Abstract
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) are rare intra-abdominal tumors arising from mesenchymal stromal cells. EGISTs are mesenchymal tumors that originate outside the GI tract and tend to have similar characteristics to GISTs. To the best of our knowledge, few cases of long standing recurrent EGIST have been reported.
PRESENTATION OF CASE
We present the case of a rare recurrent EGIST in the mesentery of a 39 year old female patient. The tumor was symptomatic at the time of complaint and measured 8.4 cm × 7.7 cm × 7.6 cm. Histological analysis revealed a spindled pattern with fusiform cells arranged in long fascicles and little atypia. Immunochemistry showed positivity for CD117 and was negative for CD34, S-100, Desmin, and MSA. B-catenin was weakly positive. A Ki-67 staining shows approximately 5% positivity revealing a low proliferative rate. The patient was doing well postoperatively and was discharged on 400 mg imanitib regimen.
DISCUSSION
While GISTs are the most common tumors of the GI tract, recurrent EGISTs of the mesentery are extremely rare. Factors that indicate poor prognosis include tumor size greater than 5 cm, mitotic rate greater than 1–5/10 HPF, presence of tumor necrosis or metastasis and most recently the c-kit mutation. Our patient had a very long time between recurrence of disease.
CONCLUSION
The current literature on EGISTs is limited. Our patient presents a very interesting case due to the time elapsed between disease recurrence and lack of metastasis or excessive growth.
Keywords: EGIST, GIST, Recurrent disease, Mesentery, CD117
1. Introduction
Gastrointestinal stromal tumors (GISTs) are rare intra-abdominal tumors arising from mesenchymal stromal cells and account for approximately 5% of GI malignancies.1 There are approximately 3300–4350 GISTs diagnosed a year and 150 of those are classified as EGISTs (extra-gastrointestinal stromal tumors).1, 2 EGISTs are mesenchymal tumors that originate outside the GI tract and tend to have similar characteristics to GISTs. EGISTs account for less than 1% of all GI malignancies.1 Here we present a case of recurrent multiple EGIST of the mesentery.
2. Presentation of case
We report the case of a 39 year old African American female presented to the emergency room with the chief complaint of low back pain superior to the rectum that radiated down the leg accompanied by nausea. A computer tomography (CT) scan was performed and revealed a cystic mass adjacent to the mid transverse colon, but not incorporated in it, measuring 8.4 cm × 7.7 cm × 7.6 cm and an adjacent solid nodule enhancing the soft tissue measuring 2.9 cm × 1.2 cm (see Fig. 1).
Fig. 1.
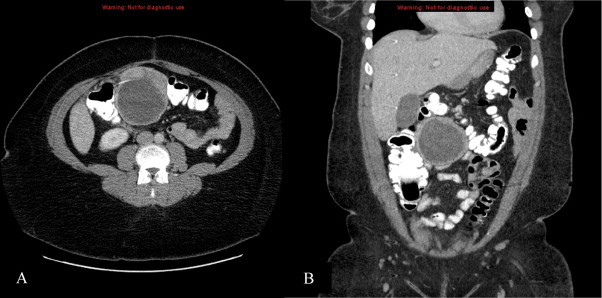
CT demonstrates cystic mass measuring 8.4 cm × 7.7 cm × 7.6 cm and an adjacent solid nodule enhancing the soft tissue measuring 2.9 cm × 1.2 cm.
The patient's past medical and surgical history were pertinent for an appendectomy, three cesarean sections and a hysterectomy. The patient also reported having undergone prior “intestinal” surgery for a similar complaint 17 years prior but denied receiving any adjuvant therapy. There was no family history of cancer. Of note, she underwent a colonoscopy in July 2011 for these complaints but her workup did not reveal a malignancy. Pertinent physical findings were a large mobile right upper quadrant mass with potential involvement of the transverse colon. The findings were highly suggestive of a malignant lesion.
On August 25th, 2011 the patient underwent surgery for an “en bloc” resection including an open extended right hemicolectomy, resection of the two mesenteric masses and creation of anileo-colic anastomosis. There was no evidence of peritoneal metastasis or ascites and the liver appeared normal. The first mass was approximately 10 cm in size and the second mass approximately 6 cm deeper with no other adenopathy or masses identified. The tumor appeared to be densely adherent to the wall of the transverse colon and to the hepatic flexure. The tumor could not be separated from the colon wall. Both masses were resected while maintaining perfusion of the gut. Evidence of a prior small bowel resection was also found confirming the patient's past surgical history.
The patient had no post-operative complications and was discharged on postoperative day five. The patient returned with a small inferior wound infection, which resolved with local care and was successfully treated with oral mefronidazole for a Clostridium difficile colitis. She is currently receiving adjuvant therapy with daily 400 mg imanitib mesylate which has been recommended for three years.
Post-operative gross examination is seen in Fig. 2. Microscopic tissue examination of the tumor revealed multiple nodules with the largest measuring 8.5 cm × 8 cm and 5 additional smaller tumors of extra gastrointestinal (soft tissue) stromal tumor (EGIST). Based on cytology, the large variable cellular tumor had a spindled pattern with fusiform cells arranged in long fascicles and little atypia. The mitotic count is 2–3 MF/50 HPF. While the tumor demonstrates areas of infarction, there is no evidence of tumor necrosis. The segment of the small bowel and colon are uninvolved by the tumor and showed various serosal adhesions. Thirty-eight reactive lymph nodes were resected but all were negative for cancer. Immunohistochemical study revealed positive staining for CD117 but negative for CD34, S-100, Desmin, and MSA. B-catenin was weakly positive. A Ki-67 staining shows approximately 5% positively revealing a low proliferative rate. Fig. 3 demonstrates positive staining for CD117 and B-catenin, a low proliferative rate for Ki-67 staining and a H&E staining of the tumor cells. Fig. 4 shows the microscopic image of EGIST grossly abutting colonic wall but demonstrating there is no involvement of the colonic wall.
Fig. 2.
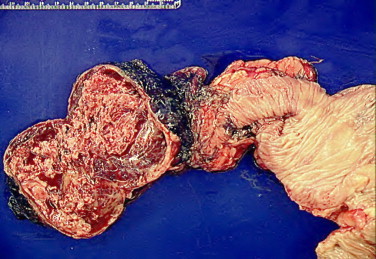
EGIST abutting colonic wall showing no involvement of the colonic wall.
Fig. 3.
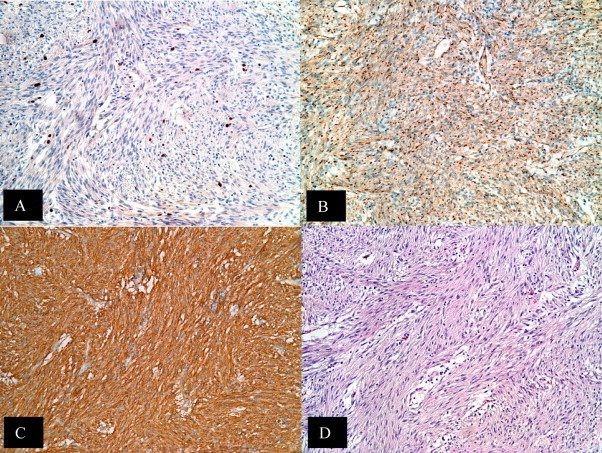
Immunohistochemical features of EGIST. Tumor cells characteristically express CD117 (A) and B-catenin (B) but Ki-67 stain shows low proliferative rate (C). A standard H&E stain of tumor cells is seen in (D).
Fig. 4.
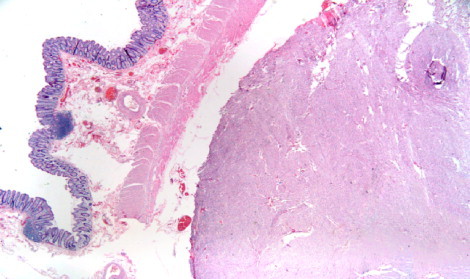
EGIST abutting colonic wall.
3. Discussion
The most common sites for GISTs are the stomach and the small intestine. The more distally a GIST is found in the GI tract, the worse the prognosis tends to be.3 EGISTs are most commonly found in the omentum, mesentery and retroperitoneum but have also been reported in the pancreas, gallbladder, retro-vaginal septum, liver, abdominal wall, periversical tissue, pharynx and posterior mediastinum.1, 2, 3, 4, 5, 6, 7 Based on reports, EGISTs tend to be much larger than GISTs and vary from 2.1 to 33 cm in diameter.3, 7, 8
3.1. Origin
EGIST is thought to arise from a common precursor of the interstitial cells of Cajal (ICC) because they both express CD117 (cluster of differentiation 117, a protein transcribed from the c-kit gene). CD117 is a transmembrane receptor protein found in hematopoietic stem cells, mast cells, germ cells and melanocytes.7, 8 ICCs are physiologically found in the myoenteric plexus of the muscularis propia of the smooth muscle in the tubular GI tract and are thought of as the pacemaker cells of the intestinal smooth muscle due to their association with peristalsis.4 The literature presents some controversy on the origin of EGISTs. One hypothesis supports the idea that GISTs and EGISTs both arise from a common precursor of the ICCs and smooth muscle cells due to the expression of CD117 while another hypothesis asserts that EGISTs are simply mural GISTs with extensive extramural growth that eventually lost connection with the gut wall.8 The second hypothesis was suggested due to the existence of primary EGISTs because ICC cells are not found outside the GI tract and thus it would be impossible for them to give rise to these tumors unless the tumors were originally in contact with the gut wall.
3.2. Clinical presentation
Most patients diagnosed with EGISTs present with symptoms of abdominal pain, an abdominal mass or GI bleeding. Less common symptoms include anorexia, dysphagia, obstruction, perforation and fever.1 There is no predilection based on gender but there is for age. Most patients are diagnosed beyond middle age but EGISTs have been reported in individuals in their twenties.2 EGISTs are typically visualized by CT or MRI.
3.3. Treatment
The current and widely accepted treatment for both GISTs and EGISTs is complete resection.1, 3, 7 Survival is correlated with the completeness of the resection and fortunately since stromal tumors do not usually invade adjacent tissue, wide margins are not necessary. “En bloc” resection is recommended if contiguous organs are involved. The most important thing to avoid intra-operatively is tumor rupture as it increases the chances of peritoneal recurrence.1 Recurrent disease is common thus adjuvant therapy is recommended as well as strict follow up. Currently, imatinib and sunitinib, both selective tyrosine kinase inhibitors, are being used for those with metastatic or recurrent disease.1, 5, 6 Other drugs such as adriamycin, doxorubicin, dacrbzine and mitoxcintrone have been used but there is little published literature assessing the level of effectiveness.1
3.4. Pathology (prognosis factors)
Approximately two-thirds of EGISTs turn out to be malignant, thus it is important to identify a list of prognostic factors for the disease despite its rarity. Based on cytology, EGISTs are classified into three cell types; spindle cell, epithelioid/round cell type or mixed. Epithelioid cell type EGISTs tend to comprise most the KIT negative EGISTs but neither cell type is predictive of malignancy (citation). There is no accepted cancer staging system but the following factors have shown to be most useful in indicating poor prognosis: size (>5 cm), mitotic rate (<1–5/10 HPF), presence of tumor necrosis or metastasis and most recently the c-kit mutation (usually in exon 11).1, 9 Recent gene profiling has revealed other useful proteins that could aid in diagnosis of EGISTs. The c-kit gene has long been used to as a useful test for identifying GISTs. However, the increasing numbers of KIT negative EGISTs inspired the search for other identifying molecular markers common to EGISTs. Platelet derived growth factor receptor alpha (PDGRF-α) has been identified in EGISTs that are resistant to imanitib.10 In addition, PKC θ, an isotype of PKC (protein kinase C), has been identified as a new marker for GISTs and is found in both KIT positive and KIT negative tumors. Lastly, DOG1, a membrane protein associated with the calcium dependent chloride channel is upregulated in 90% of GISTs and antibodies have already been developed against it.6
4. Conclusion
Despite complete resection, 50% of patients have recurrent disease within 2 years.11 We believe that the cyst removed from our patient was recurrence of the disease she had 17 years prior. This may be considered a long time for disease recurrence as well as classified as minimally aggressive in growth rate. As recommended by Winer and Raut, our patient has started a 400 mg daily treatment of imatinib mesylate, which is currently the adjuvant therapy of choice for those with recurrent GISTs disease.
Conflict of interest statement
None.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors’ contributions
Jennifer E. Rosen participated in the care of the patient and in revising the manuscript. Ilona D. Goukassian performed the literature review, drafted and revised the manuscript. Steven R. Kussman provided radiology images. Yanelba Toribio provided microscopic and gross pathology images. All authors read and approved the final manuscript.
Contributor Information
Ilona D. Goukassian, Email: Ilona.Goukassian@bmc.org.
S.R. Kussman, Email: steven.kussman@bmc.org.
Y. Toribio, Email: morena@bu.edu.
Jennifer E. Rosen, Email: Jennifer.Rosen@bmc.org.
References
- 1.Pidhorecky I., Cheney R.T., Kraybill W.G., Gibbs J.F. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Annals of Surgical Oncology. 2000;7(October (9)):705–712. doi: 10.1007/s10434-000-0705-6. [DOI] [PubMed] [Google Scholar]
- 2.Castillo-Sang M., Mancho S., Tsang A.W., Gociman B., Almaroof B., Ahmed M.Y. A malignant omental extra-gastrointestinal stromal tumor on a young man: a case report and review of the literature. World Journal of Surgical Oncology. 2008;6:50. doi: 10.1186/1477-7819-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reith J.D., Goldblum J.R., Lyles R.H., Weiss S.W. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Modern Pathology. 2000;13(May (5)):577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- 4.Agaimy A., Wuensch P.H. Gastrointestinal stromal tumours in patients with other-type cancer: a mere coincidence or an etiological association? A study of 97 GIST cases. Zeitschrift fur Gastroenterologie. 2005;43(September (9)):1025–1030. doi: 10.1055/s-2005-858378. [DOI] [PubMed] [Google Scholar]
- 5.Barros A., Linhares E., Valadao M., Goncalves R., Vilhena B., Gil C. Extragastrointestinal stromal tumors (EGIST): a series of case reports. Hepato-Gastroenterology. 2011;58(May–June (107–108)):865–868. [PubMed] [Google Scholar]
- 6.Yamamoto H., Kojima A., Nagata S., Tomita Y., Takahashi S., Oda Y. KIT-negative gastrointestinal stromal tumor of the abdominal soft tissue: a clinicopathologic and genetic study of 10 cases. American Journal of Surgical Pathology. 2011;35(September (9)):1287–1295. doi: 10.1097/PAS.0b013e3182206f15. [DOI] [PubMed] [Google Scholar]
- 7.Miettinen M., Monihan J.M., Sarlomo-Rikala M., Kovatich A.J., Carr N.J., Emory T.S. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. American Journal of Surgical Pathology. 1999;23(September (9)):1109–1118. doi: 10.1097/00000478-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Goh B.K., Chow P.K., Kesavan S.M., Yap W.M., Chung Y.F., Wong W.K. A single-institution experience with eight CD117-positive primary extragastrointestinal stromal tumors: critical appraisal and a comparison with their gastrointestinal counterparts. Journal of Gastrointestinal Surgery. 2009;13(June (6)):1094–1098. doi: 10.1007/s11605-009-0828-4. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa M., Akasaka Y., Kanai T., Takabayashi T., Miyazawa N. Clinicopathological and immunohistochemical features of extragastrointestinal stromal tumors: report of two cases. Surgery Today. 2005;35(4):336–340. doi: 10.1007/s00595-004-2932-0. [DOI] [PubMed] [Google Scholar]
- 10.Terada T. Primary extragastrointestinal stromal tumor of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Medical Oncology. 2009;26(2):233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 11.Winer J.H., Raut C.P. Management of recurrent gastrointestinal stromal tumors. Journal of Surgical Oncology. 2011;104(December (8)):915–920. doi: 10.1002/jso.21890. [DOI] [PubMed] [Google Scholar]


