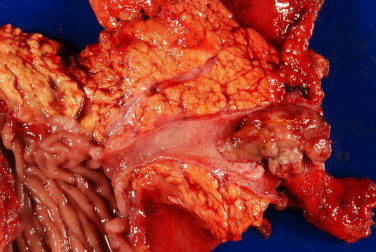
Solitary intraluminal polypoid mass arising from the common bile duct.
Fig. 3.
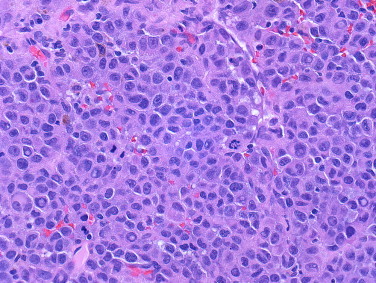
Hematoxylin and eosin staining demonstrated sheets of cells with moderate amounts of eosinophilic cytoplasm and pleomorphic nuclei with nuclear inclusions and scattered mitotic figures (original magnification 160×).
Fig. 4.
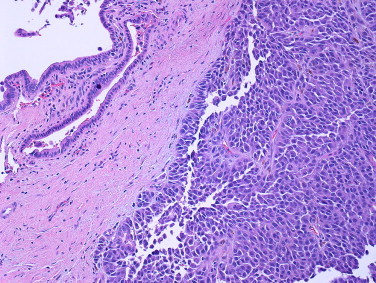
Edge of lesion in relation to overlying bile duct. Multiple areas of the lesion showed cellular discohesion (hematoxylin–eosin, original magnification 64×).
3. Discussion
The overwhelming majority of melanomas arise on cutaneous sites with a minor population arising from other sites such as the uvea and retina. The existence of melanomas arising from endodermal derivatives remains controversial, however. These melanomas are theoretically possible because melanoblasts arise from the neural crest and migrate through various tissues. Primary melanomas can presumably arise in these sites due to the existence of melanocytes in endodermal sites such as the bile duct and gallbladder.12 Ricci and colleagues further confirmed this possibility when they provided ultrastructural evidence of two distinct benign and malignant melanocytic populations in a case of primary melanoma of the gallbladder.13
The first reported case of primary melanoma of the bile duct was in 1963 by Zaide.2 The patient was a 47-year-old man with a long-standing history of jaundice. The tumor consisted of a friable, black, polypoid mass. No other primary cutaneous melanoma was identified and the patient remained in good health 6 months following surgery.
The second reported case was not until 1986 when Carstens et al.3 and subsequently Wright et al.4 described a 30-year-old previously healthy male who initially presented with indigestion, abdominal pain, and jaundice. A Whipple procedure was performed and revealed a 1.3 cm brown polypoid mass located in the common bile duct. Multiple nodules of metastatic melanoma were eventually found in the liver in the following months. The patient eventually succumbed to the disease 6 months after the onset of symptoms.
The third and fourth cases were both reported in 1991. Deugnier et al.5 described a 34-year-old woman with extrahepatic bile duct obstruction by melanoma. Her disease eventually progressed to widespread involvement of lymph nodes with accompanying subcutaneous nodules. She died approximately one and a half years after presentation. Zhang et al.12 reported the case of a 58-year-old man presenting with obstructive jaundice resulting from a mass in the intra-pancreatic portion of the common bile duct. An additional soft, black mass was found in the gallbladder. After resection, no distant metastases were identified and the patient was free of disease after 2 years.
The fifth and sixth cases were both reported by Washburn et al.6 in 1995 and Gates et al. in 1996.7 The first case was a 45-year-old previously healthy male who presented with 1 month of itching and dark urine. A Whipple procedure was performed after a mass was found in the bile duct. After resection, an intraluminal mass was found in the ampulla of Vater. The patient was discharged in good condition 25 days after surgery. The second case reported by the same group was a 43-year-old male who presented with lethargy, itching, and dark urine for 2 months. ERCP showed a 2.2 cm mass just below the bifurcation of the hepatic duct. The patient underwent resection of the extra-hepatic biliary tree.
The seventh case was reported by Wagner et al. in 20008 in a 48-year-old previously healthy male who presented with right upper quadrant pain and jaundice and was found to have a tumor in the common bile duct. The resection specimen showed a fungating, polypoid, 4.0 cm mass located in the mid-portion of the bile duct. The patient died 9 months after diagnosis.
Another case was reported in 2003 by Medina et al.9 and in 2005 by Bejarano Gonzalez et al.10 The patient was a 47-year-old man who presented with progressive jaundice for approximately 1 month. MRCP showed an obstructive tumor in the bile duct and several nodules in the gallbladder wall. The patient died approximately 2 years after presentation. In 2006, Hoshi et al.11 published the most recent case in a 55-year-old gentleman who was found to have a tumor in the middle common bile duct on MRCP. Pathological examination showed a 3 cm black tumor. No primary tumor was identified and FDG-PET showed multiple liver metastases. The patient ultimately succumbed to the disease 4 months after presentation.
Prior authors have suggested several characteristics to differentiate primary visceral from metastatic melanoma. Ricci et al.13 presented the following five criteria based on prior reported cases of primary melanoma of the gallbladder: (1) exclusion of previous melanoma, (2) absence of other involved sites, (3) solitary nature of the lesion, (4) polypoid or papillary shape, and (5) presence of a junctional component. The most important characteristic is the lack of an identifiable primary site. This requires a thorough skin, ophthalmological, gynecological, and anal proctological examination to ensure no current or history of atypical pigmented lesions. Other imaging modalities such as PET and CT are valuable in further ensuring the absence of other lesions.
Primary bile duct melanomas share common gross characteristics. A majority of the lesions have been described as solitary, polypoid, intraluminal masses either with or without pigment. The solitary nature of the lesion supports a primary site as metastatic melanoma is more likely to present as multiple, multifocal, flattened lesions in visceral sites.1 An overwhelming majority of primary melanomas of the gallbladder have also been described as solitary, polypoid lesions.13 Nonetheless, a metastatic melanoma presenting as a solitary lesion has also been reported.14
A definitive junctional component of the melanoma was not identified in this case. However, the significance of a junctional melanocytic component in these cases is a matter of debate. Some authors consider this a prerequisite for the diagnosis of primary biliary tract melanoma.15 Other authors of primary bile duct melanomas did not specifically report or require the presence of an established junctional component. The extensive ulceration or even sampling error in the current case may have prevented the identification of an in situ component. Further, while the identification of an in situ melanoma certainly corroborates a primary origin, this component is not required for the diagnosis of primary melanoma at other sites, and thus we do not consider it essential. For example, cutaneous nodular melanomas may have little or no in situ component16 and primary dermal melanomas containing no in situ component have also been reported.17
The prognosis of primary bile duct melanomas is difficult to ascertain due to the small number of reported cases and lack of long-term follow-up. Given the higher number of case reports, the prognosis of primary gallbladder melanomas is more well-studied and perhaps instructive in predicting the prognosis of primary bile duct melanomas. The median survival in primary and metastatic melanomas of the gallbladder is 20.1 months and 8.4 months,18 respectively. Overall, metastatic melanoma to visceral sites has a dismal prognosis with a 5-year survival of between 5.9 and 18%.19 The extended disease-free survival of many of the primary bile duct melanoma cases also supports the tumor being of primary origin.
4. Conclusion
Continued follow-up of the current patient will be useful in further understanding the true nature of these lesions. A durable disease-free survival would suggest that this case, similar to prior reports, truly represents a primary lesion in contrast to metastases from a regressed, unidentified primary. Additional case reports and longitudinal study will hopefully continue to clarify and contribute to the understanding of this poorly understood entity.
Conflict of interest
None declared.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
Dr. Smith reviewed the literature and wrote the manuscript. Dr. Taube contributed to the microscopic slide review and editing of the manuscript. Ms. Warczynksi, Dr. Collier, and Dr. Pawlik edited and contributed to the manuscript.
References
- 1.Martel J.P., McLean C.A., Rankin R.N. Melanoma of the gallbladder. Radiographics. 2009;29(January–February (1)):291–296. doi: 10.1148/rg.291085021. [DOI] [PubMed] [Google Scholar]
- 2.Zaide E. Malignant melanoma of the choledochus. Arquivos de Oncología. 1963;26:254–255. [PubMed] [Google Scholar]
- 3.Carstens H.B., Ghazi C., Carnighan R.H., Brewer M.S. Primary malignant melanoma of the common bile duct. Human Pathology. 1986;17(December (12)):1282–1285. doi: 10.1016/s0046-8177(86)80573-1. [DOI] [PubMed] [Google Scholar]
- 4.Wright R.A., Brewer M. Primary melanoma of the common bile duct. Southern Medical Journal. 1988;81(March (3)):396–397. doi: 10.1097/00007611-198803000-00026. [DOI] [PubMed] [Google Scholar]
- 5.Deugnier Y., Turlin B., Lehry D., Pennarun J.R., Verger P., Launois B. Malignant melanoma of the hepatic and common bile ducts. A case report and review of the literature. Archives of Pathology and Laboratory Medicine. 1991;115(September (9)):915–917. [PubMed] [Google Scholar]
- 6.Washburn W.K., Noda S., Lewis W.D., Jenkins R.L. Primary malignant melanoma of the biliary tract. Liver Transplantation and Surgery. 1995;1(March (2)):103–106. doi: 10.1002/lt.500010206. [DOI] [PubMed] [Google Scholar]
- 7.Gates J., Kane R.A., Hartnell G.G. Primary biliary tract malignant melanoma. Abdominal Imaging. 1996;21(September–October (5)):453–455. doi: 10.1007/s002619900103. [DOI] [PubMed] [Google Scholar]
- 8.Wagner M.S., Shoup M., Pickleman J., Yong S. Primary malignant melanoma of the common bile duct: a case report and review of the literature. Archives of Pathology and Laboratory Medicine. 2000;124(March (3)):419–422. doi: 10.5858/2000-124-0419-PMMOTC. [DOI] [PubMed] [Google Scholar]
- 9.Medina V., Darnell A., Bejarano N., Falco J., Musulen E., Martin J. Primary biliary tract malignant melanoma: US, CT, and MR findings. Abdominal Imaging. 2003;28(November–December (6)):842–846. doi: 10.1007/s00261-003-0051-9. [DOI] [PubMed] [Google Scholar]
- 10.Bejarano Gonzalez N., Garcia Moforte N., Darnell Martin A., Dinares Fernandez M.C., Laporte Rosello E., Navarro Soto S. Primary malignant melanoma of the common bile duct: a case report and literature review. Gastroenterologia y Hepatologia. 2005;28(August–September (7)):382–384. doi: 10.1157/13077759. [DOI] [PubMed] [Google Scholar]
- 11.Hoshi K., Saitoh Y., Anzai R., Tanno H. A case of primary malignant melanoma of the bile duct. Japanese Journal of Gastroenterological Surgery. 2006;39:317–322. [Google Scholar]
- 12.Zhang Z.D., Myles J., Pai R.P., Howard J.M. Malignant melanoma of the biliary tract: a case report. Surgery. 1991;109(March (3 Pt 1)):323–328. [PubMed] [Google Scholar]
- 13.Ricci R., Maggiano N., Martini M., Mule A.M., Pierconti F., Capelli A. Primary malignant melanoma of the gallbladder in dysplastic naevus syndrome. Virchows Archiv. 2001;438(February (2)):159–165. doi: 10.1007/s004280000336. [DOI] [PubMed] [Google Scholar]
- 14.Higgins C.M., Strutton G.M. Malignant melanoma of the gall bladder—does primary melanoma exist? Pathology. 1995;27(October (4)):312–314. doi: 10.1080/00313029500169203. [DOI] [PubMed] [Google Scholar]
- 15.Allen A. Malignant melanoma: a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953;6(January (1)):1–45. doi: 10.1002/1097-0142(195301)6:1<1::aid-cncr2820060102>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 16.Elder D.E., Elenitsas R., Murphy G.F., Xu X. Benign pigmented lesions and malignant melanoma. In: Elder D.E., Ovid Technologies I., editors. Lever's histopathology of the skin. 10th ed. Lippincott Williams & Wilkins; Philadelphia: 2009. [Google Scholar]
- 17.Swetter S.M., Ecker P.M., Johnson D.L., Harvell J.D. Primary dermal melanoma: a distinct subtype of melanoma. Archives of Dermatology. 2004;140(January (1)):99–103. doi: 10.1001/archderm.140.1.99. [DOI] [PubMed] [Google Scholar]
- 18.Dong X.D., DeMatos P., Prieto V.G., Seigler H.F. Melanoma of the gallbladder: a review of cases seen at Duke University Medical Center. Cancer. 1999;85(January (1)):32–39. [PubMed] [Google Scholar]
- 19.Kamposioras K., Pentheroudakis G., Pectasides D., Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Critical Reviews in Oncology/Hematology. 2011;78(May (2)):112–126. doi: 10.1016/j.critrevonc.2010.04.007. [DOI] [PubMed] [Google Scholar]


