Abstract
INTRODUCTION
Several conventional techniques have been developed in order to control surgical bleeding. Their greatest disadvantage, though, is their inability to control bleeding in areas where access is very difficult. In such cases the application of topical hemostatic agents may prove particularly useful.
PRESENTATION OF CASE
We describe the case of an 82-year old patient with life-threatening post-ERCP bleeding which was intraoperatively controlled with infusion of a topical gelatin matrix-thrombin hemostatic agent into the distal portion of the common bile duct.
DISCUSSION
Most iatrogenic cases of post-ERCP bleeding occur at the site of sphincterotomy at the level of the ampulla of Vater and may be relatively easily controlled by repeat endoscopy and local hemostatic measures. More rarely, however, significant and difficult to control bleeding may occur within the lower portion of the common bile duct (CBD) where routine hemostatic techniques may prove unsuccessful. Under these circumstances, we successfully employed a novel hemostatic technique using a gelatin matrix-thrombin agent in a patient with life-threatening bleeding after ERCP.
CONCLUSION
This novel technique might prove particularly useful for bleeding control in surgically challenging anatomical areas such as the lower portion of the CBD.
Keywords: Post-ERCP bleeding, Common bile duct, Gelatin matrix-thrombin, Topical hemostatic agent
1. Introduction
Several conventional techniques have been developed in order to control surgical bleeding including mechanical means (manual compression, ligation, use of tourniquet) or sealing of bleeding vessels by thermal methods (laser or electrocauterization). All these techniques may prolong operative time or produce necrotic areas with increased likelihood of infection and impaired healing.1,2 Their greatest disadvantage though is their inability to control bleeding in areas where access by the surgeon is very difficult and/or might endanger vital structures. In such cases the application of topical hemostatic agents may prove particularly useful. Several types of hemostatic agents are currently available exerting their hemostatic effect in a variety of ways. Some of them promote primary hemostasis whereas others stimulate fibrin formation or implement a biocompatible matrix in order to achieve their effect.3
Endoscopic retrograde cholangiopancreatography (ERCP) is a technically complex endoscopic procedure that may, under certain circumstances, bear significant morbidity and occasional mortality. In three recent large prospective multicenter studies the rate of severe or fatal complications ranged between 2.1 and 4%.4–6 Severe post-ERCP hemorrhage is a rare fatal complication with a reported prevalence of about 0.2%.7,8 Surgical control of this complication may prove particularly difficult due to problematic access of the bleeding area. In this article we report the case of an 82-year old woman with a life threatening post-ERCP bleeding which was controlled intraoperatively with the application of a topical hemostatic agent in the distal portion of the common bile duct (CBD).
2. Presentation of case
An 82-year old woman was admitted to our hospital due to severe acute cholecystitis, which was clinically suspected and sonographically confirmed. Initial treatment included a NPO regimen, correction of fluid and electrolytes and intravenous antibiotic therapy (tazobactam/piperacillin and metronidazole). Past medical and surgical history were remarkable of pharmacologically controlled arterial hypertension and an open partial gastrectomy due to bleeding gastric ulcer, respectively. On day 3 after admission a laborious open cholecystectomy was performed. Intraoperative findings were necrotic gangrenous cholecystitis and secondary rupture of the gallbladder with subhepatic abscess formation. On the third postoperative day the patient had ongoing bile leak at the subhepatic drain (500 mL/day), thus an ERCP was conducted. At ERCP, bile leak was recognized through the stump of the cystic duct; a temporary plastic stent was placed in the CBD and adjunctive sphincterotomy was performed.
Twelve hours post-ERCP the patient developed severe upper gastrointestinal bleeding associated with blood loss per rectum and hemodynamic instability. After initial resuscitation we performed a new ERCP in order to define the site of bleeding and attempt endoscopic control. The site of sphincterotomy was identified as the source of active arterial bleeding, however the endoscopist's attempts for local control were unsuccessful. Subsequently an urgent selective arteriography was performed in the operating room, where the source of bleeding was shown to be a branch of the gastroduodenal artery (GDA), which was embolized with coil material. The patient suffered continuous bleeding even after the embolization of the main trunk of GDA and, due to progressive hemodynamic instability, an urgent surgical exploration was considered an imperative option.
An attempt to ligate the GDA was not made at surgical exploration for two reasons. First, dissection of the arterial structures just above the duodenum would be very dangerous and could lead to inadvertent damage to the main hepatic arterial trunks and/or the supraduodenal part of the CBD. The timing of emergency operation, i.e. 6 days from initial presentation, and the extent of local inflammation due to previous gallbladder rupture were unfavorable factors for a safe dissection and ligation of the GDA. Second, at arteriography, selective coil embolization of a branch and the main trunk of GD artery, although technically successful, was unable to control bleeding. Thus, we performed a longitudinal duodenotomy which provided adequate exposure of the ampulla of Vater and the sphincter of Oddi (Fig. 1a). Arterial bleeding at the site of sphincterotomy was initially encountered after removal of fresh, non-adherent blood clots from the duodenal lumen. Flexible choledochoscopy also confirmed active intraluminal bleeding at the distal part of the CBD (Fig. 1b). We attempted bleeding control with conventional means by both suturing the site of sphincterotomy and exerting internal pressure on the bleeding vessels of the distal CBD with balloon tipped catheters. Nevertheless, bleeding was not controlled by these conventional means, so we decided to innovate. We infused a topical hemostatic agent consisting of a bovine-derived gelatin matrix and a human-derived thrombin component (FloSeal Hemostatic Matrix, Baxter International Inc.) into the distal portion of the CBD through the sphincter of Oddi. In order to keep the lumen open, we cannulated the CBD with a short piece of a Nelaton catheter (0.3 Fr) (Fig. 1c and d). This technique achieved complete hemostasis three minutes later. Then we removed the short piece of Nelaton catheter very carefully and we performed closure of the duodenotomy according to the Heineke–Mikulicz technique. Postoperatively, the patient remained stable at the ICU with no signs of gastrointestinal bleeding for the next twenty days, although she finally passed away due to pulmonary complications. During the postoperative period, we noticed only a mild increase in bilirubin (up to 3.8 mg/dL), alkaline phosphatase (up to 280 IU/L) and alanine aminotransferase (up to 110 IU/L) levels, which returned to normal within four days. This was attributed to a temporary obstruction of the CBD by both severe local inflammation and the topical hemostatic agent.
Fig. 1.
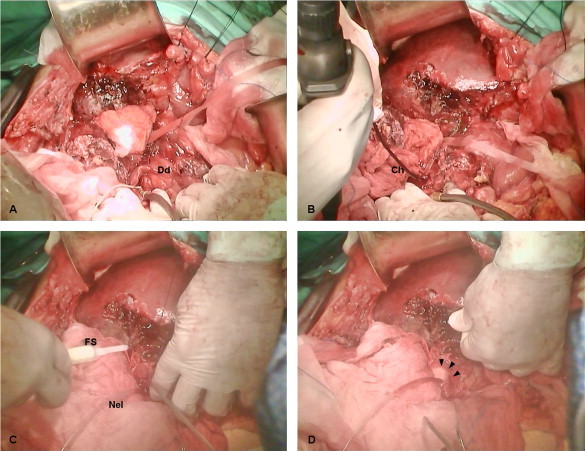
(a–d) Operative steps of post-ERCP bleeding control in our patient. (a) A longitudinal incision was made at the second part of the duodenum (Dd) providing excellent exposure of the ampulla of Vater (cannulated), (b) intraoperative inspection of the CBD with a choledochoscope (Ch); active bleeding through the cannulation site is visible, (c) cannulation of the ampulla with a Nelaton catheter (Nel) prevents occlusion of the CBD during infusion of the topical hemostatic agent (FS, FloSeal infusion device) and (d) the gelatin matrix-thrombin hemostatic has been infused in the distal part of the CBD (arrowheads), resulting in successful bleeding control.
3. Discussion
As conventional means of hemostasis seem not to be always effective in controlling bleeding from complex injuries where access to the site of bleeding is difficult, the application of topical hemostatic agents might be particularly useful in such situations.3 Several types of hemostatic agents are currently available with diverse modes of function. Floseal® consists of a bovine-derived gelatin matrix component and a human-derived thrombin component. The mechanism of action of gelatin-based hemostatic agents is not completely understood. FloSeal matrix works through a combination of mechanical and pharmacological effects. The granules in the gel swell by 10–20% when contact with blood or body fluid occurs, so that blood flow is reduced by a local tamponade effect. Additionally, the thrombin component reinforces hemostasis through a pharmacological pathway. The double action of this product renders it appropriate for local control of heavy bleeding.9–11
Several studies have been published in the pertinent literature regarding the effectiveness of FloSeal in conditions requiring bleeding control. It has been used in open and laparoscopic urological procedures, in liver trauma, in neurosurgery both in experimental studies and clinical cases.12–15 The most important application of this topical hemostatic agent is probably a number of ear, nose and throat procedures, where access to a bleeding site at these anatomical areas may be extremely difficult.16 In our case we took advantage of this specific feature of the product, i.e. its effectiveness in areas with difficult access. As bleeding from the lower part of the CBD could not be otherwise controlled, we used the special device included for the local application of the hemostatic with special care not to occlude the lumen of the CBD. This novel technique of intraluminal bleeding control has not been previously described for the surgically challenging area of CBD and sphincter of Oddi.
The application of a topical hemostatic agent onto the epithelium of the CBD was not associated with any short-term complications in our patient. However, safety of these agents remains a crucial issue as the extent of tissue reaction against them has not been fully elucidated. When implanted subdurally in rabbit model these agents appeared safe, although transient granulomatous inflammation of variable severity was described. Some of the reported mild adverse effects of gelatin-based hemostatic agents include foreign-body reactions, excessive fibrosis, fever and failure of absorption. Multiple neurological events have been described when used in laminectomies, such as spinal stenosis, meningitis, headaches, paresthesias, pain and bladder and bowel dysfunction.17 The long-term effects of FloSeal on CBD epithelium cannot be accurately predicted.
4. Conclusion
The application of topical hemostatic agents is a very useful tool for the quick and safe control of bleeding, especially in areas where surgical access is difficult and the conventional hemostatic means are only inadequately effective. Life-threatening bleeding arising in the CBD obviously belongs to these situations. Nevertheless, further studies are required both in the direction of safety documentation of these products and improvement of infusion devices as well as the effectiveness of their components.
Conflicts of interest statement
None declared.
Funding
No funding sources were available for this study.
Ethical approval
A written informed consent was obtained by the patient's husband since the patient remained at the ICU for a long time.
Author contributions
Dimitrios Dimitroulis: study design and manuscript preparation; Nikolaos P. Karidis: data collection and manuscript preparation; Efstathios Antoniou: surgical management and manuscript revision; Konstantinos Kontzoglou: surgical management and bibliographic analysis; Gregory Kouraklis: supervision of data and outcome comparison analysis.
References
- 1.Zwischenberger J.B., Brunston R.L., Jr., Swann J.R., Conti V.R. Comparison of two topical collagen-based hemostatic sponges during cardiothoracic procedures. Journal of Investigative Surgery. 1999;12:101–106. doi: 10.1080/089419399272656. [DOI] [PubMed] [Google Scholar]
- 2.Tan S.R., Tope W.D. Effectiveness of microporous polysaccharide hemospheres for achieving hemostasis in mohs micrographic surgery. Dermatologic Surgery. 2004;30:908–914. doi: 10.1111/j.1524-4725.2004.30261.x. [DOI] [PubMed] [Google Scholar]
- 3.Seyednejad H., Imani M., Jamieson T., Seifalian A.M. Topical hemostatic agents. British Journal of Surgery. 2008;95:1197–1225. doi: 10.1002/bjs.6357. [DOI] [PubMed] [Google Scholar]
- 4.Freeman M.L., Nelson D.B., Sherman S. Complications of endoscopic biliary sphincterotomy. New England Journal of Medicine. 1996;335:909–918. doi: 10.1056/NEJM199609263351301. [DOI] [PubMed] [Google Scholar]
- 5.Loperfido S., Angelini G., Benedetti G. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointestinal Endoscopy. 1998;48:1–10. doi: 10.1016/s0016-5107(98)70121-x. [DOI] [PubMed] [Google Scholar]
- 6.Masci E., Toti G., Mariani A. Complications of diagnostic and therapeutic ERCP: a prospective multicenter study. American Journal of Gastroenterology. 2001;96:417–423. doi: 10.1111/j.1572-0241.2001.03594.x. [DOI] [PubMed] [Google Scholar]
- 7.Salminen P., Laine S., Gullichsen R. Severe and fatal complications after ERCP: analysis of 2555 procedures in a single experienced center. Surgical Endoscopy. 2008;22:1965–1970. doi: 10.1007/s00464-007-9711-0. [DOI] [PubMed] [Google Scholar]
- 8.Pawa S., Al-Kawas F.H. ERCP in the management of biliary complications after cholecystectomy. Current Gastroenterology Reports. 2009;11:160–166. doi: 10.1007/s11894-009-0025-3. [DOI] [PubMed] [Google Scholar]
- 9.Oz M.C., Rondinone J.F., Shargill N.S. FloSeal Matrix: new generation topical hemostatic sealant. Journal of Cardiac Surgery. 2003;18:486–493. doi: 10.1046/j.0886-0440.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 10.Renkens K.L., Jr., Payner T.D., Leipzig T.J. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976) 2001;26:1645–1650. doi: 10.1097/00007632-200108010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Oz M.C., Cosgrove D.M., 3rd, Badduke B.R. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. The Fusion Matrix Study Group. Annals of Thoracic Surgery. 2000;69:1376–1382. doi: 10.1016/s0003-4975(00)01194-2. [DOI] [PubMed] [Google Scholar]
- 12.Richter F., Schnorr D., Deger S. Improvement of hemostasis in open and laparoscopically performed partial nephrectomy using a gelatin matrix-thrombin tissue sealant (FloSeal) Urology. 2003;61(January (1)):73–77. doi: 10.1016/s0090-4295(02)02143-x. [DOI] [PubMed] [Google Scholar]
- 13.Bak J.B., Singh A., Shekarriz B. Use of gelatin matrix thrombin tissue sealant as an effective hemostatic agent during laparoscopic partial nephrectomy. Journal of Urology. 2004;171:780–782. doi: 10.1097/01.ju.0000104800.97009.c6. [DOI] [PubMed] [Google Scholar]
- 14.Klemcke H.G. Evaluation of FloSeal as a potential intracavitary hemostatic agent. Journal of Trauma. 2006;60:385–389. doi: 10.1097/01.ta.0000204440.48338.79. [DOI] [PubMed] [Google Scholar]
- 15.Dogulu F., Durdag E., Cemil B., Kurt G., Ozgun G. The role of FloSeal in reducing epidural fibrosis in a rat laminectomy model. Neurologia i Neurochirurgia Polska. 2009;43:346–351. [PubMed] [Google Scholar]
- 16.Mathiasen R.A., Cruz R.M. Prospective, randomized, controlled clinical trial of a novel matrix hemostatic sealant in patients with acute anterior epistaxis. Laryngoscope. 2005;115:899–902. doi: 10.1097/01.MLG.0000160528.50017.3C. [DOI] [PubMed] [Google Scholar]
- 17.Buchowski J.M., Bridwell K.H., Lenke L.G., Good C.R. Epidural spinal cord compression with neurologic deficit associated with intrapedicular application of hemostatic gelatin matrix during pedicle screw insertion. Spine (Phila Pa 1976) 2009;34:E473–E477. doi: 10.1097/BRS.0b013e3181a56a21. [DOI] [PubMed] [Google Scholar]


