All cells are divided into distinct subcellular compartments, each with its own defining set of proteins. A fundamental problem in molecular cell biology is to understand how proteins that are synthesized on ribosomes in the cytoplasm reach their proper intracellular address. This process, usually called protein sorting or protein trafficking, is understood to involve information encoded in the protein sequence itself as well as the cellular machinery that decodes this information and delivers the protein to its correct location. Many of the sorting steps in a eukaryotic cell take place along the secretory pathway. At each step in the secretory pathway, carrier vesicles bud from one compartment and then fuse with the membrane of the next compartment allowing the transfer of membranes and proteins between organelles. To sort proteins, transport vesicles must be able to select the appropriate contents, accepting cargo proteins that should advance to the next compartment while excluding compartmental resident proteins that should remain in place. The companion article for this commentary concerns the function of the p24 family of vesicle proteins, which are thought to play an important part in cargo selection (1).
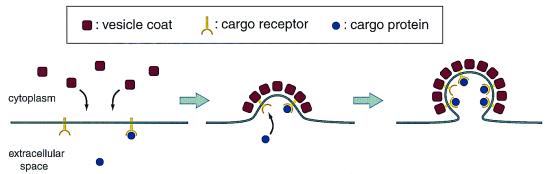
Our current understanding of how vesicles select specific cargo molecules can be traced back to studies of how extracellular proteins are transported into the cell interior by endocytosis (2). A schematic diagram of the molecular associations that drive endocytic vesicle assembly and cargo loading into these vesicles is shown in Fig. 1. The individual steps (reviewed in ref. 3) are as follows: clathrin and adaptor protein complexes form the coats of endocytic vesicles, and as these coat proteins polymerize onto the plasma membrane, the resulting deformation of the membrane into a nascent vesicle produces a structure known as a coated pit. Because membrane receptor proteins have short cytoplasmic sequences with affinity for coat proteins, the polymerized coat acts as affinity matrix to cluster receptor molecules into the coated pits. The extracellular domains of receptors, such as the low density lipoprotein receptor, in their turn bind to their corresponding ligands to collect them into the forming vesicle. Further polymerization of the coat sets the curvature of the membrane into a spherical shell, and finally, with the aid of accessory proteins such as dynamin, a completed vesicle is pinched off from the membrane. This process of coat assembly coupled to the selection of cargo by membrane receptors seems to be quite general, because cargo receptors mediate protein sorting in the trans-Golgi (4, 5) and retrieval of mistargeted proteins from the Golgi to the endoplasmic reticulum (ER; ref. 6). For ER to Golgi transport, the first vesicle trafficking step in the secretory pathway, cargo receptors have not been identified; however, some type of cargo selection mechanism is expected, because cargo is concentrated into ER-derived vesicles (7, 8), whereas organelle resident proteins are excluded (9).
Figure 1.
Molecular interactions that allow a vesicle to capture cargo proteins selectively. Vesicle coat protein complexes assemble onto the membrane to form a coated pit. Affinity of the cytosolic portion of a cargo receptor for the inside of the vesicle coat causes receptor clustering within the coated pit. The ligand binding domains of the receptors capture cargo proteins. Finally, coat polymerization drives completion of the vesicle whose lumen is enriched in cargo protein.
The p24 proteins are a conserved family of small integral membrane proteins found in eukaryotes from yeast to mammals. These proteins were first identified as abundant constituents of the COPI and COPII vesicles that operate in the early secretory pathway (10–12). (COPI vesicles carry proteins between the cisternae of the Golgi complex and from the Golgi to the ER, whereas COPII vesicles carry proteins from the ER to the Golgi.) Because of their abundance, their conservation through evolution, and the fact that they shuttle between the ER and Golgi compartments in transport vesicles, the p24 proteins are thought to be fundamental constituents of vesicles, perhaps acting as cargo receptors. One approach to define the physiological function of the p24 proteins has been to mutate the corresponding genes and then to look for associated defects in vesicular trafficking. Such genetic tests have been applied to the yeast Saccharomyces cerevisiae, but they have not yielded simple answers. Yeast strains carrying mutations in p24 genes grow normally, and overt defects in either COPI or COPII vesicle functions are not seen (11, 13). A confounding problem in the interpretation of these experiments is the redundancy of p24 genes. The S. cerevisiae genome carries eight p24 homologs, and if these proteins can substitute even partly for one another, one cannot be sure of the consequences of elimination of the p24 function until all eight homologs have been knocked out. This octuple mutant has now been constructed and has no detectable defect in the rate of ER to Golgi transport (as measured by the kinetics of carboxypeptidase Y export from the ER) or in the rate of transport from the Golgi back to the ER (as measured by the ability of a reporter protein bearing a KKXX retrieval sequence to be recycled; ref. 1). These results show that the p24 proteins are not essential in yeast for the function of either COPI or COPII vesicles.
Although they do not seem to cause a pronounced defect in vesicle trafficking, p24 gene mutations in yeast do cause subtle alterations in the secretory pathway, which may provide important clues as to their function. The EMP24 gene was the first of the yeast p24 proteins to be identified. Morphological characterization of a deletion of EMP24 revealed an approximately 2-fold reduction in the production of small vesicles, although the identity of the affected vesicle class as either COPI or COPII could not be established (10). Parallel experiments examining the kinetics of protein trafficking in the early secretory pathway revealed that deletion of either EMP24 or ERV25 (a second p24 homolog) has no observable effect on carboxypeptidase Y export and slows export from the ER of invertase and proteins linked to glycosylphosphatidylinositol (GPI) anchors (11, 13). This difference in the rates of export for different protein cargo molecules suggests that EMP24 and ERV25 might encode cargo receptors for invertase and GPI-linked proteins. However, none of the attempts to crosslink p24 proteins to cargo molecules has succeeded yet, and it may be that the connection between p24 function and cargo loading is indirect. For example, GPI anchor attachment is known to precede export from the ER, and a defect in GPI anchor synthesis or attachment in p24 mutants could be the root cause of the transport delay of GPI-linked proteins. A quite different phenotype of EMP24 and ERV25 mutants is that they secrete high levels of the ER resident proteins BiP and PDI (14). ER retention is a two-stage process, involving slow initial exit from the ER followed by signal-mediated retrieval of any ER resident protein that has escaped the ER (15). The p24 proteins seem to act at the initial stage of ER export, because an ERV25 mutation can increase BiP secretion greatly, even when the retrieval mechanism has been inactivated entirely by mutation (14). The octuple p24 mutant had defects of the same magnitude as single mutants of EMP24 or ERV25 as determined in tests for either increased secretion of BiP or a delay in secretion of invertase and the GPI-linked Gas1p (1). Taken together, the different effects of p24 protein indicate that these proteins are required to maintain the fidelity of COPII vesicle traffic—in the absence of p24 proteins, some cargo proteins are transported more slowly, whereas ER residents are more readily admitted into vesicles.
A role for p24 proteins in the fidelity of ER sorting is also indicated by genetic experiments in Caenorhabditis elegans. A genetic screen for suppressors of mutations in the gene for LIN-12, a member of the notch family of receptors involved in cell differentiation, yielded mutations in sel-9, which encodes a p24 protein (16). As it happens, the lin-12 allele used for the suppression screen lies in the luminal domain of the LIN-12 protein and causes intracellular retention, presumably because of ER quality-control processes. The sel-9 mutation apparently defeats this retention process and allows efficient transport of the defective LIN-12 protein to the cell surface. This behavior of a p24 mutant in C. elegans finds close parallels with failure of p24 mutants in yeast to retain unprocessed invertase and the ER residents BiP and PDI. A somewhat different result was obtained when the consequence of a p24 gene mutation was examined in the mouse; deletion of one of the mammalian p24 genes causes death at an early embryonic stage (17). This result does not necessarily imply that p24 proteins have a more important cellular function in mammals than they do in C. elegans or in yeast. Deletion of a p24 gene could disturb intracellular trafficking in a way that could mislocalize a crucial membrane protein with lethal consequences in a mammalian embryo, whereas a similar disturbance in sorting might have relatively little effect on C. elegans development or on a growing yeast cell.
What then do the phenotypes of p24 mutations tell us about how these proteins might act in vesicle transport? The challenge is to explain the following: p24 proteins are abundant constituents of the vesicle membrane, and their cytosolic tails interact with and powerfully nucleate assembly of both COPI and COPII vesicle coats (18, 19). Nonetheless, in the absence of p24 proteins, vesicle budding still occurs efficiently, but both ER residents and misfolded proteins seem to be more readily admitted into ER-derived vesicles. At present, the elements of the classical vesicle budding mechanism shown in Fig. 1 cannot explain the loss of sorting fidelity caused by p24 mutants. To account for the properties of p24 mutations, it is therefore necessary to hypothesize more complicated mechanisms. I will outline below three models for vesicle budding that incorporate a role for p24 proteins in cargo selection.
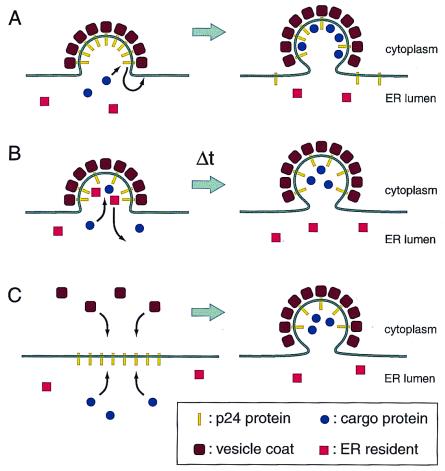
One model is that p24 proteins may sterically exclude proteins that do not belong in the vesicle (Fig. 2A). The p24 proteins are probably densely incorporated into vesicles because of the affinity of their cytosolic tails for vesicle coat proteins (19). Springer et al. (1) suggested that once incorporated into vesicles, the p24 proteins could then act as place holders to exclude proteins from the vesicle interior. The idea is that entry of a cargo protein into the vesicle would require concomitant displacement of a p24 protein. Only cargo proteins or cargo receptor proteins with a high affinity for the coat would be capable of displacing a p24 protein, whereas ER resident proteins with relatively low affinity for the vesicle interior would not be capable of displacement and would remain outside the vesicle.
Figure 2.
Three speculative mechanisms for how p24 proteins might increase the fidelity of sorting by ER vesicles. (A) Cargo exclusion. As abundant constituents of the vesicle buds that interact with coat complexes, the p24 proteins may exclude proteins such as ER residents. Only cargo proteins that have high-affinity binding sites the interior surface of the vesicle would be able to displace a p24 protein and to occupy a site within the vesicle. (B) Time delay. Vesicle coat assembly may be intrinsically faster than sorting processes. The presence of p24 proteins in the membrane may force a delay in the time of budding, allowing sorting to reach completion. (C) Membrane segregation. p24 proteins may self-associate to create a specialized domain of the membrane. This domain would be expected to be a preferred site for coat assembly and may also serve as an attachment site for sorting factors in the lumen.
Another possibility is that the p24 proteins may increase the fidelity of cargo loading by temporal control of the budding process (Fig. 2B). One version of such a timing model assumes that vesicle coat polymerization can occur so rapidly that cargo proteins and ER resident proteins will not have sufficient time to segregate on the basis of their different affinities for the vesicle interior. The p24 proteins may slow the completion of the vesicle bud, in effect holding the nascent vesicle in a coated-pit conformation, giving cargo proteins time to find their binding sites within the vesicle, while ER resident proteins will have time to diffuse away. According to this model, the p24 proteins fundamentally act as negative regulators of vesicle budding. This role is consistent with genetic data showing that deletion of p24 protein genes can suppress mutations in the genes for COPII coat subunits (14).
Finally, biochemical data show that, in both yeast and mammalian cells, multiple p24 proteins associate with one another within the membrane (20, 21); in the mammalian Golgi, p24 proteins can be found in complexes of sufficient size to contain more than 50 copies of p24 proteins (22). It is possible that these large membrane rafts of p24 proteins define subdomains of the ER and Golgi membranes that are particularly active for both vesicle formation and cargo-protein sorting (Fig. 2C). On the cytoplasmic face of the membrane, the aggregated tails of p24 proteins may serve as nucleation sites for coat protein polymerization. On the luminal side of the membrane, the p24 proteins might in some way define a subcompartment that would contain cargo proteins and exclude resident proteins. This view of p24 function finds support from the recent observation that p24 proteins contribute to the formation of vesiculotubular clusters (also called the ER Golgi intermediate compartment), a subcompartment of the ER responsible for much of the vesicular sorting between the ER and Golgi in mammalian cells (23).
To discriminate among these possible models, it will be necessary to follow cargo loading into vesicles with a spatial and temporal definition that exceeds the capabilities of current methods. However, in vitro dissection of the vesicle-budding process into increasingly refined steps and the advances in our ability to image vesicular transport of cargo in live cells give hope that some of these more subtle aspects of vesicle function may be accessible in the near future.
Footnotes
See companion article on page 4034.
References
- 1.Springer S, Chen E, Duden R, Marzioch M, Rowley A, Hamamoto S, Merchant S, Schekman R. Proc Natl Acad Sci USA. 2000;97:4034–4039. doi: 10.1073/pnas.070044097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein J L, Anderson R G, Brown M S. Nature (London) 1979;279:679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- 3.Schmid S. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 4.Kornfeld S. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 5.Marcusson E G, Horazdovsky B F, Cereghino J L, Gharakhanian E, Emr S D. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 6.Lewis M J, Pelham H R. Cell. 1992;68:353–364. doi: 10.1016/0092-8674(92)90476-s. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno M, Singer S J. Proc Natl Acad Sci USA. 1993;90:5732–5736. doi: 10.1073/pnas.90.12.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch W E, McCaffery J M, Plutner H, Farquhar M G. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 9.Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach M F, Ravazzola M, Amherdt M, Schekman R. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 10.Stamnes M A, Craighead M W, Hoe M H, Lampen N, Geromanos S, Tempst P, Rothman J E. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schimmoller F, Singer-Kruger B, Schroder S, Kruger U, Barlowe C, Riezman H. EMBO J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspeich F, Fiedler K, Helms J B, Wieland F T. J Cell Biol. 1996;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belden W J, Barlowe C. J Biol Chem. 1996;271:26939–26946. doi: 10.1074/jbc.271.43.26939. [DOI] [PubMed] [Google Scholar]
- 14.Elrod-Erickson M J, Kaiser C A. Mol Biol Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenza J C, Hardwick K G, Dean N, Pelham H R. Cell. 1990;61:1349–1357. doi: 10.1016/0092-8674(90)90698-e. [DOI] [PubMed] [Google Scholar]
- 16.Wen C, Greenwald I. J Cell Biol. 1999;145:1165–1175. doi: 10.1083/jcb.145.6.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denzel A, Otto F, Girod A, Pepperkok R, Watson R, Rosewell I, Bergeron J J, Solari R C, Owen M J. Curr Biol. 2000;10:55–58. doi: 10.1016/s0960-9822(99)00266-3. [DOI] [PubMed] [Google Scholar]
- 18.Kuehn M J, Herrmann J M, Schekman R. Nature (London) 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- 19.Bremser M, Nickel W, Schweikert M, Ravazzola M, Amherdt M, Hughes C A, Sollner T H, Rothman J E, Wieland F T. Cell. 1999;96:495–506. doi: 10.1016/s0092-8674(00)80654-6. [DOI] [PubMed] [Google Scholar]
- 20.Marzioch M, Henthorn D C, Herrmann J M, Wilson R, Thomas D Y, Bergeron J J, Solari R C, Rowley A. Mol Biol Cell. 1999;10:1923–1938. doi: 10.1091/mbc.10.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullekrug J, Suganuma T, Tang B L, Hong W, Storrie B, Nilsson T. Mol Biol Cell. 1999;10:1939–1955. doi: 10.1091/mbc.10.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominguez M, Dejgaard K, Fullekrug J, Dahan S, Fazel A, Paccaud J P, Thomas D Y, Bergeron J J, Nilsson T. J Cell Biol. 1998;140:751–765. doi: 10.1083/jcb.140.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavoie C, Paiement J, Dominguez M, Roy L, Dahan S, Gushue J N, Bergeron J J. J Cell Biol. 1999;146:285–299. doi: 10.1083/jcb.146.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]