Abstract
We explored the possibility of the cysteinyl leukotriene receptor antagonists, pranlukast and montelukast, preventing tumor cell migration through both cerebral and peripheral capillaries. To study tumor cell migration through brain capillaries, male Fisher rats were cannulated via the cisterna magna under pentobarbital anesthesia. RCN9 cells labeled with a fluorescent marker PKH67 were intravenously administered following arachidonic acid administration into the subarachnoid space, and specimens of the central nervous system were collected every 30 min for 8 h. Arachidonic acid increased the fluid volume with elevated white blood cell and RCN9 cell counts. When given 2 h before arachidonic acid administration, pranlukast, but not montelukast, reduced the fluid volume and inhibited white blood cell and RCN9 cell extravasation through the brain capillary. In addition, a Lewis lung carcinoma metastasis model in mice was used to study the inhibitory effect of pranlukast and montelukast against cancer cell extravasation through general capillaries. When mice were given food containing either pranlukast or montelukast, immediately after paw amputation, tumor metastasis was prevented by both drugs, and their survival was prolonged. These results show that pranlukast can inhibit tumor cell migration through both the brain and peripheral capillaries, whereas montelukast inhibits tumor cell migration only in the peripheral capillaries.
Keywords: pranlukast, montelukast, cysteinyl leukotriene receptor antagonist, metastasis inhibition, capillary permeability
INTRODUCTION
Metastasis is the primary cause of death in most cancer patients. Nevertheless, an anti-tumor metastasis agent is not yet available for clinical use.
It is known that selectins, integrins, cadherins, and CD44 play an important role in hematogenic tumor metastasis. Since it has been reported that the adhesion of cancer cells to vascular endothelial cells is a key step in tumor cell extravasation1, 2, many studies have focused on the inhibition of the binding of sialyl Lewis x and sialyl Lewis a antigen with E- and P-selectins on the activated endothelium by using competitive ligand inhibitors, metabolic carbohydrate mimetics, or neutralizing monoclonal antibodies. However, these efforts have not yet produced any fruitful results. Kannagi3 suggested that the inhibition of E-, P-, and L-selectin might block tumor cell extravasation.
Cysteinyl leukotriene receptor antagonists such as pranlukast, montelukast, and zafirlukast are well known inhibitors of slow-reacting substance of anaphylaxis (SRSA), a mixture of LTC4, LTD4, and LTE4, and hence, useful for treating asthma and allergy by suppressing both capillary permeability and white blood cell (WBC) extravasation. This fact led us to consider that these cysteinyl leukotriene receptor antagonists might also inhibit tumor metastasis because cell extravasation is a common event in inflammation and tumor metastasis.
In our present study, 2 animal models are proposed to investigate cell extravasation during the process of inflammation and tumor metastasis in rats and mice. In vivo morphological studies revealed useful information on the process of tumor cell extravasation. Using these animal models, our studies were mainly focused on demonstrating the anti-metastatic action of 2 leukotriene receptor antagonists, i.e., pranlukast and montelukast. Possible clinical applications of these drugs are also discussed.
MATERIALS AND METHODS
Tumor cell lines and culture conditions
A rat colon cancer cell line (RCN9) was obtained from Riken BRC Cell Bank, Tsukuba, Japan, and a mouse Lewis lung carcinoma (LLC) cell line was purchased from Health Science Research Resources Bank, Osaka, Japan. Cells were cultured at 37° C under 5% CO2 in PRMI-1640 (SIGMA, St. Louis, MO, USA) supplemented with 10% fetal bovine serum, 2 mM L-glutamine (Gibco, Grand Island, NY, USA), 100 units penicillin, and 100 μg/ml streptomycin (Gibco).
Animals
Male Fisher 344 rats (weighing approximately 400 ± 50g) and female BDF1 mice (6 weeks old) were purchased from Sankyo Lab-Service Co. (Sapporo, Japan). Animals were maintained in a ventilated, temperature-controlled (24 ± 1C°) envelopment, on a bed of wood shavings with free access to water and food and were subjected to a 12-h light-dark cycle. The present experiments were in compliance with the guidelines for animal care followed at the Sapporo Medical University School of Medicine.
Chemicals
Sodium pentobarbital (Nembutal injection), a product of Abbott Laboratories (Abbott Park, Chicago, IL) and PKH67 Green Fluorescent Cell Linker Kit, a product of Zynaxis Cell Science Inc., were purchased from Dainippon Sumitomo Pharmaceutical Co. (Osaka, Japan). Arachidonic acid (sodium salts) from porcine liver was obtained from Calbiochem (San Diego, CA, USA). Dextran (M.W. 190,000–230,000) was obtained from Nacalai Tesque Inc. (Kyoto, Japan), and 2-methylbutane was obtained from Kishida Chemical Co., Ltd. (Osaka, Japan). Malinol, a mounting agent, was obtained from Muto Pure Chemicals Co., Ltd. (Tokyo, Japan). Tissue-Tek OCT compound was obtained from Sakura Finetek (Tokyo, Japan). Sterile phosphate buffered saline (PBS), which contains 144 mg (=1.06 mM) of KH2PO4, 9000 mg (=154 mM) of NaCl, and 795 mg (=5.60 mM) of Na2 HPO4 in 1000 ml of H2O was obtained from Cambrex Charles City Inc. (IA, USA). ONON, containing 112.5 mg/capsules of pranlukast, was purchased from Ono Pharmaceutical Co. (Osaka, Japan). Singulair tablets (10 mg) that contain 10 mg/tablet of montelukast and are a product of Merck & Co. Inc. (White House Station, NJ, USA) were purchased from Banyu Pharmaceutical Co., Ltd. (Tokyo, Japan).
Instruments
A cryostat (Tissue Tek Cryo 2000; Bayer Co., NY, USA), fluorescence microscopes (ProvisAX x 80; Olympus, Tokyo, Japan and Axiovert S 100; Zeiss, Germany), and a stereotaxic unit (Type SRS-6; Narishige Scientific Instrument Lab, Tokyo, Japan) were used.
Miscellany
Polyethylene tube (SP-10; I.D.: 0.28 mm; O.D.: 0.61 mm) was obtained from Natsume Seisakusho Co. (Tokyo, Japan).
The morphological clarification of tumor cell extravasation in vivo
1. RCN9 cell extravasation through the brain capillary
Rats were anesthetized using sodium pentobarbital (50 mg/kg i.p.). A femoral vein was exposed for the intravenous injection of cancer cells. The rats were then placed in a stereotaxic unit. An 18-gauge needle was inserted through the dura via the cisterna magna, and a cannula (SP-10) was introduced into the subarachnoid space (Figure 1A). The cannula was fixed with cyanoacrylic glue to the surrounding peripheral tissue. Arachidonic acid (816 ng/2 μl of PBS) was administered through the cannula, and subsequently, specimens of the CNS (CNS specimens) were collected.
Figure 1.
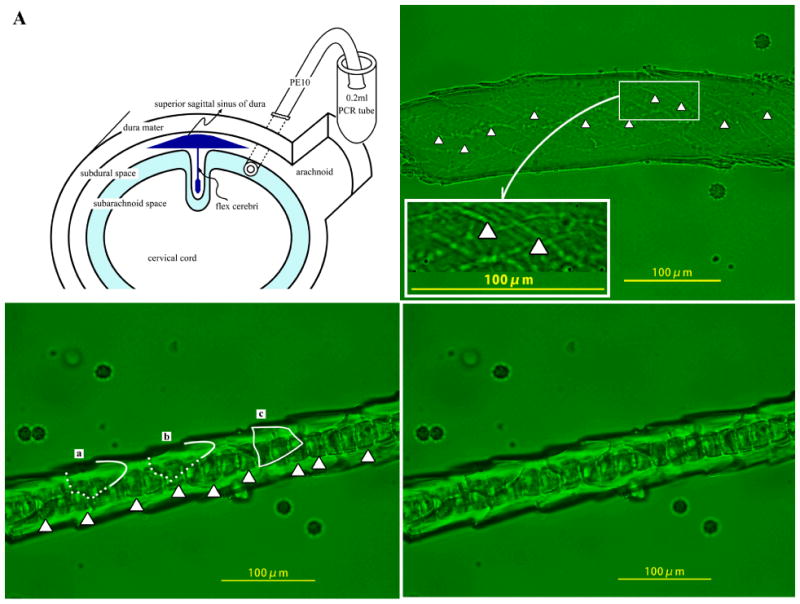
An SP-10 polyethylene tube inserted into rat cisterna magna was used for administration of arachidonic acid and collection of cerebrospinal specimens (A). Capillary of pia mater was observed by fluoromicroscopy 30 min after intracerebrospinal injection of arachidonic acid in rats administered with (C-2) or without (B) RCN9 cells. Arrowheads show the endothelial cell junction (B). Contour of RCN9 cells is traced (C-1).
RCN9 cells that originated from the Fisher rats were stained with a fluorescent marker PKH 67 linker kit. These cells (between 1.88 × 104 and 2.43 × 104) were suspended in PBS, which amounts to approximately 1/100th of the number of neutrophils in the body of each rat, were intravenously administered immediately before arachidonic acid injection to the subarachnoid space. RCN9 cells in the CNS specimen were examined under a fluorescent microscope (Axisovert S 100), and the cells with a flat, square shape were counted.
2. RCN9 cell extravasation in the general capillary
RCN9 cells were intravenously administered immediately before intradermal injection of 0.1 ml of 0.01% dextran into the rat’s hind paw; this induces edema without WBC extravasation. The fresh tissues of the paw were harvested 15–30 min after dextran injection and carefully embedded into OCT compound in a plastic mold, while taking care to avoid trapping air bubbles. The mold was covered with a piece of flat metal, soaked in 2-methylbutane, and frozen with liquid nitrogen for 30 seconds. Then, 10-μm-thick frozen sections were obtained using the cryostat, mounted on the slides, dried on the heating plate for 30 seconds and finally fixed with a mounting agent (malinol). These frozen sections of the rat paw were examined for the presence of RCN9 cells under a fluorescent microscope (ProvisAX x 80).
The pharmacological studies evaluating cysteinyl leukotriene receptor antagonists in the arachidonic acid-induced inflammation and tumor metastasis
Pranlukast (450 mg/kg) or montelukast (10 mg/kg) was administered orally 2 h before arachidonic acid injection into the subarachnoidal space. Equipotent doses of each cysteinyl leukotriene receptor antagonist were chosen taking into account the daily dose recommended for clinical use. RCN9 cells were intravenously administered immediately before arachidonic acid injection. A CNS specimen was collected every 30 min after arachidonic acid injection and the volume of CNS specimen, WBC counts, and RCN9 cell counts were measured for a period of 8 h.
Effect of cysteinyl leukotriene receptor antagonists on a spontaneous metastasis model of LLC cells
LLC cells (5 × 105) were inoculated into the footpad of BDF1 mice for a passage culture. When the diameter of tumors reached 10 mm, the tumor-cell-inoculated foot was amputated under ether anesthesia. Then, the mice were given powdered food containing pranlukast (0 mg/kg, 7.5 mg/kg, 41.25 mg/kg, 225 mg/kg, 450 mg/kg, and 675 mg/kg) or montelukast (0 mg/kg, 0.178 mg/kg, and 10 mg/kg). These doses were adjusted mg/kg/day by changing the drug concentration in the powdered food. The survival rate was evaluated 4 months after amputation and used as an indicator for anti-metastatic effect of these leukotriene receptor antagonists on peripheral spontaneous metastasis.
Statistical analysis
All data were summarized, and we analyzed the total volume of the CNS specimen and counts of WBC and RCN9 cells collected over an 8-h experimental period. Comparison between the means of multiple groups were analyzed by the Mann-Whitney’s U-test using SPSS 16.0J for Windows (SPSS Inc. Chicago, IL, USA) in pharmacological studies for evaluating cysteinyl leukotriene receptor antagonists on arachidonic acid-induced inflammation and tumor metastasis.
Effect of cysteinyl leukotriene receptor antagonists on a spontaneous metastasis model of LLC cells was analyzed by plotting Kaplan-Meier survival curves4 with log-rank and Wilcoxon test using PRISM ver.5 software package (GraphPad Software, Inc., La Jolla, CA, USA). Significance was set at P < 0.05.
RESULTS
Morphological studies
1) RCN9 cell extravasation through the brain capillary
The morphological changes in the brain capillaries were studied by intravenous injection of the fluorescent marker solution free of RCN9 cells and subsequent injection of arachidonic acid into the subarachnoidal space (Figure 1B). A piece of the brain capillary was obtained in the CNS specimen within 30 min of arachidonic acid injection. Arrowheads pointing towards the irregular gaps indicate the openings of the blood brain barrier caused by arachidonic acid. Arachidonic acid induced RCN9 cell extravasation in the brain capillary (Figures 1C-1 and 1C-2). The process of transmigration of cancer cells from the brain capillaries was revealed morphologically by comparing the abovementioned images. The outlines of cancer cells at the inside and the outside of the brain capillary are traced by the dotted and the solid lines, respectively (Figure 1C-1), on the original picture (Figure 1C-2). RCN9 cells (a and b) in Figure 1C-1 are shown escaping from the brain capillary. RCN9 cell c in Figure 1C-1 has extravasated. As observed in the CNS specimen, the RCN9 cell after extravasation is flat and square shaped. Dark square-shaped substances shown with arrowheads in Figure 1C-1 are neutrophils adhered to the endothelial cells. Figures 2A and 2B are representative images of RCN9 cells obtained within 30 min after extravasations from the brain capillary in the CNS specimen.
Figure 2.

Representative images of RCN9 cells (A and B) in the CNS specimen within 30 min after extravasations.
2) RCN9 cell extravasation through the general capillary
The morphological changes of cancer cells were examined in the general capillary in the rat paw edema induced by dextran. Figure 3A shows RCN9 cells attached to the inside of the peripheral capillary. Arrowheads show RCN9 cells that were adhered to the gaps of endothelial cells. The shape of the RCN9 cells had changed from spherical to flat and square before transmigration through the junctions of endothelial cells. Figure 3B shows RCN9 cells (shown by arrowheads) during the transmigration through the general capillary; however, neither white blood cell adhesion nor the transmigration in the peripheral capillary is induced by dextran (Figures 3A and 3B).
Figure 3.
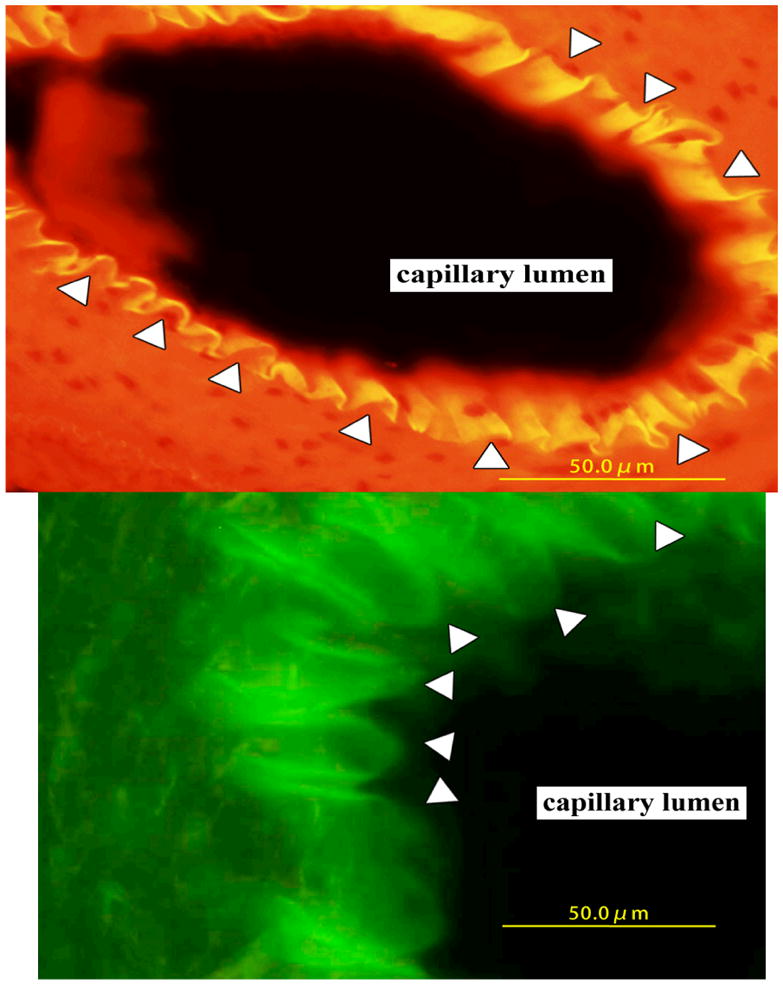
Histological section of dextran-induced edema in rat foot pad. Arrowheads show RCN9 cells. The general capillary is shown with (A) or without orange color filter (B).
The pharmacological studies evaluating cysteinyl leukotriene receptor antagonists in increased capillary permeability and WBC extravasation in the inflammation induced by arachidonic acid
Arachidonic acid (816 ng/2 μl) injection into the subarachnoidal space immediately increased capillary permeability, which continued throughout an 8-h experimental period (Figure 4A). On the other hand, WBC counts started to increase 30–60 min after arachidonic acid injection, maintaining its high level until the end of the experimental period (Figure 4A). Remarkably, pranlukast inhibited both increased capillary permeability (Figures 4B and 5A) and WBC extravasation (Figures 4B and 5B) induced by arachidonic acid in the brain capillary, whereas montelukast exerted no such inhibitory effect under otherwise identical experimental conditions (Figure 4C), instead, it caused an enhancement of both capillary permeability and WBC extravasation (Figure 5A)
Figure 4.
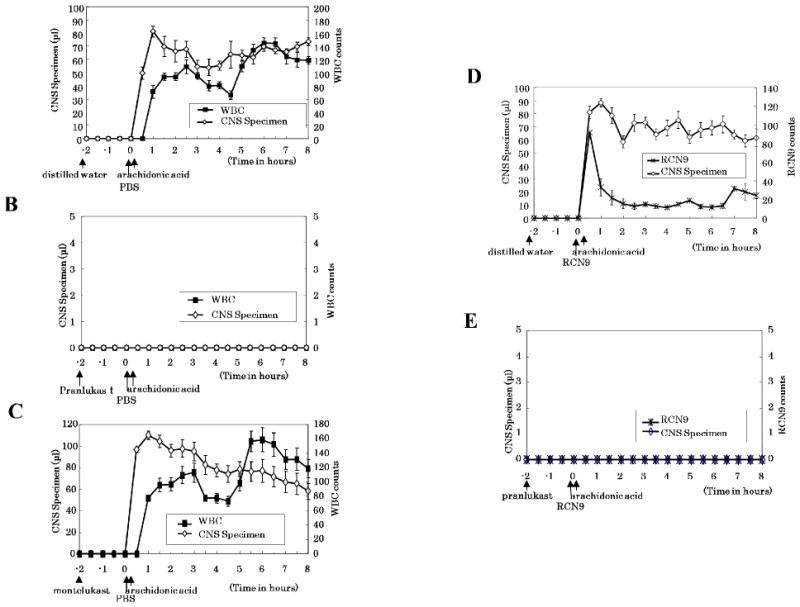
Time-dependent effect of pranlukast and montelukast on the arachidonic acid-induced CNS specimen (μl) (◇), counts of WBC (■) (A, B, C), and RCN9 cells (×) (D, E) are shown. Symbols represent the mean and the s.e.m. n = 6. Rats were pretreated with pranlukast (B, E), montelukast (C), or distilled water (A, D), and CNS specimen was collected every 30 min up to 8 h after arachidonic acid injection.
Figure 5.
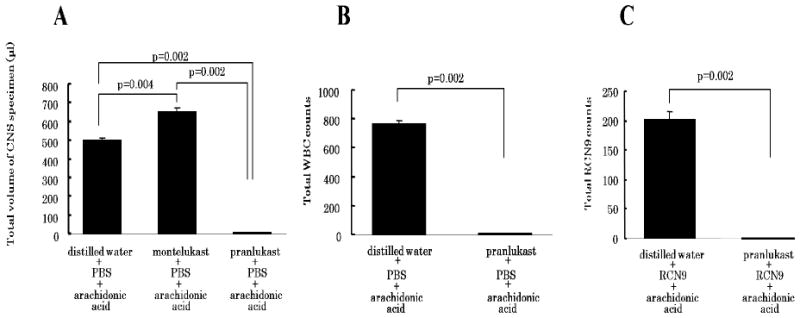
The summary of the results of pharmacological effects of cysteinyl leukotriene receptor antagonists on inflammation and tumor cell migration induced by arachidonic acid in the brain capillary. Total volume of cerebrospinal specimen (A) and counts of WBC (B) and RCN9 cells (C) examined over an 8-h experimental period are calculated from Figure 3(A–E). Bars represent the mean and the s.e.m. n = 6
The effect of cysteinyl leukotriene receptor antagonists on RCN9 cell extravasation induced by arachidonic acid
When RCN9 cells were intravenously injected immediately before arachidonic acid to the subarachnoidal space, RCN9 cell extravasation was observed in the CNS specimen immediately after arachidonic acid injection (Figure 4D). The RCN9 cell counts peaked at 30 min after arachidonic acid injection and then decreased sharply (Figure 4D). Both, increased capillary permeability and RCN9 cell extravasation were inhibited by pretreatment with pranlukast (450 mg/kg) (Figures 4E and 5A), but not with montelukast (10 mg/kg) (data not shown).
The effect of leukotriene receptor antagonist on a spontaneous metastasis model of Lewis lung carcinoma cells
The lung metastasis experiment was performed to determine the survival rate of mice for up to 4 months after paw amputation. Lung cancer metastasis was proven by gross examination of the lung after the death of mice. All the mice that did not receive the cysteinyl leukotriene receptor antagonist died within 1 month. The survival rates of mice treated with pranlukast (7.5–675 mg/kg) (Figure 6A and Table 1) and montelukast (0.178–10 mg/kg) (Figure 6B and Table 1) treatments were as high as 66 – 100%, although they were not dose dependent.
Figure 6.
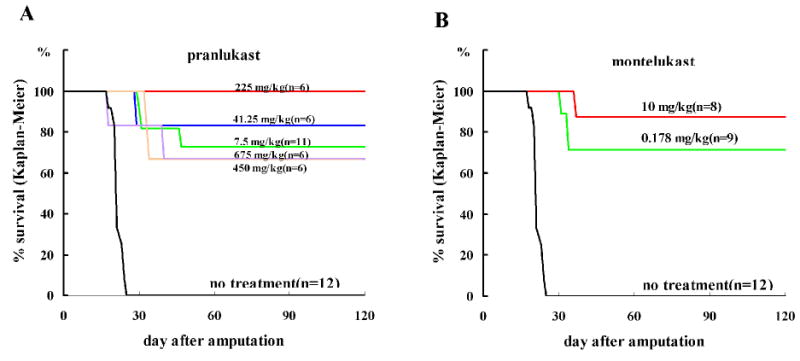
Effect of pranlukast (A) and montelukast (B) on the Kaplan-Meier survival curve of a spontaneous metastasis model in mouse for 4 months after amputation of the paw inoculated with Lewis Lung Carcinoma cells. Log rank test and Wilcoxon test are shown as inset. n = 6–12
Table 1.
Summary of Statistics of Kaplan-Meier Survival Curves
| dose(mg/kg) | log rank test | Gehan-Breslow Wilcoxon test |
|---|---|---|
| pranlukast | ||
| 7.50 | p < 0.0001 | p < 0.0001 |
| 41.25 | p < 0.0001 | p < 0.0006 |
| 225.00 | p < 0.0001 | p < 0.0006 |
| 450.00 | p <0.0001 | p < 0.0006 |
| 675.00 | p < 0.0001 | p < 0.0001 |
|
| ||
| montelukast | ||
| 0.175 | p < 0.0001 | p < 0.0001 |
| 10.00 | p < 0.0001 | p < 0.0001 |
DISCUSSION
A quantitative method to determine increased capillary permeability of the brain microvasculature and extravasation of both WBCs and tumor cells is described in this paper. This method includes subarachnoidal injection of arachidonic acid followed by collection of CNS effluent from the cisterna magna. We assume that the CSF effluent consists mainly of inflammatory exudates from the brain capillary. It provides reliable data because collecting CNS specimens from the same set of animals at given time intervals can eliminate statistical variations that could arise while sampling between individual animals. This method was first applied to determine the phase that is inhibited by drugs during the process of inflammation and tumor metastasis. Furthermore, this method can be used as a tool to clarify the mode of action of the anti-inflammatory and/or anti-tumor metastatic drugs.
Our in vivo morphological studies on both the brain and general capillary revealed that cancer cells can pass through narrow gaps in between the capillary endothelial cells by flattening themselves. The ability of cancer cells to change their shape from spherical to flat and square may facilitate transmigration through the gap between endothelial cells shortly after arachidonic acid administration; the transmigration of WBCs had a delay of over 30 min. The fact that cancer cell extravasation started immediately after a noxious stimulus such as arachidonic acid injection suggests the possibility of causing acute metastasis directly through the capillary rather than forming emboli as generally believed. Therefore, it may be necessary for cancer patients to start taking cysteinyl leukotriene receptor antagonist drugs before the surgery to inhibit this form of acute metastasis.
Our morphological studies do not focus on the effect of cysteinyl leukotriene receptor antagonists on dextran-induced cancer cell extravasation since dextran-induced paw edema is not recognized as an inflammation because of a lack of WBC extravasation. The only reason of using dextran-induced edema is to demonstrate how cancer cells change their shape during transmigration through the general capillary without the effects of other factors such as WBC extravasation.
This is the first report showed that pranlukast, but not montelukast, is able to inhibit increased capillary permeability and extravasation of both WBCs and tumor cells through the brain capillary. As the blood brain barrier has continuous tight junctions, drug penetration into the brain must depend on transcellular rather than paracellular transport. Therefore, it is necessary to overcome this limitation of the blood brain barrier to deliver anti-inflammatory agents and/or anti-tumor metastasis agents that otherwise do not cross the blood brain barrier, and for this reason, there is no drug available for the clinical treatment of encephalic inflammation and brain tumor 5. Drugs that open the blood brain barrier would cause extravasation of blood components from the brain capillary to the CNS because of the large difference between blood pressure (80 – 140 mm Hg) and intracranial pressure (7–15 mm Hg), thereby giving rise to adverse physical conditions in the patients. Since cysteinyl leukotriene receptors are present at the outside of the brain capillary, cysteinyl leukotriene receptor antagonists that do not, or only minimally, cross the blood brain barrier 6 should have no effect on the brain capillary. In fact, the present study demonstrated that montelukast, which is known as a pure cysteinyl leukotriene receptor antagonist, failed to inhibit the extravasation of both WBCs and cancer cells in the brain capillary. The results of our study suggest that arachidonic acid-induced capillary permeability might be further increased by pretreatment with montelukast because of an unknown mechanism. This unexpected finding should be confirmed by future studies.
The inhibitory effect of pranlukast against both inflammation and tumor metastasis through the brain capillary might be attributed to some particular mode of action via peripheral and/or the interior of the blood vessel, including the endothelial cells and the capillary lumen. Pranlukast might block VEGF7, 8 and/or glycoprotein in the blood vessels, because over 98.9% of pranlukast binds to plasma proteins after oral administration in rats9. Therefore, it is possible that pranlukast inhibits not only tumor metastasis but also tumor growth.
Because of the effect of leukotriene receptor antagonists on a spontaneous metastasis model of the LLC cells, both montelukast and pranlukast treatments produced increased survival rates, suggesting that these leukotriene receptor antagonists may inhibit peripheral tumor metastasis. Since the inhibitory effect of pranlukast and montelukast on the permeability in the general capillary have been well documented and used for the treatment of asthma and allergy10–16, the inhibitory effect on the general capillary was not tested in the present study.
Hoff et al. reported that the adhesion of cancer cells to vascular endothelial cells is considered to be a key step in tumor cell extravasation1, 2, Kobayashi et al.17 clarified that metastasis is inhibited by the prevention of cancer cell adhesion to the endothelial cells because of the blocking E-selectin expression, and Kannagi3 proposed that inhibiting the expression of P-, E-, and L-selectins induces a blockade of metastasis. We speculate that pranlukast suppresses expression of all selectins on the vascular endothelial cells, thereby inhibiting tumor cell adhesion and extravasation.
While the process of tumor metastasis and inflammation, once started, is progressive and consecutive, enhanced capillary permeability is a prerequisite, enabling cell extravasation through the capillary. Hence, suppressing the capillary permeability with pranlukast is considered to be the most effective means of preventing these pathological events. Because the mediators that increase capillary permeability are same to those that activate E- and P-selectin on the vascular endothelial cells pranlukast might block not only leukotriene receptor but also the mediators.
Cimetidine was first reported as an anti-tumor metastatic drug in 197918. Matsumoto19 reported that cimetidine had a beneficial effect on survival in colorectal cancer patients, the inhibitory effect of cimetidine (100 mg/kg administered intramuscularly) on increased capillary permeability was studied using our rat model and was found to be potent. It suggests that cimetidine might be useful in inhibiting acute metastasis during surgeries for colorectal cancer patients. Although no severe adverse effects were described in their study, long-term use of cimetidine has, in general, adverse side effects, such as inhibition of P450 system, headache, and gynecomastia. In contrast pranlukast has long been used for treating asthma chronically with little adverse effects20
The clinical applications of pranlukast, as suggested by the present study, include prevention of systemic tumor metastasis and treatment of brain edema, secondary injuries in cerebral circulation failure, and systemic inflammation such as sepsis.
Acknowledgments
We wish to thank Dr. Tomoko Sonoda, Ph.D., for her statistical analysis and Mr. Mikio Fukuda for drawing the figures.
References
- 1.Hoff SD, Matsushita Y, Ota DM, Cleary KR, Yamori T, Hakomori S, Irimura T. Increased expression of sialyl-dimeric LeX antigen in liver metastases of human colorectal carcinoma. Cancer Res. 1989;49:6883–6888. [PubMed] [Google Scholar]
- 2.Hoff SD, Irimura T, Matsushita Y, Ota DM, Cleary KR, Hakomori S. Metastatic potential of colon carcinoma: Expression of ABO/Lewis-related antigens. Arch Surg. 1990;125:206–209. doi: 10.1001/archsurg.1990.01410140084013. [DOI] [PubMed] [Google Scholar]
- 3.Kannagi R. Roles of cell adhesion molecules in cancer metastasis. Nippon Rinsho. 2003;61(suppl 8):87–93. (in Japanese) [Google Scholar]
- 4.Kaplan EMP. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 5.Wilkinson R. Pharmakokinetics: The dynamics of drug absorption, distribution, and elimination. In: Hardman GJ, Limbird EL, editors. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 10. New York NY: McGraw-Hill; 2001. pp. 3–29. [Google Scholar]
- 6.Ishido(A) M, Shibakawa K, Nakao Y, Sawada M, Aishita H. Studies on the metabolic fate of leukotriene antagonist ONO-1078 (1): Absorption, distribution and excretion after single administration to rats. Yakubutsudoutai. 1993;8:3–26. (in Japanese) [Google Scholar]
- 7.Kanazawa H, Yoshikawa T, Hirata K, Yoshikawa J. Effects of pranlukast administration on vascular endothelial growth factor levels in asthmatic patients. Chest. 2004;125:1700–1705. doi: 10.1378/chest.125.5.1700. [DOI] [PubMed] [Google Scholar]
- 8.Lee KS, Kim SR, Park HS, Jin GY, Lee YC. Cysteinyl leukotriene receptor antagonist regulates vascular permeability by reducing vascular endothelial growth factor expression. J Allergy Clin Immunol. 2004;114:1093–1099. doi: 10.1016/j.jaci.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 9.Ishido(B) M, Shibakawa K, Takamoto M, Kajiwara I, Sawada M, Aishita H. Studies on the metabolic fate of leukotriene antagonist ONO-1078 (4); Metabolism and protein binding. Yakubutsudoutai. 1993;8:49–66. (in Jananese) [Google Scholar]
- 10.Bochnowicz S, Underwood DC. Dose-dependent mediation of leukotriene D4-induced airway microvascular leakage and bronchoconstriction in the guinea pig. Prostaglandins Leukot Essent Fatty Acids. 1995;52:403–411. doi: 10.1016/0952-3278(95)90069-1. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel SE. Arachidonic acid metabolites: mediators of inflammation in asthma. Pharmacotherapy. 1997;17:3S–12S. [PubMed] [Google Scholar]
- 12.Tan RA, Spector SL. Antileukotriene agents. Curr Opin Pulm Med. 1997;3:215–20. doi: 10.1097/00063198-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Liu YC, Khawaja AM, Rogers DF. Effects of the cysteinyl leukotriene receptor antagonists pranlukast and zafirlukast on tracheal mucus secretion in ovalbumin-sensitized guinea-pigs in vitro. Br J Pharmacol. 1998;124:563–571. doi: 10.1038/sj.bjp.0701886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bisgaard H. Pathophysiology of the cysteinyl leukotrienes and effects of leukotriene receptor antagonists in asthma. Allergy. 2001;56(Suppl 66):7–11. doi: 10.1034/j.1398-9995.56.s66.2.x. [DOI] [PubMed] [Google Scholar]
- 15.Hele DJ, Birrell MA, Webber SE, Foster ML, Belvisi MG. Mediator involvement in antigen-induced bronchospasm and microvascular leakage in the airways of ovalbumin sensitized Brown Norway rats. Br J Pharmacol. 2001;132:481–488. doi: 10.1038/sj.bjp.0703847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters-Golden M, Henderson WR., Jr The role of leukotrienes in allergic rhinitis. Ann Allergy Asthma Immunol. 2005;94:609–618. doi: 10.1016/S1081-1206(10)61317-8. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi K, Matsumoto S, Morishima T, Kawabe T, Okamoto T. Cimetidine inhibits cancer cell adhesion to endothelial cells and prevents metastasis by blocking E-selectin expression. Cancer Res. 2000;60:3978–3984. [PubMed] [Google Scholar]
- 18.Armitage OJ, Sidner DR. Antitumor effect of cimetidine? The Lancet. 1979;313:882–883. doi: 10.1016/s0140-6736(79)91306-0. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto S, Imaeda Y, Umemoto S, Kobayashi K, Suzuki H, Okamoto T. Cimetidine increases survival of colorectal cancer patients with high levels of sialyl Lewis-X and sialyl Lewis -A epitope expression on tumor cells. Brit J Can. 2002;86:161–167. doi: 10.1038/sj.bjc.6600048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obase Y, Shimoda T, Matsuse H, Kondo Y, Machida I, Kawano T, Saeki S, Tamori S, Mitsuta-Izaki K, Matsuno N, Fukushima C, Kohno S. The position of pranlukast, a cysteinyl leukotriene receptor antagonist, in the long-term treatment of asthma. 5-year follow-up study. Respiration. 2004;71:225–232. doi: 10.1159/000077419. [DOI] [PubMed] [Google Scholar]


